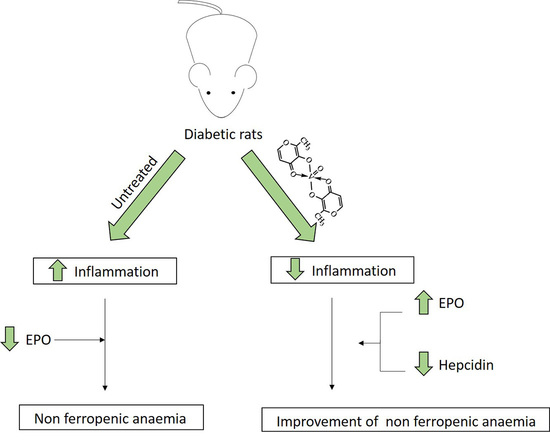
Vanadium Decreases Hepcidin mRNA Gene Expression in STZ-Induced Diabetic Rats, Improving the Anemic State
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Determination of Total Metal Concentrations
2.3. Determination of Biochemical and Pro-Inflammatory Parameters
2.4. Measurement of Hematological Parameters
2.5. Analysis of Hepcidin Gene Expression in Liver Samples by RT-qPCR
2.6. Ferroportin Expression in HepG2 Cells
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. Effect of V (IV) Treatment on Leptin and Other Inflammatory Parameters
4.2. Effect of V(IV) Treatment on the mRNA Expression Level of Hepcidin in Liver and Level of Fe, Transferrin, and Ferritin in Serum and Ferroportin Expression in Hepatic Cells
4.3. Effect of V(IV) Treatment on Hematological Parameters
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, K.H.; Orvig, C. Vanadium in diabetes: 100 years from Phase 0 to Phase I. J. Inorg. Biochem. 2006, 100, 1925–1935. [Google Scholar] [CrossRef]
- Ścibior, A.; Kurus, J. Vanadium and Oxidative Stress Markers—In Vivo Model: A Review. Curr. Med. Chem. 2019, 26, 5456–5500. [Google Scholar] [CrossRef]
- Treviño, S.; Díaz, A.; Sánchez-Lara, E.; Sanchez-Gaytan, B.L.; Perez-Aguilar, J.M.; González-Vergara, E. Vanadium in Biological Action: Chemical, Pharmacological Aspects, and Metabolic Implications in Diabetes Mellitus. Biol. Trace Elem. Res. 2019, 188, 68–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and possible benefits in the light of a comprehensive overview of its pharmacotoxicological mechanisms and multi-applications with a summary of further research trends. J. Trace Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef]
- Thompson, K.H.; Lichter, J.; LeBel, C.; Scaife, M.C.; McNeill, J.H.; Orvig, C. Vanadium treatment of type 2 diabetes: A view to the future. J. Inorg. Biochem. 2009, 103, 554–558. [Google Scholar] [CrossRef]
- López-Viseras, M.E.; Fernández, B.; Hilfiker, S.; González, C.S.; González, J.L.; Calahorro, A.J.; Colacio, E.; Rodríguez-Diéguez, A. In vivo potential antidiabetic activity of a novel zinc coordination compound based on 3-carboxy-pyrazole. J. Inorg. Biochem. 2014, 131, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Rivas-García, L.; Quiles, J.L.; Varela-López, A.; Arredondo, M.; Lopez, P.; Diéguez, A.R.; Montes-Bayon, M.; Aranda, P.; Llopis, J.; Sánchez-González, C. In vitro study of the protective effect of manganese against vanadium-mediated nuclear and mitochondrial DNA damage. Food Chem. Toxicol. 2020, 135, 110900. [Google Scholar] [CrossRef] [PubMed]
- Oster, M.H.; Llobet, J.M.; Domingo, J.L.; German, J.B.; Keen, C.L. Vanadium treatment of diabetic Sprague-Dawley rats results in tissue vanadium accumulation and pro-oxidant effects. Toxicology 1993, 83, 115–130. [Google Scholar] [CrossRef]
- Ward, D.T.; Hamilton, K.; Burnand, R.; Smith, C.P.; Tomlinson, D.R.; Riccardi, D. Altered expression of iron transport proteins in streptozotocin-induced diabetic rat kidney. Biochim. Biophys. Acta 2005, 1740, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Yigitturk, G.; Acara, A.C.; Erbas, O.; Oltulu, F.; Yavasoglu, N.U.K.; Uysal, A.; Yavasoglu, A. The antioxidant role of agomelatine and gallic acid on oxidative stress in STZ induced type I diabetic rat testes. Biomed. Pharmacother. 2017, 87, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.-Y.; Zhang, F.; Hu, X.-T.; Chen, J.; Tang, R.-X.; Zheng, K.-Y.; Song, Y.-J. Depression can be prevented by astaxanthin through inhibition of hippocampal inflammation in diabetic mice. Brain Res. 2017, 1657, 262–268. [Google Scholar] [CrossRef]
- Thomas, M.C. Anemia in diabetes: Marker or mediator of microvascular disease? Nat. Clin. Pr. Nephrol. 2007, 3, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, U. Anemia, Diabetes, and Chronic Kidney Disease. Diabetes Care 2009, 32, 1320–1326. [Google Scholar] [CrossRef] [Green Version]
- Niu, H.S.; Chang, C.H.; Niu, C.S.; Cheng, J.T.; Lee, K.S. Erythropoietin ameliorates hyperglycemia in type 1-like diabetic rats. Drug Des. Devel. Ther. 2016, 10, 1877–1884. [Google Scholar] [PubMed] [Green Version]
- Ścibior, A.; Zaporowska, H.; Ostrowski, J. Selected Haematological and Biochemical Parameters of Blood in Rats After Subchronic Administration of Vanadium and/or Magnesium in Drinking Water. Arch. Environ. Contam. Toxicol. 2006, 51, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Ścibior, A.; Adamczyk, A.; Gołębiowska, D.; Niedźwiecka, I. Effect of 12-week vanadate and magnesium co-administration on chosen haematological parameters as well as on some indices of iron and copper metabolism and biomarkers of oxidative stress in rats. Environ. Toxicol. Pharmacol. 2012, 34, 235–252. [Google Scholar] [CrossRef]
- Ścibior, A.; Adamczyk, A.; Gołębiowska, D.; Niedźwiecka, I.; Fornal, E.; Scibior, A.; Adamczyk, A.; Gołębiowska, D.; Niedźwiecka, I.; Fornal, E.; et al. The influence of combined magnesium and vanadate administration on the level of some elements in selected rat organs: V–Mg interactions and the role of iron-essential protein (DMT-1) in the mechanism underlying altered tissues iron level. Metallomics 2014, 6, 907–920. [Google Scholar] [CrossRef]
- Chakraborty, A.; Chatterjee, M. Enhanced erythropoietin and suppression of γ-glutamyl transpeptidase (GGT) activity in murine lymphoma following administration of vanadium. Neoplasma 1994, 41, 291–296. [Google Scholar]
- Aguirre, M.V.; Juaristi, J.A.; Alvarez, M.A.; Brandan, N.C. Characteristics of in vivo murine erythropoietic response to sodium orthovanadate. Chem. Biol. Interact. 2005, 156, 55–68. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Iron imports. IV. Hepcidin and regulation of body iron metabolism. Am. J. Physiol. Liver Physiol. 2006, 290, G199–G203. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganz, T.; Nemeth, E. Hepcidin and disorders of iron metabolism. Annu. Rev. Med. 2011, 62, 347–360. [Google Scholar] [CrossRef]
- Sánchez-González, C.; Rivas-García, L.; López-Chaves, C.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J.; Gómez-Aracena, J.; Vera-Ramírez, L.; Montes-Bayon, M.; Sanz-Medel, A.; et al. Exposure to bis(maltolato)oxovanadium(IV) increases levels of hepcidin mRNA and impairs the homeostasis of iron but not that of manganese. Food Chem. Toxicol. 2014, 73, 113–118. [Google Scholar] [CrossRef]
- Ścibior, A.; Hus, I.; Mańko, J.; Jawniak, D. Evaluation of the level of selected iron-related proteins/receptors in the liver of rats during separate/combined vanadium and magnesium administration. J. Trace Elem. Med. Biol. 2020, 61, 126550. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gonzalez, C.; Moreno, L.; Lopez-Chaves, C.; Nebot, E.; Pietschmann, P.; Rodriguez-Nogales, A.; Galvez, J.; Montes-Bayon, M.; Sanz-Medel, A.; Llopis, J.; et al. Effect of vanadium on calcium homeostasis, osteopontin mRNA expression, and bone microarchitecture in diabetic rats. Metallomics 2017, 9, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gonzalez, C.; Bermudez-Peña, C.; Guerrero-Romero, F.; Trenzado, C.E.C.E.; Montes-Bayon, M.; Sanz-Medel, A.; Llopis, J. Effect of bis(maltolato)oxovanadium (IV) (BMOV) on selenium nutritional status in diabetic streptozotocin rats. Br. J. Nutr. 2012, 108, 893–899. [Google Scholar] [CrossRef] [Green Version]
- Giampieri, F.; Quiles, J.L.J.L.J.L.; Orantes-Bermejo, F.J.F.J.F.J.; Gasparrini, M.; Forbes-Hernandez, T.Y.T.Y.; Sánchez-González, C.; Llopis, J.; Rivas-García, L.; Afrin, S.; Varela-López, A.; et al. Are by-products from beeswax recycling process a new promising source of bioactive compounds with biomedical properties? Food Chem. Toxicol. 2018, 112, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Di Bella, L.M.; Alampi, R.; Biundo, F.; Toscano, G.; Felice, M.R. Copper chelation and interleukin-6 proinflammatory cytokine effects on expression of different proteins involved in iron metabolism in HepG2 cell line. BMC Biochem. 2017, 18, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, C.; Torres, M.; Bermúdez-Peña, M.C.; Aranda, P.; Montes-Bayón, M.; Sanz-Medel, A.; Llopis, J. Bioavailability, tissue distribution and hypoglycaemic effect of vanadium in magnesium-deficient rats. Magnes. Res. 2011, 24, 196–208. [Google Scholar] [CrossRef]
- Bermúdez-Peña, M.C.; López-Chaves, C.; Llopis, J.; Guerrero-Romero, F.; Montes-Bayón, M.; Sanz-Medel, A.; Sánchez-González, C. Aggravation by vanadium of magnesium deficiency in STZ-induced diabetic rats. Magnes. Res. Off. Organ Int. Soc. Dev. Res. Magnes. 2013, 26, 74–82. [Google Scholar] [CrossRef]
- Kirel, B.; Doğruel, N.; Korkmaz, Ü.; Kiliç, F.S.; Özdamar, K.; Uçar, B. Serum leptin levels in type 1 diabetic and obese children: Relation to insulin levels. Clin. Biochem. 2000, 33, 475–480. [Google Scholar] [CrossRef]
- Jéquier, E. Leptin Signaling, Adiposity, and Energy Balance. Ann. N. Y. Acad. Sci. 2006, 967, 379–388. [Google Scholar] [CrossRef]
- Chen, M.D.; Yang, V.C.; Alexander, P.S.; Lin, P.Y.; Song, Y.M. Effects of selected minerals on leptin secretion in streptozotocin-induced hyperglycemic mice. Exp. Biol. Med. (Maywood) 2001, 226, 836–840. [Google Scholar] [CrossRef]
- Wilsey, J.; Matheny, M.K.; Scarpace, P.J. Oral Vanadium Enhances the Catabolic Effects of Central Leptin in Young Adult Rats. Endocrinology 2006, 147, 493–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levina, A.; Lay, P.A. Vanadium(V/IV)–Transferrin Binding Disrupts the Transferrin Cycle and Reduces Vanadium Uptake and Antiproliferative Activity in Human Lung Cancer Cells. Inorg. Chem. 2020, 59, 16143–16153. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, C.; López-Chaves, C.; Trenzado, C.E.; Aranda, P.; López-Jurado, M.; Gómez-Aracena, J.; Montes-Bayón, M.; Sanz-Medel, A.; Llopis, J. Changes in Iron Metabolism and Oxidative Status in STZ-Induced Diabetic Rats Treated with Bis(maltolato) Oxovanadium (IV) as an Antidiabetic Agent. Sci. World J. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [Green Version]
- O´Connell, N. Anaemia: A silent complication of diabetes. Diabetes Voice 2003, 48, 25–27. [Google Scholar]
- Urrutia, P.; Aguirre, P.; Esparza, A.; Tapia, V.; Mena, N.P.; Arredondo, M.; González-Billault, C.; Núñez, M.T. Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J. Neurochem. 2013, 126, 541–549. [Google Scholar] [CrossRef]
- Jain, S.K.; Rains, J.; Croad, J.; Larson, B.; Jones, K. Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid. Redox Signal. 2009, 11, 241–249. [Google Scholar] [CrossRef] [Green Version]
- Bartnikas, T.B.T.B. Known and potential roles of transferrin in iron biology. BioMetals 2012, 25, 677–686. [Google Scholar] [CrossRef] [Green Version]
- Kristiansen, O.P.; Mandrup-Poulsen, T. Interleukin-6 and Diabetes: The Good, the Bad, or the Indifferent? Diabetes 2005, 54, S114–S124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, R.; Lei, S.; Xia, Z.-Y.; Wu, Y.; Meng, Q.; Zhan, L.; Su, W.; Liu, H.; Xu, J.; Liu, Z.; et al. Selective inhibition of PTEN preserves ischaemic post-conditioning cardioprotection in STZ-induced Type 1 diabetic rats: Role of the PI3K/Akt and JAK2/STAT3 pathways. Clin. Sci. (Lond) 2016, 130, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Elliott, S.; Pham, E.; Macdougall, I.C. Erythropoietins: A common mechanism of action. Exp. Hematol. 2008, 36, 1573–1584. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gonzalez, C.; Bermudez-Peña, C.; Trenzado, C.E.; Goenaga-Infante, H.; Montes-Bayon, M.; Sanz-Medel, A.; Llopis, J. Changes in the antioxidant defence and in selenium concentration in tissues of vanadium exposed rats. Metallomics 2012, 4, 814–819. [Google Scholar] [CrossRef] [PubMed]



| 1st Week | 5th Week | |||||||
|---|---|---|---|---|---|---|---|---|
| Groups | Food Intake (g/day) | Water Intake (g/day) | Diuresis (mL/day) | Glucose Level (mmol/L) | Food Intake (g/day) | Water Intake (g/day) | Diuresis (mL/day) | Glucose Level (mmol/L) |
| C | 14.1 ± 4.0 | 17.1 ± 4.7 | 16.6 ± 4.3 | 5.0 ± 0.3 | 15.0 ± 02.0 | 16.6 ± 4.3 | 13.0 ± 7.5 | 4.7 ± 0.3 |
| DM | 21.8 ± 6.0 * | 156.0 ± 33.0 * | 90.4 ± 23.0 * | 13.9 ± 2.1 * | 30.0 ± 0.9 * | 324.0 ± 26.0 * | 203.0 ± 43.0 * | 14.6 ± 1.2 * |
| DMV | 26.5 ± 3.9 * | 167.0 ± 33.0 * | 95.7 ± 21.0 * | 17.0 ± 2.0 * | 22.6 ± 1.2 * | 191.0 ± 41.0 * | 187.0 ± 46.0 * | 19.6 ± 1.7 * |
| Groups | C | DM | DMV | p Test |
|---|---|---|---|---|
| Leptin (ng/mL) | 16 ± 4 | 2.6 ± 1.0 * | 3.2 ± 1.2 * | p < 0.001 |
| CRP (mg/L) | 2.4 ± 1.5 | 5.5 ± 1.6 * | 5.7 ± 1.4 * | p < 0.01 |
| IL-6 (pg/mL) | 127 ± 48 | 193 ± 54 * | 197 ± 56 * | p < 0.05 |
| IL-1β (pg/mL) | 79 ± 23 | 121 ± 33 * | 89 ± 18 | NS |
| TNF-α (ng/mL) | 7.7 ± 2.0 | 11.2 ± 2.2 * | 10.1 ± 4.5 | p < 0.05 |
| Groups | C | DM | DMV | p Test |
|---|---|---|---|---|
| V (μg/L) | 2.4 ± 0.5 | 6.2 ± 1.2 * | 455 ± 96 *,† | p < 0.001 |
| Fe (mg/L) | 1.8 ± 0.1 | 2.1 ± 0.7 | 2.7 ± 0.6 * | p < 0.01 |
| Transferrin (mg/mL) | 6.0 ± 1.4 | 5.0 ± 1.5 | 6.9 ± 0.9 | NS |
| TSAT (%) | 23 ± 8 | 30 ± 8 | 28 ± 5 | NS |
| Ferritin (ng/mL) | 211 ± 51 | 236 ± 18 | 223 ± 31 | NS |
| Groups | C | DM | DMV | p Test |
|---|---|---|---|---|
| Erythrocytes (×106 cells/mm3) | 9.1 ± 0.9 | 8.0 ± 0.3 * | 8.8 ± 0.4 † | p < 0.05 |
| Hb (g/dL) | 16 ± 3 | 13 ± 1 * | 15 ± 1 † | p < 0.05 |
| Hct (%) | 47 ± 8 | 44 ± 3 | 47 ± 3 | NS |
| MCV (fL) | 52 ± 5 | 55 ± 2 | 53 ± 4 | NS |
| MCH (pg) | 18 ± 1 | 16 ± 1 | 17 ± 2 | NS |
| Leucocytes (×103 cells/mm3) | 4.8 ± 1.2 | 4.1 ± 2.4 | 2.9 ± 0.5 * | p < 0.05 |
| Lymphocytes (×103 cells/mm3) | 4.3 ± 1.8 | 2.6 ± 0.9 * | 1.9 ± 0.4 * | p < 0.01 |
| Platelets (×103 cells/mm3) | 679 ± 260 | 513 ± 121 | 646 ± 122 | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-González, C.; Rivas-García, L.; Rodríguez-Nogales, A.; Algieri, F.; Gálvez, J.; Aranda, P.; Montes-Bayón, M.; Llopis, J. Vanadium Decreases Hepcidin mRNA Gene Expression in STZ-Induced Diabetic Rats, Improving the Anemic State. Nutrients 2021, 13, 1256. https://doi.org/10.3390/nu13041256
Sánchez-González C, Rivas-García L, Rodríguez-Nogales A, Algieri F, Gálvez J, Aranda P, Montes-Bayón M, Llopis J. Vanadium Decreases Hepcidin mRNA Gene Expression in STZ-Induced Diabetic Rats, Improving the Anemic State. Nutrients. 2021; 13(4):1256. https://doi.org/10.3390/nu13041256
Chicago/Turabian StyleSánchez-González, Cristina, Lorenzo Rivas-García, Alba Rodríguez-Nogales, Francesca Algieri, Julio Gálvez, Pilar Aranda, María Montes-Bayón, and Juan Llopis. 2021. "Vanadium Decreases Hepcidin mRNA Gene Expression in STZ-Induced Diabetic Rats, Improving the Anemic State" Nutrients 13, no. 4: 1256. https://doi.org/10.3390/nu13041256