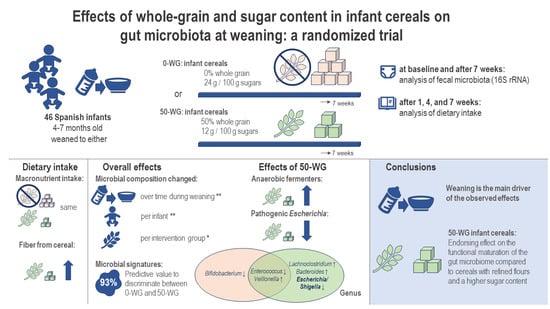
Effects of Whole-Grain and Sugar Content in Infant Cereals on Gut Microbiota at Weaning: A Randomized Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Participants and Experimental Design
2.3. Intervention Products
2.4. Intestinal Microbiota Analysis
2.5. Bioinformatics
2.6. Assessment of Dietary Intake
2.7. Statistical Analysis on Fecal Microbiota Composition
3. Results
3.1. Study Logistics and Subject Characteristics
3.2. Changes in Dietary Intake during the Study Period
3.3. Overall Effects of Introduction of Cereals as First Weaning Foods on Microbiota Composition
3.4. Effect of Infant Cereals with Different Whole Grain and Sugar Contents on Microbiota Composition
3.5. Effect of Infant Cereals with Different Whole Grain and Sugar Contents on Microbiota Diversity
3.6. Correlations between Microbial, Anthropometric, Demographic, and Dietary Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kuczynski, J.; Lauber, C.L.; Walters, W.A.; Parfrey, L.W.; Clemente, J.C.; Gevers, D.; Knight, R. Experimental and analytical tools for studying the human microbiome. Nat. Rev. Genet. 2011, 13, 47–58. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Mercado, A.I.; Navarro-Oliveros, M.; Robles-Sanchez, C.; Plaza-Diaz, J.; Saez-Lara, M.J.; Munoz-Quezada, S.; Fontana, L.; Abadia-Molina, F. Microbial Population Changes and Their Relationship with Human Health and Disease. Microorganisms 2019, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Francino, M.P. Early development of the gut microbiota and immune health. Pathogens 2014, 3, 769. [Google Scholar] [CrossRef] [Green Version]
- Plaza-Diaz, J.; Fernandez-Caballero, J.A.; Chueca, N.; Garcia, F.; Gomez-Llorente, C.; Saez-Lara, M.J.; Fontana, L.; Gil, A. Pyrosequencing analysis reveals changes in intestinal microbiota of healthy adults who received a daily dose of immunomodulatory probiotic strains. Nutrients 2015, 7, 3999. [Google Scholar] [CrossRef] [Green Version]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Immune-Mediated Mechanisms of Action of Probiotics and Synbiotics in Treating Pediatric Intestinal Diseases. Nutrients 2018, 10, 42. [Google Scholar] [CrossRef] [Green Version]
- Rowland, I.; Gibson, G.; Heinken, A.; Scott, K.; Swann, J.; Thiele, I.; Tuohy, K. Gut microbiota functions: Metabolism of nutrients and other food components. Eur. J. Nutr. 2018, 57, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, B.; Gueimonde, M.; Pena, A.S.; Bernardo, D. Intestinal microbiota as modulators of the immune system. J. Immunol. Res. 2015, 2015, 159094. [Google Scholar] [CrossRef]
- Blanton, L.V.; Charbonneau, M.R.; Salih, T.; Barratt, M.J.; Venkatesh, S.; Ilkaveya, O.; Subramanian, S.; Manary, M.J.; Trehan, I.; Jorgensen, J.M. Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 2016, 351, 6275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Funkhouser, L.J.; Bordenstein, S.R. Mom knows best: The universality of maternal microbial transmission. PLoS Biol. 2013, 11, e1001631. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Palacio, S.D.; Montes, S.A.; Mancabelli, L. The first microbial colonizers of the human gut: Composition, activities, and health implications of the infant gut microbiota. Microbiol. Mol. Biol. Rev. 2017, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Doare, K.; Holder, B.; Bassett, A.; Pannaraj, P.S. Mother’s milk: A purposeful contribution to the development of the infant microbiota and immunity. Front. Immunol. 2018, 9, 361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hascoët, J.-M.; Hubert, C.; Rochat, F.; Legagneur, H.; Gaga, S.; Emady-Azar, S.; Steenhout, P.G. Effect of formula composition on the development of infant gut microbiota. J. Pediatric Gastroenterol. Nutr. 2011, 52, 756–762. [Google Scholar] [CrossRef]
- Stewart, C.J.; Ajami, N.J.; O’Brien, J.L.; Hutchinson, D.S.; Smith, D.P.; Wong, M.C.; Ross, M.C.; Lloyd, R.E.; Doddapaneni, H.; Metcalf, G.A. Temporal development of the gut microbiome in early childhood from the TEDDY study. Nature 2018, 562, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, Z.; Zhang, W.; Zhang, C.; Zhang, Y.; Mei, H.; Zhuo, N.; Wang, H.; Wang, L.; Wu, D. Comparison of gut microbiota in exclusively breast-fed and formula-fed babies: A study of 91 term infants. Sci. Rep. 2020, 10, 15792. [Google Scholar] [CrossRef]
- Borewicz, K.; Suarez-Diez, M.; Hechler, C.; Beijers, R.; de Weerth, C.; Arts, I.; Penders, J.; Thijs, C.; Nauta, A.; Lindner, C. The effect of prebiotic fortified infant formulas on microbiota composition and dynamics in early life. Sci. Rep. 2019, 9, 2434. [Google Scholar] [CrossRef] [Green Version]
- Laursen, M.F.; Bahl, M.I.; Michaelsen, K.F.; Licht, T.R. First foods and gut microbes. Front. Microbiol. 2017, 8, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butte, N.F.; Fox, M.K.; Briefel, R.R.; Siega-Riz, A.M.; Dwyer, J.T.; Deming, D.M.; Reidy, K.C. Nutrient intakes of US infants, toddlers, and preschoolers meet or exceed dietary reference intakes. J. Am. Diet. Assoc. 2010, 110, S27–S37. [Google Scholar] [CrossRef]
- Freeman, V.; van’t Hof, M.; Haschke, F.; Group, E.-G.S. Patterns of milk and food intake in infants from birth to age 36 months: The Euro-growth study. J. Pediatric Gastroenterol. Nutr. 2000, 31, S76–S85. [Google Scholar] [CrossRef]
- Klerks, M.; Roman, S.; Bernal, M.J.; Haro-Vicente, J.F.; Sanchez-Siles, L.M. Complementary Feeding Practices and Parental Pressure to Eat among Spanish Infants and Toddlers: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 1982. [Google Scholar] [CrossRef]
- AECOSAN. Consumo Gramos/día (Base: Población Infantil de 12-35 Meses). Available online: https://www.aesan.gob.es/AECOSAN/docs/documentos/seguridad_alimentaria/evaluacion_riesgos/Consumo_12_36_meses.pdf (accessed on 15 April 2021).
- Bernal-Cava, M.J.; Klerks, M.; Haro-Vicente, J.F.; Bodenstab, S.; Sanchez-Siles, L.M. Mapping out Commercial Infant Cereals: An Overview From 8 Countries. In Proceedings of the 6th Nutrition & Growth Congress, Valencia, Spain, 7–9 March 2019. [Google Scholar]
- Klerks, M.; Bernal, M.J.; Roman, S.; Bodenstab, S.; Gil, A.; Sanchez-Siles, L.M. Infant Cereals: Current Status, Challenges, and Future Opportunities for Whole Grains. Nutrients 2019, 11, 473. [Google Scholar] [CrossRef] [Green Version]
- Ye, E.Q.; Chacko, S.A.; Chou, E.L.; Kugizaki, M.; Liu, S. Greater whole-grain intake is associated with lower risk of type 2 diabetes, cardiovascular disease, and weight gain. J. Nutr. 2012, 142, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Commission, E. Commission Directive 2006/125/EC of 5 December 2006 on processed cereal-based foods and baby foods for infants and young children. OJ L 2006, 339, 16–35. [Google Scholar]
- Gong, L.; Cao, W.; Chi, H.; Wang, J.; Zhang, H.; Liu, J.; Sun, B. Whole cereal grains and potential health effects: Involvement of the gut microbiota. Food Res. Int. 2018, 103, 84–102. [Google Scholar] [CrossRef]
- Jefferson, A.; Adolphus, K. The effects of intact cereal grain fibers, including wheat bran on the gut microbiota composition of healthy adults: A systematic review. Front. Nutr. 2019, 6, 33. [Google Scholar] [CrossRef] [Green Version]
- Do, M.H.; Lee, E.; Oh, M.-J.; Kim, Y.; Park, H.-Y. High-glucose or-fructose diet cause changes of the gut microbiota and metabolic disorders in mice without body weight change. Nutrients 2018, 10, 761. [Google Scholar] [CrossRef] [Green Version]
- Rosas-Villegas, A.; Sánchez-Tapia, M.; Avila-Nava, A.; Ramírez, V.; Tovar, A.R.; Torres, N. Differential effect of sucrose and fructose in combination with a high fat diet on intestinal microbiota and kidney oxidative stress. Nutrients 2017, 9, 393. [Google Scholar] [CrossRef] [PubMed]
- Koecher, K.J.; McKeown, N.M.; Sawicki, C.M.; Menon, R.S.; Slavin, J.L. Effect of whole-grain consumption on changes in fecal microbiota: A review of human intervention trials. Nutr. Rev. 2019, 77, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Siles, L.M.; Bernal, M.J.; Gil, D.; Bodenstab, S.; Haro-Vicente, J.F.; Klerks, M.; Plaza-Diaz, J.; Gil, Á. Are Sugar-Reduced and Whole Grain Infant Cereals Sensorially Accepted at Weaning? A Randomized Controlled Cross-Over Trial. Nutrients 2020, 12, 1883. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Cohen, D.B.; Ravel, J.; Abdo, Z.; Forney, L.J. Evaluation of methods for the extraction and purification of DNA from the human microbiome. PLoS ONE 2012, 7, e33865. [Google Scholar] [CrossRef] [Green Version]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glockner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Caporaso, J.G. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2′s q2-feature-classifier plugin. Microbiome 2018, 6, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. General principles for the collection of national food consumption data in the view of a pan-European dietary survey. EFSA J. 2009, 7, 1435. [Google Scholar] [CrossRef]
- European Food Safety Authority. Guidance on the EU Menu methodology. EFSA J. 2014, 12, 3944. [Google Scholar]
- Bernal-Cava, M.J.; Haro-Vicente, J.F.; Ros, G.; Roman, S.; Sanchez-Siles, L.M. Homemade vs commercial infant food: A nutritional comparison on dietary fibre. In Proceedings of the 50th Annual Meeting ESPGHAN, Prague, Czech Republic, 10 May 2017; p. 819. [Google Scholar]
- Carbajal Azcona, Á. Manual de Nutrición y Dietética; Departamento de Nutrición-M-008157: Madrid, Spain, 2013. [Google Scholar]
- USDA. Child and Adult Care Food Program: Meal pattern revisions related to the Healthy, Hunger-Free Kids Act of 2010. Final Rule. Fed Reg 2016, 81, 24348. [Google Scholar]
- BEDCA. Available online: https://www.bedca.net/bdpub/ (accessed on 14 February 2021).
- Rivera-Pinto, J.; Egozcue, J.J.; Pawlowsky-Glahn, V.; Paredes, R.; Noguera-Julian, M.; Calle, M.L. Balances: A new perspective for microbiome analysis. MSystems 2018, 3. [Google Scholar] [CrossRef] [Green Version]
- Vandeputte, D.; Kathagen, G.; D’hoe, K.; Vieira-Silva, S.; Valles-Colomer, M.; Sabino, J.; Wang, J.; Tito, R.Y.; De Commer, L.; Darzi, Y. Quantitative microbiome profiling links gut community variation to microbial load. Nature 2017, 551, 507. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Package ‘vegan’. Community Ecol. Package Version 2013, 2, 1–295. [Google Scholar]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package ‘corrplot’. Statistician 2017, 56, e24. [Google Scholar]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Moore, R.E.; Townsend, S.D. Temporal development of the infant gut microbiome. Open Biol. 2019, 9, 190128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallani, M.; Amarri, S.; Uusijarvi, A.; Adam, R.; Khanna, S.; Aguilera, M.; Gil, A.; Vieites, J.M.; Norin, E.; Young, D. Determinants of the human infant intestinal microbiota after the introduction of first complementary foods in infant samples from five European centres. Microbiology 2011, 157, 1385–1392. [Google Scholar] [CrossRef] [Green Version]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4578–4585. [Google Scholar] [CrossRef] [Green Version]
- Vallès, Y.; Artacho, A.; Pascual-García, A.; Ferrús, M.L.; Gosalbes, M.J.; Abellán, J.J.; Francino, M.P. Microbial succession in the gut: Directional trends of taxonomic and functional change in a birth cohort of Spanish infants. PLoS Genet. 2014, 10, e1004406. [Google Scholar] [CrossRef] [Green Version]
- Laursen, M.F.; Andersen, L.B.; Michaelsen, K.F.; Mølgaard, C.; Trolle, E.; Bahl, M.I.; Licht, T.R. Infant gut microbiota development is driven by transition to family foods independent of maternal obesity. Msphere 2016, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guittar, J.; Shade, A.; Litchman, E. Trait-based community assembly and succession of the infant gut microbiome. Nat. Commun. 2019, 10, 512. [Google Scholar] [CrossRef] [Green Version]
- Wexler, H.M. Bacteroides: The good, the bad, and the nitty-gritty. Clin. Microbiol. Rev. 2007, 20, 593–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627. [Google Scholar] [CrossRef]
- Baxter, N.T.; Schmidt, A.W.; Venkataraman, A.; Kim, K.S.; Waldron, C.; Schmidt, T.M. Dynamics of human gut microbiota and short-chain fatty acids in response to dietary interventions with three fermentable fibers. MBio 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izydorczyk, M.S.; Biliaderis, C.G. Cereal arabinoxylans: Advances in structure and physicochemical properties. Carbohydr. Polym. 1995, 28, 33–48. [Google Scholar] [CrossRef]
- Andersson, A.A.; Andersson, R.; Piironen, V.; Lampi, A.M.; Nystrom, L.; Boros, D.; Fras, A.; Gebruers, K.; Courtin, C.M.; Delcour, J.A.; et al. Contents of dietary fibre components and their relation to associated bioactive components in whole grain wheat samples from the HEALTHGRAIN diversity screen. Food Chem. 2013, 136, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Henrion, M.; Francey, C.; Lê, K.-A.; Lamothe, L. Cereal B-glucans: The impact of processing and how it affects physiological responses. Nutrients 2019, 11, 1729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergstrom, A.; Skov, T.H.; Bahl, M.I.; Roager, H.M.; Christensen, L.B.; Ejlerskov, K.T.; Molgaard, C.; Michaelsen, K.F.; Licht, T.R. Establishment of intestinal microbiota during early life: A longitudinal, explorative study of a large cohort of Danish infants. Appl. Environ. Microbiol. 2014, 80, 2889–2900. [Google Scholar] [CrossRef] [Green Version]
- Avershina, E.; Lundgard, K.; Sekelja, M.; Dotterud, C.; Storro, O.; Oien, T.; Johnsen, R.; Rudi, K. Transition from infant- to adult-like gut microbiota. Environ. Microbiol. 2016, 18, 2226–2236. [Google Scholar] [CrossRef] [PubMed]
- Finn, K.; Callen, C.; Bhatia, J.; Reidy, K.; Bechard, L.J.; Carvalho, R. Importance of dietary sources of iron in infants and toddlers: Lessons from the FITS study. Nutrients 2017, 9, 733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theurich, M.A.; Zaragoza-Jordana, M.; Luque, V.; Gruszfeld, D.; Gradowska, K.; Xhonneux, A.; Riva, E.; Verduci, E.; Poncelet, P.; Damianidi, L. Commercial complementary food use amongst European infants and children: Results from the EU Childhood Obesity Project. Eur. J. Nutr. 2020, 59, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- World Health Organization. Sustainable Healthy Diets: Guiding Principles; Food & Agriculture Organization: Roma, Italy, 2019. [Google Scholar]
- Theurich, M.A.; Koletzko, B.; Grote, V. Nutritional Adequacy of Commercial Complementary Cereals in Germany. Nutrients 2020, 12, 1590. [Google Scholar] [CrossRef]
- European Commission. JRC Technical Reports: Feeding Infants and Young Children, a Compilation of National Food-Based Dietary Guidelines and Specific Products. Available online: https://ec.europa.eu/jrc/sites/jrcsh/files/processed_cereal_baby_food_online.pdf (accessed on 15 April 2021).
- NHS Website for England. Starchy Foods and Carbohydrates. Eat Well. Available online: https://www.nhs.uk/live-well/eat-well/starchy-foods-and-carbohydrates/ (accessed on 15 April 2021).
- AEP. Recomendaciones de la Asociación Española de Pediatría Sobre la Alimentación Complementaria 2018. Available online: https://www.aeped.es/sites/default/files/documentos/recomendaciones_aep_sobre_alimentacio_n_complementaria_nov2018_v3_final.pdf/ (accessed on 10 January 2020).
- Norwegian Directorate of Health. Nordic Nutrition Recommendations. 2011. Available online: https://www.helsedirektoratet.no/faglige-rad/kostradene-og-naeringsstoffer/kostrad-for-befolkningen#grove-kornprodukter-hver-dag (accessed on 15 April 2021).
- Danish Veterinary and Food Administration (DVFA). The Official Dietary Advice—Good for Health and Climate. Available online: https://altomkost.dk/raad-og-anbefalinger/de-officielle-kostraad-godt-for-sundhed-og-klima/spis-mad-med-fuldkorn/ (accessed on 15 April 2021).
- Swedish National Food Agency (SNFA). Swedish Dietary Guidelines. Available online: https://www.livsmedelsverket.se/globalassets/publikationsdatabas/rapporter/2015/rapp-hanteringsrapport-engelska-omslag--inlaga--bilagor-eng-version.pdf (accessed on 15 April 2021).
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025. 9th Edition. 2020. Available online: https://www.dietaryguidelines.gov/sites/default/files/2020-12/Dietary_Guidelines_for_Americans_2020-2025.pdf (accessed on 15 April 2021).
- Haro-Vicente, J.F.; Bernal-Cava, M.J.; Lopez-Fernandez, A.; Ros-Berruezo, G.; Bodenstab, S.; Sanchez-Siles, L.M. Sensory Acceptability of Infant Cereals with Whole Grain in Infants and Young Children. Nutrients 2017, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Fidler Mis, N.; Braegger, C.; Bronsky, J.; Campoy, C.; Domellöf, M.; Embleton, N.D.; Hojsak, I.; Hulst, J.; Indrio, F.; Lapillonne, A. Sugar in infants, children and adolescents: A position paper of the European Society for Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 65, 681–696. [Google Scholar] [CrossRef] [Green Version]
- Lonnerdal, B. Dietary factors influencing zinc absorption. J. Nutr. 2000, 130, 1378S–1383S. [Google Scholar] [CrossRef] [PubMed]
- Persson, L.; Lundström, M.; Lönnerdal, B.; Hernell, O. Are weaning foods causing impaired iron and zinc status in 1-year-old Swedish infants? A cohort study. Acta Paediatr. 1998, 87, 618–622. [Google Scholar] [CrossRef]
- Lind, T.; Persson, L.Å.; Lönnerdal, B.; Stenlund, H.; Hernell, O. Effects of weaning cereals with different phytate content on growth, development and morbidity: A randomized intervention trial in infants from 6 to 12 months of age. Acta Paediatr. 2004, 93, 1575–1582. [Google Scholar] [CrossRef]
- Lind, T.; Lönnerdal, B.; Persson, L.-Å.; Stenlund, H.; Tennefors, C.; Hernell, O. Effects of weaning cereals with different phytate contents on hemoglobin, iron stores, and serum zinc: A randomized intervention in infants from 6 to 12 mo of age. Am. J. Clin. Nutr. 2003, 78, 168–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domellöf, M.; Braegger, C.; Campoy, C.; Colomb, V.; Decsi, T.; Fewtrell, M.; Hojsak, I.; Mihatsch, W.; Molgaard, C.; Shamir, R. Iron requirements of infants and toddlers. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 119–129. [Google Scholar] [CrossRef]
- Sjödin, K.S.; Domellöf, M.; Lagerqvist, C.; Hernell, O.; Lönnerdal, B.; Szymlek-Gay, E.A.; Sjödin, A.; West, C.E.; Lind, T. Administration of ferrous sulfate drops has significant effects on the gut microbiota of iron-sufficient infants: A randomised controlled study. Gut 2019, 68, 2095–2097. [Google Scholar]
- Seyoum, Y.; Baye, K.; Humblot, C. Iron homeostasis in host and gut bacteria—A complex interrelationship. Gut Microbes 2021, 13, 1–19. [Google Scholar] [CrossRef]
- Paganini, D.; Uyoga, M.A.; Kortman, G.A.; Cercamondi, C.I.; Moretti, D.; Barth-Jaeggi, T.; Schwab, C.; Boekhorst, J.; Timmerman, H.M.; Lacroix, C. Prebiotic galacto-oligosaccharides mitigate the adverse effects of iron fortification on the gut microbiome: A randomised controlled study in Kenyan infants. Gut 2017, 66, 1956–1967. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Nutritional Content (Per 100 g) | 0-WG | 50-WG |
|---|---|---|
| Energy (kcal) | 376 | 375 |
| Protein (g) | 9.8 | 10 |
| Carbohydrates (g) | 79.5 | 75 |
| Sugars (g) | 24 | 12 |
| Fat (g) | 1.3 | 2.0 |
| Fiber (g) | 4.0 | 7.2 |
| Calcium (mg) | 160 | 160 |
| Zinc (mg) | 6.23 | 8.16 |
| Iron (mg) | 0.63 | 1.09 |
| Phytate (mg) | 143.51 | 176.83 |
| Intervention Group | p-Value | ||
|---|---|---|---|
| 0-WG n = 18 | 50-WG n = 25 | ||
| Gender infant n (%) | 0.897 | ||
| Female | 9 (50%) | 12 (48%) | |
| Male | 9 (50%) | 13 (52%) | |
| Birth weight (g) | 3259 ± 339 | 3276 ± 446 | 0.898 |
| Age (months) | 5.16 ± 0.31 | 5.23 ± 0.41 | 0.496 |
| Weight at inclusion (g) | 7449 ± 909 | 7399 ± 789 | 0.850 |
| Length (cm) | 64.3 ± 2.9 | 65.3 ± 2.6 | 0.243 |
| Head circumference (cm) | 42.5 ± 1.5 | 42.6 ± 1.3 | 0.828 |
| Formula-fed at inclusion n (%) | 18 (100%) | 25 (100%) | - |
| Type of formula: | 0.416 | ||
| Containing prebiotic fiber (GOS or FOS) | 15 (83%) | 20 (80%) | |
| Containing probiotics | 2 (11%) | 1 (4%) | |
| Without prebiotic fiber or probiotics | 1 (6%) | 4 (16%) | |
| Dietary Factor | Intervention Group | Study Visit Week 1 | Study Visit Week 4 | Study Visit Week 7 End of Intervention |
|---|---|---|---|---|
| Infant cereal intake (g/100 g) | 0-WG | 28.9 ± 19.2 | 39.3 ± 20.8 | 34.5 ± 16.8 |
| 50-WG | 37.9 ± 34.7 | 38.3 ± 27.4 | 38.9 ± 24.9 | |
| Sugar from cereals (g) | 0-WG | 6.9 ± 4.6 | 9.3 ± 4.9 | 8.2 ± 4.1 |
| 50-WG | 4.7 ± 4.1 | 4.7 ± 3.2 # | 4.9 ± 3.9 # | |
| Fiber from cereals (g) | 0-WG | 1.1 ± 0.8 | 1.6 ± 0.8 | 1.4 ± 0.7 |
| 50-WG | 2.7 ± 2.5 # | 2.7 ± 1.9 # | 2.7 ± 1.7 # | |
| Energy (kcal/day) | 0-WG | 686.6 ± 190.9 | 683.3 ± 197.7 | 699.8 ± 188.5 |
| 50-WG | 760.4 ± 171.6 | 783.8 ± 223.7 | 797.7 ± 196.8 | |
| Protein (g/day) | 0-WG * | 14.7 ± 4.6 | 15.3 ± 5.7 | 17.7 ± 5.1 |
| 50-WG * | 15.9 ± 3.9 | 16.6 ± 5.8 | 20.1 ± 5.8 | |
| Carbohydrates (g/day) | 0-WG * | 89.2 ± 26.8 | 101.3 ± 31.1 | 107.7± 30.9 |
| 50-WG * | 97.0 ± 26.9 | 110.1 ± 38.0 | 118.3 ± 34.8 | |
| Sugar (g/day) | 0-WG | 60.8 ± 20.2 | 60.8 ± 20.9 | 59.8 ± 17.9 |
| 50-WG | 68.1 ± 17.7 | 68.4 ± 18.6 | 62.2 ± 16.3 | |
| Fat (g/day) | 0-WG * | 29.1 ± 9.1 | 23.9 ± 5.5 | 21.7 ± 6.6 |
| 50-WG * | 33.6 ± 7.5 | 29.9 ± 7.6 # | 26.9 ± 6.6 # | |
| Saturated fat (g/day) | 0-WG * | 12.4 ± 7.1 | 10.2 ± 6.2 | 8.6 ± 3.9 |
| 50-WG * | 13.1 ± 2.8 | 11.5 ± 2.9 | 9.8 ± 2.9 | |
| Fiber (g/day) | 0-WG * | 4.4 ± 3.8 | 5.4 ± 2.8 | 7.2 ± 1.9 |
| 50-WG * | 4.9 ± 3.2 | 6.2 ± 3.4 | 8.4 ± 4.1 |
| Bacterial Variables | 0-WG | 50-WG | ||
|---|---|---|---|---|
| Phylum | Baseline (n = 18) | 7 Weeks (n = 18) | Baseline (n = 25) | 7 Weeks (n = 25) |
| Actinobacteria | 47.5 (21.3–66.7) | 33.2 (7.0–61.2) * | 35.2 (2.2 -80.2) | 34.9 (2.7–77.6) |
| Bacteroidetes | 0.4 (0.0–6.1) | 1.3 (0.0–26.6) * | 0.6 (0.0–43.3) | 2.3 (0.0–38.1) * |
| Firmicutes | 29.5 (11.0–55.7) | 33.3 (17.3–58.0) | 39.1 (14.0–74.6) | 43.4 (12.4–74.0) |
| Proteobacteria | 15.2 (0.1–44.8) | 21.6 (0.7–39.5) | 15.2 (1.9–37.0) | 10.3 (0.1–32.3) * |
| Verrucomicrobia | 0.01 (0.0–19.3) | 0.04 (0.0–34.7) | 0.01 (0.0–20.6) | 0.01 (0.0–6.7) |
| Species richness | 30 (13–70) | 35 (20–68) | 34 (17 -56) | 30 (18–63) |
| Sequences | 88,539 (61,442–107,505) | 93,989 (67,552–134,141) | 88,521 (59,156–124,053) | 86,305 (58,310–113,512) |
| Unclassified sequences derived from bacteria | 0.0 (0.0–0.05) | 0.04 (0.02–0.1) | 0.03 (0.01–0.08) | 0.04 (0.01–0.07) |
| Bacterial Variables | 0-WG | 50-WG | ||
|---|---|---|---|---|
| Genus | Baseline (n = 18) | 7 weeks (n = 18) | Baseline (n = 25) | 7 weeks (n = 25) |
| Bifidobacterium | 39.9 (7.5–66.3) | 28.4 (6.6–59.2) * | 28.4 (0.5–69.3) | 28.7 (1.6–73.8) |
| Escherichia-Shigella | 11.2 (0.0–41.4) | 18.8 (0.1–39.1) | 10.5 (1.3–34.5) | 6.3 (0.06–28.4) * |
| Blautia | 0.001 (0.0–3.9) | 0.001 (0.0–11.9) | 0.001 (0.0–7.6) | 0.3 (0.0–1.9) |
| Streptococcus | 1.5 (0.2–8.3) | 1.3 (0.1–17.6) | 2.2 (0.4–14.3) | 1.5 (0.2–23.6) |
| Enterococcus | 2.7 (0.3–20.5) | 0.9 (0.0–9.3) * | 1.5 (0.0–23.4) | 0.6 (0.0–8.8) * |
| Veillonella | 0.8 (0.0–16.1) | 8.9 (0.0–25.7) * | 4.4 (0.0–35.9) | 9.3 (0.7–46.2) * |
| Clostridium sensustricto 1 | 0.2 (0.0–2.5) | 0.9 (0.0–5.2) | 0.4 (0.0–3.1) | 0.5 (0.0–3.3) |
| Eggerthella | 0.1 (0.0–1.2) | 0.2 (0.0–0.9) | 0.5 (0.0–1.4) | 0.4(0.0–2.1) |
| Granulicatella | 0.02 (0.0–1.0) | 0.06 (0.0–0.2) | 0.04 (0.0–0.6) | 0.001 (0.0–0.4) |
| Gemella | 0.02 (0.0–0.3) | 0.04 (0.0–0.4) | 0.09 (0.0–0.5) | 0.1 (0.0–0.3) |
| Lachnoclostridium | 0.04 (0.0–5.2) | 0.3 (0.0–1.6) | 0.001 (0.0–3.2) | 0.7 (0.0–3.1) * |
| Collinsella | 0.07 (0.0–30.0) | 0.01 (0.0–25.6) | 0.04 (0.0–27.2) | 0.03 (0.0–20.9) |
| Akkermansia | 0.01 (0.0–19.2) | 0.04 (0.0–34.7) | 0.01 (0.0–20.6) | 0.01 (0.0–6.7) |
| Ruminococcus gnavus group | 7.0 (0.0–31.1) | 4.2 (0.0–27.0) | 8.1 (0.0–61.0) | 6.6 (0.0–29.6) |
| Bacteroides | 0.3 (0.0–4.7) | 1.2 (0.0–26.6) | 0.2 (0.0–43.2) | 2.1 (0.0–37.8) * |
| Rothia | 0.002 (0.0–0.2) | 0.002 (0.0–0.2) | 0.004 (0.0–0.2) | 0.003 (0.0–0.08) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaza-Diaz, J.; Bernal, M.J.; Schutte, S.; Chenoll, E.; Genovés, S.; Codoñer, F.M.; Gil, A.; Sanchez-Siles, L.M. Effects of Whole-Grain and Sugar Content in Infant Cereals on Gut Microbiota at Weaning: A Randomized Trial. Nutrients 2021, 13, 1496. https://doi.org/10.3390/nu13051496
Plaza-Diaz J, Bernal MJ, Schutte S, Chenoll E, Genovés S, Codoñer FM, Gil A, Sanchez-Siles LM. Effects of Whole-Grain and Sugar Content in Infant Cereals on Gut Microbiota at Weaning: A Randomized Trial. Nutrients. 2021; 13(5):1496. https://doi.org/10.3390/nu13051496
Chicago/Turabian StylePlaza-Diaz, Julio, Maria Jose Bernal, Sophie Schutte, Empar Chenoll, Salvador Genovés, Francisco M. Codoñer, Angel Gil, and Luis Manuel Sanchez-Siles. 2021. "Effects of Whole-Grain and Sugar Content in Infant Cereals on Gut Microbiota at Weaning: A Randomized Trial" Nutrients 13, no. 5: 1496. https://doi.org/10.3390/nu13051496