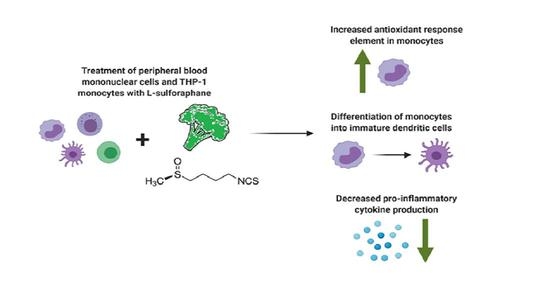
Examination of Novel Immunomodulatory Effects of L-Sulforaphane
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Study Samples
2.3. PBMC Culture
2.4. Flow Cytometry
2.5. Chemokine and Cytokine Measurements
2.6. THP-1 Cell Culture
2.7. Antioxidant Response Element (ARE)-Luciferase Reporter Assay
2.8. Statistical Analysis
3. Results
3.1. LSF and its Metabolites Reduced Chemokines and Cytokines
3.2. LSF Effects on Immune Cell Phenotype
3.3. Immunomodulatory Effects of LSF on DC Populations
3.4. LSF Increases Nrf2-ARE Activity in THP-1 Monocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fahey, J.W.; Talalay, P. Antioxidant functions of sulforaphane: A potent inducer of Phase II detoxication enzymes. Food Chem. Toxicol. 1999, 37, 973–979. [Google Scholar] [CrossRef]
- Ho, E.; Clarke, J.D.; Dashwood, R.H. Dietary sulforaphane, a histone deacetylase inhibitor for cancer prevention. J. Nutr. 2009, 139, 2393–2396. [Google Scholar] [CrossRef] [PubMed]
- Yanaka, A. Role of Sulforaphane in Protection of Gastrointestinal Tract Against H. pylori and NSAID-Induced Oxidative Stress. Curr. Pharm. Des. 2017, 23, 4066–4075. [Google Scholar] [CrossRef] [Green Version]
- Heiss, E.; Herhaus, C.; Klimo, K.; Bartsch, H.; Gerhäuser, C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J. Biol. Chem. 2001, 276, 32008–32015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myzak, M.C.; Karplus, P.A.; Chung, F.L.; Dashwood, R.H. A novel mechanism of chemoprotection by sulforaphane: Inhibition of histone deacetylase. Cancer Res. 2004, 64, 5767–5774. [Google Scholar] [CrossRef] [Green Version]
- Pal, S.; Konkimalla, V.B. Sulforaphane regulates phenotypic and functional switching of both induced and spontaneously differentiating human monocytes. Int. Immunopharmacol. 2016, 35, 85–98. [Google Scholar] [CrossRef]
- Qu, X.; Pröll, M.; Neuhoff, C.; Zhang, R.; Cinar, M.U.; Hossain, M.M.; Tesfaye, D.; Große-Brinkhaus, C.; Salilew-Wondim, D.; Tholen, E.; et al. Sulforaphane epigenetically regulates innate immune responses of porcine monocyte-derived dendritic cells induced with lipopolysaccharide. PLoS ONE 2015, 10, e0121574. [Google Scholar]
- Thejass, P.; Kuttan, G. Immunomodulatory activity of Sulforaphane, a naturally occurring isothiocyanate from broccoli (Brassica oleracea). Phytomedicine 2007, 14, 538–545. [Google Scholar] [CrossRef]
- Deng, Z.; Rong, Y.; Teng, Y.; Mu, J.; Zhuang, X.; Tseng, M.; Samykutty, A.; Zhang, L.; Yan, J.; Miller, D.; et al. Broccoli-Derived Nanoparticle Inhibits Mouse Colitis by Activating Dendritic Cell AMP-Activated Protein Kinase. Mol. Ther. 2017, 25, 1641–1654. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.V.; Warin, R.; Xiao, D.; Powolny, A.A.; Stan, S.D.; Arlotti, J.A.; Zeng, Y.; Hahm, E.R.; Marynowski, S.W.; Bommareddy, A.D.; et al. Sulforaphane inhibits prostate carcinogenesis and pulmonary metastasis in TRAMP mice in association with increased cytotoxicity of natural killer cells. Cancer Res. 2009, 69, 2117–2125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harvey, C.J.; Thimmulappa, R.K.; Sethi, S.; Kong, X.; Yarmus, L.; Brown, R.H.; Feller-Kopman, D.; Wise, R.; Biswal, S. Targeting Nrf2 signaling improves bacterial clearance by alveolar macrophages in patients with COPD and in a mouse model. Sci. Transl. Med. 2011, 3, 78ra32. [Google Scholar] [CrossRef] [Green Version]
- Neumann, M.; Naumann, M. Beyond IkappaBs: Alternative regulation of NF-kappaB activity. FASEB J. 2007, 21, 2642–2654. [Google Scholar] [CrossRef] [PubMed]
- Afonina, I.S.; Zhong, Z.; Karin, M.; Beyaert, R. Limiting inflammation-the negative regulation of NF-κB and the NLRP3 inflammasome. Nat. Immunol. 2017, 18, 861–869. [Google Scholar] [CrossRef]
- Bessler, H.; Djaldetti, M. Broccoli and human health: Immunomodulatory effect of sulforaphane in a model of colon cancer. Int. J. Food Sci. Nutr. 2018, 69, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.A.; Shelar, S.B.; Dang, T.M.; Lee, B.N.; Yang, H.; Ong, S.M.; Ng, H.L.; Chui, W.K.; Wong, S.C.; Chew, E.H. Sulforaphane and its methylcarbonyl analogs inhibit the LPS-stimulated inflammatory response in human monocytes through modulating cytokine production, suppressing chemotactic migration and phagocytosis in a NF-κB- and MAPK-dependent manner. Int. Immunopharmacol. 2015, 24, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Subedi, L.; Lee, J.H.; Yumnam, S.; Ji, E.; Kim, S.Y. Anti-Inflammatory Effect of Sulforaphane on LPS-Activated Microglia Potentially through JNK/AP-1/NF-κB Inhibition and Nrf2/HO-1 Activation. Cells 2019, 8, 194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Pröll, M.J.; Salilew-Wondim, D.; Zhang, R.; Tesfaye, D.; Fan, H.; Cinar, M.U.; Große-Brinkhaus, C.; Tholen, E.; Islam, M.A.; et al. LPS-induced expression of CD14 in the TRIF pathway is epigenetically regulated by sulforaphane in porcine pulmonary alveolar macrophages. Innate Immun. 2016, 22, 682–695. [Google Scholar] [CrossRef]
- Vuong, L.D.; Nguyen, Q.N.; Truong, V.L. Anti-inflammatory and anti-oxidant effects of combination between sulforaphane and acetaminophen in LPS-stimulated RAW 264.7 macrophage cells. Immunopharmacol. Immunotoxicol. 2019, 41, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Bewley, M.A.; Budd, R.C.; Ryan, E.; Cole, J.; Collini, P.; Marshall, J.; Kolsum, U.; Beech, G.; Emes, R.D.; Tcherniaeva, I.; et al. Opsonic Phagocytosis in Chronic Obstructive Pulmonary Disease Is Enhanced by Nrf2 Agonists. Am. J. Respir. Crit Care Med. 2018, 198, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; de Mooij, T.; Peterson, T.E.; Kaptzan, T.; Johnson, A.J.; Daniels, D.J.; Parney, I.F. Modulating glioma-mediated myeloid-derived suppressor cell development with sulforaphane. PLoS ONE 2017, 12, e0179012. [Google Scholar] [CrossRef]
- Atwell, L.L.; Hsu, A.; Wong, C.P.; Stevens, J.F.; Bella, D.; Yu, T.-W.; Pereira, C.B.; Löhr, C.V.; Christensen, J.M.; Dashwood, R.H.; et al. Absorption and chemopreventive targets of sulforaphane in humans following consumption of broccoli sprouts or a myrosinase-treated broccoli sprout extract. Mol. Nutr. Food Res. 2015, 59, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Dinkova-Kostova, A.T.; Wade, K.L.; Zhang, Y.; Shapiro, T.A.; Talalay, P. Quantitative determination of dithiocarbamates in human plasma, serum, erythrocytes and urine: Pharmacokinetics of broccoli sprout isothiocyanates in humans. Clin. Chim. Acta 2002, 316, 43–53. [Google Scholar] [CrossRef]
- Sivapalan, T.; Melchini, A.; Saha, S.; Needs, P.W.; Traka, M.H.; Tapp, H.; Dainty, J.R.; Mithen, R.F. Bioavailability of Glucoraphanin and Sulforaphane from High-Glucoraphanin Broccoli. Mol. Nutr. Food Res. 2018, 62, e1700911. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, M.; Klöpping-Ketelaars, I.W.; van den Berg, R.; Vaes, W.H. Bioavailability and kinetics of sulforaphane in humans after consumption of cooked versus raw broccoli. J. Agric. Food Chem. 2008, 56, 10505–10509. [Google Scholar] [CrossRef] [PubMed]
- Schall, T.J.; Jongstra, J.; Dyer, B.J.; Jorgensen, J.; Clayberger, C.; Davis, M.M.; Krensky, A.M. A human T cell-specific molecule is a member of a new gene family. J. Immunol. 1988, 141, 1018–1025. [Google Scholar]
- Krensky, A.M.; Ahn, Y.T. Mechanisms of disease: Regulation of RANTES (CCL5) in renal disease. Nat. Clin. Pract. Nephrol. 2007, 3, 164–170. [Google Scholar] [CrossRef] [Green Version]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Posch, W.; Lass-Flörl, C.; Wilflingseder, D. Generation of Human Monocyte-derived Dendritic Cells from Whole Blood. J. Vis. Exp. 2016, 118, 54968. [Google Scholar] [CrossRef] [PubMed]
- Boyette, L.B.; Macedo, C.; Hadi, K.; Elinoff, B.D.; Walters, J.T.; Ramaswami, B.; Chalasani, G.; Taboas, J.M.; Lakkis, F.G.; Metes, D.M. Phenotype, function, and differentiation potential of human monocyte subsets. PLoS ONE 2017, 12, e0176460. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, R.T.; Wartman, M.A.; Bailey, H.H.; Gipp, J.J. Constitutive and beta-naphthoflavone-induced expression of the human gamma-glutamylcysteine synthetase heavy subunit gene is regulated by a distal antioxidant response element/TRE sequence. J. Biol. Chem. 1997, 272, 7445–7454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; O’Connor, T.; Yamamoto, M. Keap1 regulates both cytoplasmic-nuclear shuttling and degradation of Nrf2 in response to electrophiles. Genes Cells 2003, 8, 379–391. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Cole, R.N.; Itoh, K.; Wakabayashi, N.; Katoh, Y.; Yamamoto, M.; Talalay, P. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 2002, 99, 11908–11913. [Google Scholar] [CrossRef] [Green Version]
- Bobilev, I.; Novik, V.; Levi, I.; Shpilberg, O.; Levy, J.; Sharoni, Y.; Studzinski, G.P.; Danilenko, M. The Nrf2 transcription factor is a positive regulator of myeloid differentiation of acute myeloid leukemia cells. Cancer Biol. 2011, 11, 317–329. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, B.; Yuan, X.; Yao, Y.; Zhao, H.; Sun, X.; Zheng, Q. Isoliquiritigenin-induced effects on Nrf2 mediated antioxidant defence in the HL-60 cell monocytic differentiation. Cell Biol. Int. 2013, 37, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Shimizu, R.; Romeo, P.H.; Yamamoto, M.; Motohashi, H. Keap1-Nrf2 system regulates cell fate determination of hematopoietic stem cells. Genes Cells 2014, 19, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Marín, E.; Cuturi, M.C.; Moreau, A. Tolerogenic Dendritic Cells in Solid Organ Transplantation: Where Do We Stand? Front. Immunol. 2018, 9, 274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chow, K.V.; Sutherland, R.M.; Zhan, Y.; Lew, A.M. Heterogeneity, functional specialization and differentiation of monocyte-derived dendritic cells. Immunol. Cell Biol. 2017, 95, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Sauter, A.; Yi, D.H.; Li, Y.; Roersma, S.; Appel, S. The Culture Dish Surface Influences the Phenotype and Cytokine Production of Human Monocyte-Derived Dendritic Cells. Front. Immunol. 2019, 10, 2352. [Google Scholar] [CrossRef]
- Yeang, H.X.A.; Hamdam, J.M.; Al-Huseini, L.M.; Sethu, S.; Djouhri, L.; Walsh, J.; Kitteringham, N.; Park, B.K.; Goldring, C.E.; Sathish, J.G. Loss of transcription factor nuclear factor-erythroid 2 (NF-E2) p45-related factor-2 (Nrf2) leads to dysregulation of immune functions, redox homeostasis, and intracellular signaling in dendritic cells. J. Biol. Chem. 2012, 287, 10556–10564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Huseini, L.M.; Yeang, H.X.A.; Sethu, S.; Alhumeed, N.; Hamdam, J.M.; Tingle, Y.; Djouhri, L.; Kitteringham, N.; Park, B.K.; Goldring, C.E.; et al. Nuclear factor-erythroid 2 (NF-E2) p45-related factor-2 (Nrf2) modulates dendritic cell immune function through regulation of p38 MAPK-cAMP-responsive element binding protein/activating transcription factor 1 signaling. J. Biol. Chem. 2013, 288, 22281–22288. [Google Scholar] [CrossRef] [Green Version]
- Unger, W.W.J.; Laban, S.; Kleijwegt, F.S.; van der Slik, A.R.; Roep, B.O. Induction of Treg by monocyte-derived DC modulated by vitamin D3 or dexamethasone: Differential role for PD-L1. Eur. J. Immunol. 2009, 39, 3147–3159. [Google Scholar] [CrossRef]
- Raker, V.K.; Domogalla, M.P.; Steinbrink, K. Tolerogenic Dendritic Cells for Regulatory T Cell Induction in Man. Front. Immunol. 2015, 6, 569. [Google Scholar] [CrossRef] [Green Version]
- Maldonado, R.A.; von Andrian, U.H. How tolerogenic dendritic cells induce regulatory T cells. Adv. Immunol. 2010, 108, 111–165. [Google Scholar] [PubMed] [Green Version]
- López-Relaño, J.; Martín-Adrados, B.; Real-Arévalo, I.; Lozano-Bartolomé, J.; Abós, B.; Sánchez-Ramón, S.; Alonso, B.; del Moral, M.G.; Martínez-Naves, E. Monocyte-Derived Dendritic Cells Differentiated in the Presence of Lenalidomide Display a Semi-Mature Phenotype, Enhanced Phagocytic Capacity, and Th1 Polarization Capability. Front. Immunol. 2018, 9, 1328. [Google Scholar] [CrossRef]
- Domogalla, M.P.; Rostan, P.V.; Raker, V.K.; Steinbrink, K. Tolerance through Education: How Tolerogenic Dendritic Cells Shape Immunity. Front. Immunol. 2017, 8, 1764. [Google Scholar] [CrossRef] [Green Version]
- Van Erp, A.E.; van Kampen, M.R.; van Kasteren, P.B.; de Wit, J. Viral Infection of Human Natural Killer Cells. Viruses 2019, 11, 243. [Google Scholar] [CrossRef] [Green Version]
- Hammer, Q.; Rückert, T.; Romagnani, C. Natural killer cell specificity for viral infections. Nat. Immunol. 2018, 19, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Waisman, A.; Lukas, D.; Clausen, B.E.; Yogev, N. Dendritic cells as gatekeepers of tolerance. Semin. Immunopathol. 2017, 39, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Ha, S.-J. Generation of Tolerogenic Dendritic Cells and Their Therapeutic Applications. Immune Netw. 2016, 16, 52–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benham, H.; Nel, H.J.; Law, S.C.; Mehdi, A.M.; Street, S.; Ramnoruth, N.; Pahau, H.; Lee, B.T.; Ng, J.; Brunck, M.E.; et al. Citrullinated peptide dendritic cell immunotherapy in HLA risk genotype-positive rheumatoid arthritis patients. Sci. Transl. Med. 2015, 7, 290ra87. [Google Scholar] [CrossRef] [Green Version]
- Anderson, A.E.; Swan, D.J.; Wong, O.Y.; Buck, M.; Eltherington, O.; Harry, R.A.; Patterson, A.M.; Pratt, A.G.; Reynolds, G.; Doran, J.P.; et al. Tolerogenic dendritic cells generated with dexamethasone and vitamin D3 regulate rheumatoid arthritis CD4(+) T cells partly via transforming growth factor-β1. Clin. Exp. Immunol. 2017, 187, 113–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kryczanowsky, F.; Raker, V.; Graulich, E.; Domogalla, M.P.; Steinbrink, K. IL-10-Modulated Human Dendritic Cells for Clinical Use: Identification of a Stable and Migratory Subset with Improved Tolerogenic Activity. J. Immunol. 2016, 197, 3607–3617. [Google Scholar] [CrossRef] [PubMed]
- Boks, M.A.; Kager-Groenland, J.R.; Haasjes, M.S.; Zwaginga, J.J.; van Ham, S.M.; Brinke, A.t. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction--A comparative study of human clinical-applicable DC. Clin. Immunol. 2012, 142, 332–342. [Google Scholar] [CrossRef] [PubMed]

 10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: CD, cluster of differentiation; DMSO, dimethyl sulfoxide; IMQ, imiquimod; IQR, inter-quartile range; LPS, lipopolysaccharide; LSF, L-sulforaphane; TLR, toll-like receptor.
10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: CD, cluster of differentiation; DMSO, dimethyl sulfoxide; IMQ, imiquimod; IQR, inter-quartile range; LPS, lipopolysaccharide; LSF, L-sulforaphane; TLR, toll-like receptor.
 10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: CD, cluster of differentiation; DMSO, dimethyl sulfoxide; IMQ, imiquimod; IQR, inter-quartile range; LPS, lipopolysaccharide; LSF, L-sulforaphane; TLR, toll-like receptor.
10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: CD, cluster of differentiation; DMSO, dimethyl sulfoxide; IMQ, imiquimod; IQR, inter-quartile range; LPS, lipopolysaccharide; LSF, L-sulforaphane; TLR, toll-like receptor.
 10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: CD, cluster of differentiation; DC, dendritic cell, DMSO, dimethyl sulfoxide; HLA-DR, Human Leukocyte Antigen–DR isotype; IMQ, imiquimod; IQR, inter-quartile range; Lin, lineage; LPS, lipopolysaccharide; LSF, L-sulforaphane; moDC, monocyte-derived dendritic cell; NK, natural killer cell; TLR, toll-like receptor.
10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: CD, cluster of differentiation; DC, dendritic cell, DMSO, dimethyl sulfoxide; HLA-DR, Human Leukocyte Antigen–DR isotype; IMQ, imiquimod; IQR, inter-quartile range; Lin, lineage; LPS, lipopolysaccharide; LSF, L-sulforaphane; moDC, monocyte-derived dendritic cell; NK, natural killer cell; TLR, toll-like receptor.
 10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: CD, cluster of differentiation; DC, dendritic cell, DMSO, dimethyl sulfoxide; HLA-DR, Human Leukocyte Antigen–DR isotype; IMQ, imiquimod; IQR, inter-quartile range; Lin, lineage; LPS, lipopolysaccharide; LSF, L-sulforaphane; moDC, monocyte-derived dendritic cell; NK, natural killer cell; TLR, toll-like receptor.
10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: CD, cluster of differentiation; DC, dendritic cell, DMSO, dimethyl sulfoxide; HLA-DR, Human Leukocyte Antigen–DR isotype; IMQ, imiquimod; IQR, inter-quartile range; Lin, lineage; LPS, lipopolysaccharide; LSF, L-sulforaphane; moDC, monocyte-derived dendritic cell; NK, natural killer cell; TLR, toll-like receptor.
 10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: CD, cluster of differentiation; DC, dendritic cell, DMSO, dimethyl sulfoxide; HLA-DR, Human Leukocyte Antigen – DR isotype; IQR, inter-quartile range; Lin, lineage; LPS, lipopolysaccharide; LSF, L-sulforaphane; moDC, monocyte-derived dendritic cell; PBMC, peripheral blood mononuclear cells; pDC, plasmacytoid dendritic cells.
10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: CD, cluster of differentiation; DC, dendritic cell, DMSO, dimethyl sulfoxide; HLA-DR, Human Leukocyte Antigen – DR isotype; IQR, inter-quartile range; Lin, lineage; LPS, lipopolysaccharide; LSF, L-sulforaphane; moDC, monocyte-derived dendritic cell; PBMC, peripheral blood mononuclear cells; pDC, plasmacytoid dendritic cells.
 10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: CD, cluster of differentiation; DC, dendritic cell, DMSO, dimethyl sulfoxide; HLA-DR, Human Leukocyte Antigen – DR isotype; IQR, inter-quartile range; Lin, lineage; LPS, lipopolysaccharide; LSF, L-sulforaphane; moDC, monocyte-derived dendritic cell; PBMC, peripheral blood mononuclear cells; pDC, plasmacytoid dendritic cells.
10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: CD, cluster of differentiation; DC, dendritic cell, DMSO, dimethyl sulfoxide; HLA-DR, Human Leukocyte Antigen – DR isotype; IQR, inter-quartile range; Lin, lineage; LPS, lipopolysaccharide; LSF, L-sulforaphane; moDC, monocyte-derived dendritic cell; PBMC, peripheral blood mononuclear cells; pDC, plasmacytoid dendritic cells.
 10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: DMSO, dimethyl sulfoxide; IL, interleukin; IQR, inter-quartile range; LSF, L-sulforaphane; MCP-1, monocyte chemoattractant protein-1; RANTES, PBMC, peripheral blood mononuclear cells; Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted; TNF-α, tumour necrosis factor-alpha.
10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: DMSO, dimethyl sulfoxide; IL, interleukin; IQR, inter-quartile range; LSF, L-sulforaphane; MCP-1, monocyte chemoattractant protein-1; RANTES, PBMC, peripheral blood mononuclear cells; Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted; TNF-α, tumour necrosis factor-alpha.
 10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: DMSO, dimethyl sulfoxide; IL, interleukin; IQR, inter-quartile range; LSF, L-sulforaphane; MCP-1, monocyte chemoattractant protein-1; RANTES, PBMC, peripheral blood mononuclear cells; Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted; TNF-α, tumour necrosis factor-alpha.
10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: DMSO, dimethyl sulfoxide; IL, interleukin; IQR, inter-quartile range; LSF, L-sulforaphane; MCP-1, monocyte chemoattractant protein-1; RANTES, PBMC, peripheral blood mononuclear cells; Regulated upon Activation, Normal T Cell Expressed and Presumably Secreted; TNF-α, tumour necrosis factor-alpha.

 10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: ARE, antioxidant response element; CI, confidence interval; DMSO; dimethyl sulfoxide; LSF, L-sulforaphane.
10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: ARE, antioxidant response element; CI, confidence interval; DMSO; dimethyl sulfoxide; LSF, L-sulforaphane.
 10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: ARE, antioxidant response element; CI, confidence interval; DMSO; dimethyl sulfoxide; LSF, L-sulforaphane.
10 µM LSF, • 50 µM LSF, • DMSO. Abbreviation: ARE, antioxidant response element; CI, confidence interval; DMSO; dimethyl sulfoxide; LSF, L-sulforaphane.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazarakis, N.; Anderson, J.; Toh, Z.Q.; Higgins, R.A.; Do, L.A.H.; Luwor, R.B.; Snibson, K.J.; Karagiannis, T.C.; Licciardi, P.V. Examination of Novel Immunomodulatory Effects of L-Sulforaphane. Nutrients 2021, 13, 602. https://doi.org/10.3390/nu13020602
Mazarakis N, Anderson J, Toh ZQ, Higgins RA, Do LAH, Luwor RB, Snibson KJ, Karagiannis TC, Licciardi PV. Examination of Novel Immunomodulatory Effects of L-Sulforaphane. Nutrients. 2021; 13(2):602. https://doi.org/10.3390/nu13020602
Chicago/Turabian StyleMazarakis, Nadia, Jeremy Anderson, Zheng Quan Toh, Rachel A. Higgins, Lien Anh Ha Do, Rodney B. Luwor, Kenneth J. Snibson, Tom C. Karagiannis, and Paul V. Licciardi. 2021. "Examination of Novel Immunomodulatory Effects of L-Sulforaphane" Nutrients 13, no. 2: 602. https://doi.org/10.3390/nu13020602