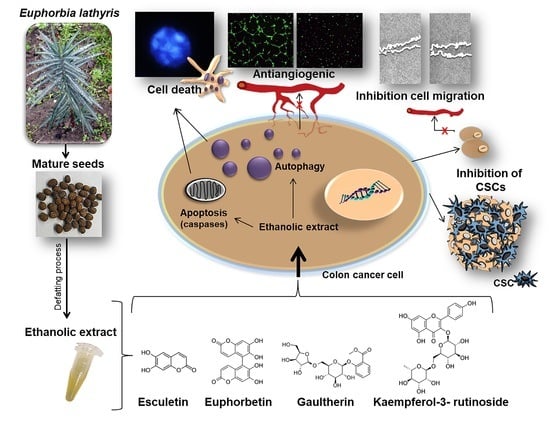
Antitumor Effect of the Ethanolic Extract from Seeds of Euphorbia lathyris in Colorectal Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Ethanolic Extract
2.3. Assessment of Total Polyphenol Content
2.4. Antioxidant Activity
2.5. Chromatographic Studies
2.6. Cell Culture
2.7. Cell Viability Assay
2.8. Western Blot Analysis
2.9. Wound-Healing Assay
2.10. Cell Cycle Analysis
2.11. Alpha-Tubulin Immunofluorescence Assay
2.12. Angiogenesis Assays
2.13. Real Time PCR
2.14. Lysotracker Labeling
2.15. Statistic Studies
3. Results
3.1. Analysis of Yield and Antioxidant Activity
3.2. Mass Spectrometry Analysis of Bioactive Compounds from the Ethanolic Extract of Euphorbia Lathyris
3.3. Effect of the Ethanolic Extract of Euphorbia Lathyris on Cell Viability
3.4. Effect of the Ethanolic Extract of Euphorbia Lathyris on the Cell Cycle
3.5. Analysis of Cell Migration
3.6. Modulation of Angiogenesis by the Ethanolic Extract of Euphorbia Lathyris
3.7. Analysis of Alteration of Stemness Markers
3.8. Molecular Analysis of Cell Death Induction by the Ethanolic Extract of Euphorbia lathyris
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- The Lancet. GLOBOCAN 2018: Counting the toll of cancer. Lancet 2018, 392, 985. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.H.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef] [Green Version]
- Fernández Montes, A.; Martínez Lago, N.; Covela Rúa, M.; de la Cámara Gómez, J.; González Villaroel, P.; Méndez Méndez, J.C.; Jorge Fernández, M.; Salgado Fernández, M.; Reboredo López, M.; Quintero Aldana, G.; et al. Efficacy and safety of FOLFIRI/aflibercept in second-line treatment of metastatic colorectal cancer in a real-world population: Prognostic and predictive markers. Cancer Med. 2019, 8, 882–889. [Google Scholar] [CrossRef]
- Goyal, S.; Gupta, N.; Chatterjee, S.; Nimesh, S. Natural Plant Extracts as Potential Therapeutic Agents for the Treatment of Cancer. Curr. Top. Med. Chem. 2017, 17, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Grace, O.M.; Saslis-Lagoudakis, C.H.; Nilsson, N.; Simonsen, H.T.; Rønsted, N. Global medicinal uses of Euphorbia L. (Euphorbiaceae). J. Ethnopharmacol. 2015, 176, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Remy, S.; Litaudon, M. Macrocyclic Diterpenoids from Euphorbiaceae as A Source of Potent and Selective Inhibitors of Chikungunya Virus Replication. Molecules 2019, 24, 2336. [Google Scholar] [CrossRef] [Green Version]
- Ul-Haq, I.; Ullah, N.; Bibi, G.; Kanwal, S.; Sheeraz Ahmad, M.; Mirza, B. Antioxidant and Cytotoxic Activities and Phytochemical Analysis of Euphorbia wallichii Root Extract and its Fractions. Iran J. Pharm. Res. 2012, 11, 241–249. [Google Scholar] [PubMed]
- Reddy, B.S.; Rao, N.R.; Vijeepallam, K.; Pandy, V. Phytochemical, pharmacological and biological profiles of tragia species (family: Euphorbiaceae). Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 105–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, M.; Saslis-Lagoudakis, C.H.; Grace, O.M.; Nilsson, N.; Simonsen, H.T.; Horn, J.W.; Rønsted, N. Evolutionary prediction of medicinal properties in the genus Euphorbia L. Sci. Rep. 2016, 6, 30531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalho, S.D.; Pinto, M.E.F.; Ferreira, D.; Bolzani, V.S. Biologically Active Orbitides from the Euphorbiaceae Family. Planta Med. 2018, 84, 558–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Wu, Y.; Dalal, S.; Cassera, M.B.; Yue, J.-M. Euphorbesulins A–P, Structurally Diverse Diterpenoids from Euphorbia esula. J. Nat. Prod. 2016, 79, 1952–1961. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Fu, Z.; Bi, Y.; Zheng, J.; Wang, L.; He, X.; Li, F.; Lei, X.; Ren, Q. Chinese herbal medicine Euphorbia esula extract induces apoptosis and inhibits the proliferation, migration and invasion of multidrug resistant gastric carcinoma cells. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2018, 35, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.-Y.; Han, X.-D.; Wang, A.-H.; Liu, X.-B. Apoptosis of human gastric carcinoma cells induced by Euphorbia esula latex. World J. Gastroenterol. 2016, 22, 3564–3572. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.S.; de Oliveira, T.L.; Kanunfre, C.C.; Paludo, K.S.; Minozzo, B.R.; Prestes, A.P.; Wang, M.; Fernandes, D.; Santos, F.A.; Manda, V.K.; et al. Pharmacokinetics and cytotoxic study of euphol from Euphorbia umbellata (Bruyns) Pax latex. Phytomedicine 2018, 47, 105–112. [Google Scholar] [CrossRef]
- Silva, V.A.O.; Rosa, M.N.; Tansini, A.; Oliveira, R.J.S.; Martinho, O.; Lima, J.P.; Pianowski, L.F.; Reis, R.M. In vitro screening of cytotoxic activity of euphol from Euphorbia tirucalli on a large panel of human cancer-derived cell lines. Exp. Ther. Med. 2018, 16, 557–566. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Yu, X.; Wang, L.; Zhang, F.; Zhang, Y. Research Progress on Chemical Constituents and Anticancer Pharmacological Activities of Euphorbia lunulata Bunge. BioMed Res. Int. 2020, 2020, 3618941. [Google Scholar] [CrossRef]
- Zhou, M.; Ma, Q.; He, L.; Chen, Y.-H.; Zhu, B.-Y.; Wang, J.-H.; Yang, Q.; Liu, S.; Ma, L.-M. Cytotoxic jatrophane diterpenoids from the aerial parts of Euphorbia helioscopia. J. Asian Nat. Prod. Res. 2020, 1–7. [Google Scholar] [CrossRef]
- Shi, Q.-W.; Su, X.-H.; Kiyota, H. Chemical and pharmacological research of the plants in genus Euphorbia. Chem. Rev. 2008, 108, 4295–4327. [Google Scholar] [CrossRef] [PubMed]
- Vasas, A.; Hohmann, J. Euphorbia diterpenes: Isolation, structure, biological activity, and synthesis (2008–2012). Chem. Rev. 2014, 114, 8579–8612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latansio de Oliveira, T.; Fontana, P.D.; Bavia, L.; Cruz, L.S.; Crisma, A.R.; Sassaki, G.L.; Alencar Menezes, L.R.; Wang, M.; Beltrame, F.L.; Messias-Reason, I.J. Effects of Euphorbia umbellata extracts on complement activation and chemotaxis of neutrophils. J. Ethnopharmacol. 2021, 265, 113348. [Google Scholar] [CrossRef]
- Martins, C.G.; Appel, M.H.; Coutinho, D.S.S.; Soares, I.P.; Fischer, S.; de Oliveira, B.C.; Fachi, M.M.; Pontarolo, R.; Bonatto, S.J.R.; Fernandes, L.C.; et al. Consumption of latex from Euphorbia tirucalli L. promotes a reduction of tumor growth and cachexia, and immunomodulation in Walker 256 tumor-bearing rats. J. Ethnopharmacol. 2020, 255, 112722. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.G.; Zhan, Z.J.; Yang, S.P.; Yue, J.-M. Lathyranoic Acid A: First Secolathyrane Diterpenoid in Nature from Euphorbia lathyris. Org. Lett. 2005, 7, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, G.; Huang, J.; Zhang, C.; Zhang, L.; Zhang, K.; Li, P.; Lin, R.; Wang, J. Lathyrane-type diterpenoids from the seeds of Euphorbia lathyris. Phytochemistry 2014, 104, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Neto, S.; Duarte, N.; Pedro, C.; Spengler, G.; Molnár, J.; Ferreira, M.-J.U. Effective MDR reversers through phytochemical study of Euphorbia boetica. Phytochem. Anal. 2019, 30, 498–511. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Chen, X.; Li, T.; Xu, J.; Ma, Y. A myrsinol diterpene isolated from a traditional herbal medicine, LANGDU reverses multidrug resistance in breast cancer cells. J. Ethnopharmacol. 2016, 194, 1–5. [Google Scholar] [CrossRef]
- Bicchi, C.; Appendino, G.; Cordero, C.; Rubiolo, P.; Ortelli, D.; Veuthey, J.L. HPLC-UV and HPLC-positive-ESI-MS analysis of the diterpenoid fraction from caper spurge (Euphorbia lathyris) seed oil. Phytochem. Anal. 2001, 12, 255–262. [Google Scholar] [CrossRef]
- Wei, J.; Lu, L.; Mei-cai, D.; Hua-wu, S.; Run-hua, L. Studies on chemical constituents in seeds of Euphorbia lathyris. Chin. Tradit. Herb. Drugs 2010, 41, 181–187. [Google Scholar]
- Teng, Y.-N.; Wang, Y.; Hsu, P.-L.; Xin, G.; Zhang, Y.; Morris-Natschke, S.L.; Goto, M.; Lee, K.-H. Mechanism of action of cytotoxic compounds from the seeds of Euphorbia lathyris. Phytomedicine 2018, 41, 62–66. [Google Scholar] [CrossRef]
- Fang, X.; Yan, B.; Duan, F.; Li, C.; Zhang, C.; Wang, Y. Chemical composition and biological activity analysis of semen Euphorbiae petroleum ether extracts. Chemistry 2014, 6, 745–749. [Google Scholar]
- Fan, L.; Zhu, H.; Tao, W.; Liu, L.; Shan, X.; Zhao, M.; Sun, D. Euphorbia factor L2 inhibits TGF-β-induced cell growth and migration of hepatocellular carcinoma through AKT/STAT3. Phytomedicine 2019, 62, 152931. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhen, Y.-Q.; Gao, F.; Huang, S.; Zhou, X.-L. Five New Diterpenoids from the Seeds of Euphorbia lathyris. Chem. Biodivers. 2018, 15, e1800386. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Zhang, T.; Wang, Q. The phytochemistry, pharmacokinetics, pharmacology and toxicity of Euphorbia semen. J. Ethnopharmacol. 2018, 227, 41–55. [Google Scholar] [CrossRef]
- Kapravelou, G.; Martínez, R.; Andrade, A.M.; Chaves, C.L.; López-Jurado, M.; Aranda, P.; Arrebola, F.; Cañizares, F.J.; Galisteo, M.; Porres, J.M. Improvement of the antioxidant and hypolipidaemic effects of cowpea flours (Vigna unguiculata) by fermentation: Results of in vitro and in vivo experiments. J. Sci. Food Agric. 2015, 95, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Duh, P.-D.; Tu, Y.-Y.; Yen, G.-C. Antioxidant Activity of Water Extract of Harng Jyur (Chrysanthemum morifolium Ramat). LWT Food Sci. Technol. 1999, 32, 269–277. [Google Scholar] [CrossRef]
- Ortiz, R.; Prados, J.; Melguizo, C.; Rama, A.R.; Segura, A.; Rodríguez-Serrano, F.; Boulaiz, H.; Hita, F.; Martinez-Amat, A.; Madeddu, R.; et al. The cytotoxic activity of the phage E protein suppress the growth of murine B16 melanomas in vitro and in vivo. J. Mol. Med. 2009, 87, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef] [Green Version]
- Vaghari-Tabari, M.; Majidinia, M.; Moein, S.; Qujeq, D.; Asemi, Z.; Alemi, F.; Mohamadzadeh, R.; Targhazeh, N.; Safa, A.; Yousefi, B. MicroRNAs and colorectal cancer chemoresistance: New solution for old problem. Life Sci. 2020, 259, 118255. [Google Scholar] [CrossRef]
- Koklesova, L.; Liskova, A.; Samec, M.; Qaradakhi, T.; Zulli, A.; Smejkal, K.; Kajo, K.; Jakubikova, J.; Behzadi, P.; Pec, M.; et al. Genoprotective activities of plant natural substances in cancer and chemopreventive strategies in the context of 3P medicine. EPMA J. 2020, 11, 261–287. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, C.; Jin, Q.; Jang, H.; Lee, D.; Lee, H.-J.; Shin, J.W.; Han, S.B.; Hong, J.T.; Kim, Y.; et al. Diterpenoids from the Roots of Euphorbia fischeriana with Inhibitory Effects on Nitric Oxide Production. J. Nat. Prod. 2016, 79, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-G.; Li, Z.-L.; Bai, J.; Meng, D.-L.; Li, N.; Pei, Y.-H.; Zhao, F.; Hua, H.-M. Anti-inflammatory diterpenoids from the roots of Euphorbia ebracteolata. J. Nat. Prod. 2014, 77, 792–799. [Google Scholar] [CrossRef]
- Ricigliano, V.A.; Sica, V.P.; Knowles, S.L.; Diette, N.; Howarth, D.G.; Oberlies, N.H. Bioactive diterpenoid metabolism and cytotoxic activities of genetically transformed Euphorbia lathyris roots. Phytochemistry 2020, 179, 112504. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.; Meng, Q.; Tian, Q.; Niu, Y.; Niu, W. Phytochemicals of Euphorbia lathyris L. and Their Antioxidant Activities. Molecules 2017, 22, 1335. [Google Scholar] [CrossRef] [Green Version]
- Nam, K.-A.; Lee, S.-K. Evaluation of Cytotoxic Potential of Natural Products in Cultured Human Cancer Cells. Nat. Prod. Sci. 2000, 6, 183–188. [Google Scholar]
- Wang, C.J.; Hsieh, Y.J.; Chu, C.Y.; Lin, Y.L.; Tseng, T.H. Inhibition of cell cycle progression in human leukemia HL-60 cells by esculetin. Cancer Lett. 2002, 183, 163–168. [Google Scholar] [CrossRef]
- Park, S.; Park, S.; Lim, J.; Choi, Y.; Kim, W.; Moon, S. PRIME PubMed | Esculetin inhibits cell proliferation through the Ras/ERK1/2 pathway in human colon cancer cells. Oncol. Rep. 2011, 25, 223–230. [Google Scholar]
- Turkekul, K.; Colpan, R.D.; Baykul, T.; Ozdemir, M.D.; Erdogan, S. Esculetin Inhibits the Survival of Human Prostate Cancer Cells by Inducing Apoptosis and Arresting the Cell Cycle. J. Cancer Prev. 2018, 23, 10–17. [Google Scholar] [CrossRef]
- Chang, H.-T.; Chou, C.-T.; Lin, Y.-S.; Shieh, P.; Kuo, D.-H.; Jan, C.-R.; Liang, W.-Z. Esculetin, a natural coumarin compound, evokes Ca2+ movement and activation of Ca2+-associated mitochondrial apoptotic pathways that involved cell cycle arrest in ZR-75-1 human breast cancer cells. Tumor Biol. 2016, 37, 4665–4678. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Sawney, S.; Saini, V.; Steffi, C.; Tiwari, M.; Saluja, D. Esculetin induces antiproliferative and apoptotic response in pancreatic cancer cells by directly binding to KEAP1. Mol. Cancer 2016, 15, 64. [Google Scholar] [CrossRef] [Green Version]
- Choi, Y.J.; Lee, C.M.; Park, S.-H.; Nam, M.J. Esculetin induces cell cycle arrest and apoptosis in human colon cancer LoVo cells. Environ. Toxicol. 2019, 34, 1129–1136. [Google Scholar] [CrossRef]
- Kim, A.D.; Han, X.; Piao, M.J.; Hewage, S.R.K.M.; Hyun, C.L.; Cho, S.J.; Hyun, J.W. Esculetin induces death of human colon cancer cells via the reactive oxygen species-mediated mitochondrial apoptosis pathway. Environ. Toxicol. Pharmacol. 2015, 39, 982–989. [Google Scholar] [CrossRef]
- Wang, X.; Yang, C.; Zhang, Q.; Wang, C.; Zhou, X.; Zhang, X.; Liu, S. In vitro anticancer effects of esculetin against human leukemia cell lines involves apoptotic cell death, autophagy, G0/G1 cell cycle arrest and modulation of Raf/MEK/ERK signalling pathway. J. BUON 2019, 24, 1686–1691. [Google Scholar] [PubMed]
- Park, S.L.; Won, S.Y.; Song, J.-H.; Lee, S.-Y.; Kim, W.-J.; Moon, S.-K. Esculetin Inhibits VEGF-Induced Angiogenesis Both In Vitro and In Vivo. Am. J. Chin. Med. 2016, 44, 61–76. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, J.; Li, X.; Tian, R.; Shang, K.; Dong, X.; Cao, B. Angiogenesis is promoted by exosomal DPP4 derived from 5-fluorouracil-resistant colon cancer cells. Cancer Lett. 2021, 497, 190–201. [Google Scholar] [CrossRef] [PubMed]
- Hirata, A.; Hatano, Y.; Niwa, M.; Hara, A.; Tomita, H. Heterogeneity of Colon Cancer Stem Cells. Adv. Exp. Med. Biol. 2019, 1139, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xia, L.; Wang, H.; Oyang, L.; Su, M.; Liu, Q.; Lin, J.; Tan, S.; Tian, Y.; Liao, Q.; et al. Cancer stem cells in progression of colorectal cancer. Oncotarget 2017, 9, 33403–33415. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Euphorbia Lathyris | Yield * | Total Polyphenols ** | Antioxidant Activity ** |
|---|---|---|---|
| Ethanolic extract from defatted flour of mature seeds | 117.5 ± 9.22 | 33.5 ± 5.79 | 23.0 ± 1.92 |
| Compound | MF | [M-H]- | RT | PPM | % Conf. |
|---|---|---|---|---|---|
| Esculetin | C9H6O4 | 177.0181 | 2.62 | −4 | 90–100 |
| 177.0185 | 2.61 | −1.7 | |||
| Euforbetin | C18H10O8 | 353.0298 | 3.17 | −0.6 | 90–100 |
| 353.0301 | 3.14 | 1.1 | |||
| Gaultherin | C19H26O12 | 445.1349 | 1.89 | 0.7 | 90–100 |
| 445.1351 | 1.81 | 1.1 | |||
| Carnosol | C20H26O4 | 329.1744 | 6.1 | −2.7 | 90–100 |
| 329.175 | 6.11 | −0.9 | |||
| Kaempferol-3-rutinóside | C27H30O15 | 593.151 | 3.86 | 0.7 | 90–100 |
| 593.152 | 3.87 | 2.4 |
| Esculetin | Kaempferol-3-Rutinoside | |
|---|---|---|
| ppm (mg/L) | 267.7 ± 34.2 | 25.8 ± 3.27 |
| mg compound/100 mg extract | 0.41 ± 0.12 | 0.03 ± 0.002 |
| IC50 (μg/mL) | |||
|---|---|---|---|
| T84 | HCT15 | CCD18 | |
| Ethanolic extract from defatted flour of mature seeds | 16.3 ± 2.54 | 72.9 ± 1.27 | 266.0 ± 18.5 |
| 5-Fu (µM) | 2.68 ± 0.16 | 6.58 ± 0.35 | 7.35 ± 0.41 |
| IC50 (μg/mL) | ||||||
|---|---|---|---|---|---|---|
| SF-268 | SK-N-SH | A-172 | LN-229 | PANC-1 | MCF-7 | |
| Ethanolic extract from defatted flour of mature seeds | 39.3 ± 13.2 | 71.4 ± 13.6 | 18.6 ± 1.64 | 70.5 ± 4.48 | 185.8 ± 25.8 | 89.6 ± 6.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mesas, C.; Martínez, R.; Ortíz, R.; Galisteo, M.; López-Jurado, M.; Cabeza, L.; Perazzoli, G.; Melguizo, C.; Porres, J.M.; Prados, J. Antitumor Effect of the Ethanolic Extract from Seeds of Euphorbia lathyris in Colorectal Cancer. Nutrients 2021, 13, 566. https://doi.org/10.3390/nu13020566
Mesas C, Martínez R, Ortíz R, Galisteo M, López-Jurado M, Cabeza L, Perazzoli G, Melguizo C, Porres JM, Prados J. Antitumor Effect of the Ethanolic Extract from Seeds of Euphorbia lathyris in Colorectal Cancer. Nutrients. 2021; 13(2):566. https://doi.org/10.3390/nu13020566
Chicago/Turabian StyleMesas, Cristina, Rosario Martínez, Raúl Ortíz, Milagros Galisteo, María López-Jurado, Laura Cabeza, Gloria Perazzoli, Consolación Melguizo, Jesús M. Porres, and Jose Prados. 2021. "Antitumor Effect of the Ethanolic Extract from Seeds of Euphorbia lathyris in Colorectal Cancer" Nutrients 13, no. 2: 566. https://doi.org/10.3390/nu13020566