Probiotics Prevent Hypertension in a Murine Model of Systemic Lupus Erythematosus Induced by Toll-Like Receptor 7 Activation
Abstract
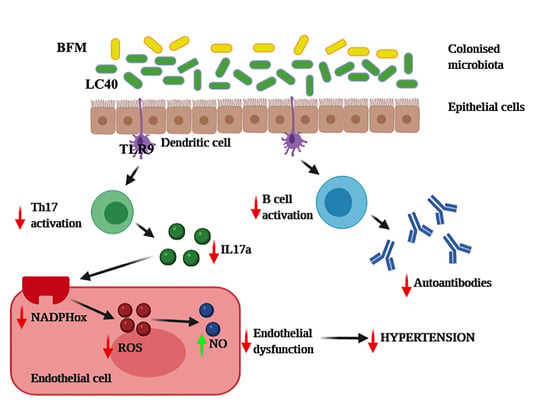
:1. Introduction
2. Materials and Methods
2.1. Probiotic Preparation and Administration
2.2. Animals and Experimental Groups
2.3. Blood Pressure Measurements
2.4. Plasma Determinations
2.5. Morphological Variables
2.6. Vascular Reactivity
2.7. NADPH Oxidase Activity
2.8. In Situ Detection of Vascular Reactive Oxygen Species (ROS) Content
2.9. Quantitative Polymerase Chain Reaction (qPCR)
2.10. Flow Cytometry
2.11. DNA Extraction, 16S rRNA Gene Amplification and Bioinformatics
2.12. Reagents
2.13. Statistical Analysis
3. Results
3.1. Probiotics Improve Intestinal Integrity without Preventing Gut Dysbiosis in IMQ-Treated Mice
3.2. Probiotics Attenuate Lupus Disease Activity and Modulate Immune Response
3.3. Probiotics Prevent Endothelial Dysfunction, Oxidative Stress and Hypertension
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Johnson, B.M.; Gaudreau, M.C.; Al-Gadban, M.M.; Gudi, R.; Vasu, C. Impact of dietary deviation on disease progression and gut microbiome composition in lupus-prone SNF1 mice. Clin. Exp. Immunol. 2015, 181, 323–337. [Google Scholar] [CrossRef] [Green Version]
- Sanz, I.; Lee, F.E. B cells as therapeutic targets in SLE. Nat. Rev. Rheumatol. 2010, 6, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Lech, M.; Anders, H.J. The pathogenesis of lupus nephritis. J. Am. Soc. Nephrol. 2013, 24, 1357–1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frostegård, J. Systemic lupus erythematosus and cardiovascular disease. Lupus 2008, 17, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Al-Herz, A.; Ensworth, S.; Shojania, K.; Esdaile, J.M. Cardiovascular risk factor screening in systemic lupus erythematosus. J. Rheumatol. 2003, 30, 493–496. [Google Scholar]
- Ocampo-Piraquive, V.; Nieto-Aristizábal, I.; Cañas, C.A.; Tobón, G.J. Mortality in systemic lupus erythematosus: Causes, predictors and interventions. Expert Rev. Clin. Immunol. 2018, 14, 1043–1053. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Somers, E.C.; Brook, R.D.; Kehrer, C.; Pfenninger, D.; Lewis, E.; Chakrabarti, A.; Richardson, B.C.; Shelden, E.; McCune, W.J.; et al. Endothelial cell apoptosis in systemic lupus erythematosus: A common pathway for abnormal vascular function and thrombosis propensity. Blood 2004, 103, 3677–3683. [Google Scholar] [CrossRef]
- Bruce, I.N.; Gladman, D.D.; Ibañez, D.; Urowitz, M.B. Single photon emission computed tomography dual isotope myocardial perfusion imaging in women with systemic lupus erythematosus. II. Predictive factors for perfusion abnormalities. J. Rheumatol. 2003, 30, 288–291. [Google Scholar] [PubMed]
- Sun, W.; Jiao, Y.; Cui, B.; Gao, X.; Xia, Y.; Zhao, Y. Immune complexes activate human endothelium involving the cell-signaling HMGB1-RAGE axis in the pathogenesis of lupus vasculitis. Lab. Investig. 2013, 93, 626–638. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Kravvariti, E.; Konstantonis, G.; Tentolouris, N.; Sfikakis, P.P.; Protogerou, A. Subclinical atherosclerosis in Systemic Lupus Erythematosus: Comparable risk with Diabetes Mellitus and Rheumatoid Arthritis. Autoimmun. Rev. 2017, 16, 308–312. [Google Scholar] [CrossRef]
- Chen, J.Q.; Szodoray, P.; Zeher, M. Toll-Like Receptor Pathways in Autoimmune Diseases. Clin. Rev. Allergy Immunol. 2016, 50, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.W.; Tang, W.; Zuo, J.P. Toll-like receptors: Potential targets for lupus treatment. Acta Pharmacol. Sin. 2015, 36, 1395–1407. [Google Scholar] [CrossRef]
- Weidenbusch, M.; Kulkarni, O.P.; Anders, H.J. The innate immune system in human systemic lupus erythematosus. Clin. Sci. 2017, 131, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Yokogawa, M.; Takaishi, M.; Nakajima, K.; Kamijima, R.; Fujimoto, C.; Kataoka, S.; Terada, Y.; Sano, S. Epicutaneous application of toll-like receptor 7 agonists leads to systemic autoimmunity in wild-type mice: A new model of systemic Lupus erythematosus. Arthritis Rheumatol. 2014, 66, 694–706. [Google Scholar] [CrossRef] [PubMed]
- Crow, M.K. Type I interferon in the pathogenesis of lupus. J. Immunol. 2014, 192, 5459–5468. [Google Scholar] [CrossRef]
- Liu, Y.; Seto, N.L.; Carmona-Rivera, C.; Kaplan, M.J. Accelerated model of lupus autoimmunity and vasculopathy driven by toll-like receptor 7/9 imbalance. Lupus Sci. Med. 2018, 5, e000259. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Visitación, N.; Toral, M.; Sánchez, M.; Gómez-Guzmán, M.; O’valle, F.; Jiménez, R.; Duarte, J.; Romero, M. Toll-like receptor 7-driven lupus autoimmunity induces hypertension and vascular alterations in mice. J. Hypertens. 2020, 38, 1322–1335. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.M.; Edwards, M.R.; Mu, Q.; Yu, Y.; Vieson, M.D.; Reilly, C.M.; Ahmed, S.A.; Bankole, A.A. Gut Microbiota in Human Systemic Lupus Erythematosus and a Mouse Model of Lupus. Appl. Environ. Microbiol. 2018, 84, e02288-17. [Google Scholar] [CrossRef] [Green Version]
- Toral, M.; Robles-Vera, I.; Romero, M.; de la Visitación, N.; Sánchez, M.; O’Valle, F.; Rodriguez-Nogales, A.; Gálvez, J.; Duarte, J.; Jiménez, R. CECT5716: A novel alternative for the prevention of vascular disorders in a mouse model of systemic lupus erythematosus. FASEB J. 2019, 33, 10005–10018. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Liao, X.; Sparks, J.B.; Luo, X.M. Dynamics of gut microbiota in autoimmune lupus. Appl. Environ. Microbiol. 2014, 80, 7551–7560. [Google Scholar] [CrossRef] [Green Version]
- de la Visitación, N.; Robles-Vera, I.; Toral, M.; Gómez-Guzmán, M.; Sánchez, M.; Moleón, J.; González-Correa, C.; Martín-Morales, N.; O’Valle, F.; Jiménez, R.; et al. Gut microbiota contributes to the development of hypertension in a genetic mouse model of systemic lupus erythematosus. Br. J. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Zegarra-Ruiz, D.F.; El Beidaq, A.; Iñiguez, A.J.; Lubrano Di Ricco, M.; Manfredo Vieira, S.; Ruff, W.E.; Mubiru, D.; Fine, R.L.; Sterpka, J.; Greiling, T.M.; et al. A Diet-Sensitive Commensal Lactobacillus Strain Mediates TLR7-Dependent Systemic Autoimmunity. Cell Host Microbe 2019, 25, 113–127.e116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de la Visitación, N.; Robles-Vera, I.; Toral, M.; Duarte, J. Protective Effects of Probiotic Consumption in Cardiovascular Disease in Systemic Lupus Erythematosus. Nutrients 2019, 11, 2676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toral, M.; Gómez-Guzmán, M.; Jiménez, R.; Romero, M.; Sánchez, M.; Utrilla, M.P.; Garrido-Mesa, N.; Rodríguez-Cabezas, M.E.; Olivares, M.; Gálvez, J.; et al. The probiotic Lactobacillus coryniformis CECT5711 reduces the vascular pro-oxidant and pro-inflammatory status in obese mice. Clin. Sci. 2014, 127, 33–45. [Google Scholar] [CrossRef]
- Romero, M.; Toral, M.; Robles-Vera, I.; Sánchez, M.; Jiménez, R.; O’Valle, F.; Rodriguez-Nogales, A.; Pérez-Vizcaino, F.; Gálvez, J.; Duarte, J. Activation of Peroxisome Proliferator Activator Receptor β/δ Improves Endothelial Dysfunction and Protects Kidney in Murine Lupus. Hypertension 2017, 69, 641–650. [Google Scholar] [CrossRef]
- Edgar, R.C.; Flyvbjerg, H. Error filtering, pair assembly and error correction for next-generation sequencing reads. Bioinformatics 2015, 31, 3476–3482. [Google Scholar] [CrossRef] [Green Version]
- Antharam, V.C.; Li, E.C.; Ishmael, A.; Sharma, A.; Mai, V.; Rand, K.H.; Wang, G.P. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J. Clin. Microbiol. 2013, 51, 2884–2892. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [Green Version]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef] [Green Version]
- Elinav, E.; Henao-Mejia, J.; Flavell, R.A. Integrative inflammasome activity in the regulation of intestinal mucosal immune responses. Mucosal Immunol. 2013, 6, 4–13. [Google Scholar] [CrossRef]
- Harrison, O.J.; Srinivasan, N.; Pott, J.; Schiering, C.; Krausgruber, T.; Ilott, N.E.; Maloy, K.J. Epithelial-derived IL-18 regulates Th17 cell differentiation and Foxp3⁺ Treg cell function in the intestine. Mucosal Immunol. 2015, 8, 1226–1236. [Google Scholar] [CrossRef] [Green Version]
- Wofsy, D.; Chiang, N.Y.; Greenspan, J.S.; Ermak, T.H. Treatment of murine lupus with monoclonal antibody to L3T4. I. Effects on the distribution and function of lymphocyte subsets and on the histopathology of autoimmune disease. J. Autoimmun. 1988, 1, 415–431. [Google Scholar] [CrossRef]
- Niess, J.H.; Brand, S.; Gu, X.; Landsman, L.; Jung, S.; McCormick, B.A.; Vyas, J.M.; Boes, M.; Ploegh, H.L.; Fox, J.G.; et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 2005, 307, 254–258. [Google Scholar] [CrossRef] [Green Version]
- Mora, J.R.; von Andrian, U.H. Differentiation and homing of IgA-secreting cells. Mucosal Immunol. 2008, 1, 96–109. [Google Scholar] [CrossRef]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef]
- Toral, M.; Romero, M.; Rodríguez-Nogales, A.; Jiménez, R.; Robles-Vera, I.; Algieri, F.; Chueca-Porcuna, N.; Sánchez, M.; de la Visitación, N.; Olivares, M.; et al. Lactobacillus fermentum Improves Tacrolimus-Induced Hypertension by Restoring Vascular Redox State and Improving eNOS Coupling. Mol. Nutr. Food Res. 2018, 62, e1800033. [Google Scholar] [CrossRef]
- Gómez-Guzmán, M.; Toral, M.; Romero, M.; Jiménez, R.; Galindo, P.; Sánchez, M.; Zarzuelo, M.J.; Olivares, M.; Gálvez, J.; Duarte, J. Antihypertensive effects of probiotics Lactobacillus strains in spontaneously hypertensive rats. Mol. Nutr. Food Res. 2015, 59, 2326–2336. [Google Scholar] [CrossRef]
- Robles-Vera, I.; Toral, M.; de la Visitación, N.; Sánchez, M.; Gómez-Guzmán, M.; Romero, M.; Yang, T.; Izquierdo-Garcia, J.L.; Jiménez, R.; Ruiz-Cabello, J.; et al. Probiotics Prevent Dysbiosis and the Rise in Blood Pressure in Genetic Hypertension: Role of Short-Chain Fatty Acids. Mol. Nutr. Food Res. 2020, 64, e1900616. [Google Scholar] [CrossRef]
- Robles-Vera, I.; de la Visitación, N.; Toral, M.; Sánchez, M.; Romero, M.; Gómez-Guzmán, M.; Yang, T.; Izquierdo-García, J.L.; Guerra-Hernández, E.; Ruiz-Cabello, J.; et al. Probiotic Bifidobacterium breve prevents DOCA-salt hypertension. FASEB J. 2020, 34, 13626–13640. [Google Scholar] [CrossRef] [PubMed]
- de la Visitación, N.; Robles-Vera, I.; Toral, M.; O’Valle, F.; Moleon, J.; Gómez-Guzmán, M.; Romero, M.; Duarte, M.; Sánchez, M.; Jiménez, R.; et al. Lactobacillus fermentum CECT5716 prevents renal damage in the NZBWF1 mouse model of systemic lupus erythematosus. Food Funct. 2020, 11, 5266–5274. [Google Scholar] [CrossRef]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef] [Green Version]
- Mu, Q.; Zhang, H.; Liao, X.; Lin, K.; Liu, H.; Edwards, M.R.; Ahmed, S.A.; Yuan, R.; Li, L.; Cecere, T.E.; et al. Control of lupus nephritis by changes of gut microbiota. Microbiome 2017, 5, 73. [Google Scholar] [CrossRef] [PubMed]
- Mack, D.R.; Michail, S.; Wei, S.; McDougall, L.; Hollingsworth, M.A. Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 1999, 276, G941–G950. [Google Scholar] [CrossRef]
- Mathis, K.W.; Wallace, K.; Flynn, E.R.; Maric-Bilkan, C.; LaMarca, B.; Ryan, M.J. Preventing autoimmunity protects against the development of hypertension and renal injury. Hypertension 2014, 64, 792–800. [Google Scholar] [CrossRef] [Green Version]
- Green, N.M.; Marshak-Rothstein, A. Toll-like receptor driven B cell activation in the induction of systemic autoimmunity. Semin. Immunol. 2011, 23, 106–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Good, M.; Sodhi, C.P.; Ozolek, J.A.; Buck, R.H.; Goehring, K.C.; Thomas, D.L.; Vikram, A.; Bibby, K.; Morowitz, M.J.; Firek, B.; et al. Lactobacillus rhamnosus HN001 decreases the severity of necrotizing enterocolitis in neonatal mice and preterm piglets: Evidence in mice for a role of TLR9. Am. J. Physiol. Gastrointest Liver Physiol. 2014, 306, G1021–G1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermudez-Brito, M.; Muñoz-Quezada, S.; Gomez-Llorente, C.; Matencio, E.; Bernal, M.J.; Romero, F.; Gil, A. Cell-free culture supernatant of Bifidobacterium breve CNCM I-4035 decreases pro-inflammatory cytokines in human dendritic cells challenged with Salmonella typhi through TLR activation. PLoS ONE 2013, 8, e59370. [Google Scholar] [CrossRef]
- Tschudi, M.R.; Mesaros, S.; Lüscher, T.F.; Malinski, T. Direct in situ measurement of nitric oxide in mesenteric resistance arteries. Increased decomposition by superoxide in hypertension. Hypertension 1996, 27, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Makhezer, N.; Ben Khemis, M.; Liu, D.; Khichane, Y.; Marzaioli, V.; Tlili, A.; Mojallali, M.; Pintard, C.; Letteron, P.; Hurtado-Nedelec, M.; et al. NOX1-derived ROS drive the expression of Lipocalin-2 in colonic epithelial cells in inflammatory conditions. Mucosal Immunol. 2019, 12, 117–131. [Google Scholar] [CrossRef] [Green Version]
- Kassan, M.; Galan, M.; Partyka, M.; Trebak, M.; Matrougui, K. Interleukin-10 released by CD4(+)CD25(+) natural regulatory T cells improves microvascular endothelial function through inhibition of NADPH oxidase activity in hypertensive mice. Arterioscler Thromb. Vasc. Biol. 2011, 31, 2534–2542. [Google Scholar] [CrossRef] [Green Version]








| mRNA Targets | Descriptions | Sense | Antisense |
|---|---|---|---|
| eNOS | endothelial nitric oxide synthase | ATGGATGAGCCAACTCAAGG | TGTCGTGTAATCGGTCTTGC |
| IFN-γ | Interferon-gamma | GCCCTCTCTGGCTGTTACTG | CCAAGAGGAGGCTCTTTCCT |
| TNF-α | Tumor necrosis factor-alpha | CTACTCCCAGGTTCTCTTCAA | GCAGAGAGGAGGTTGACTTTC |
| Muc-2 | Mucin-2 | GATAGGTGGCAGACAGGAGA | GCTGACGAGTGGTTGGTGAATG |
| Muc-3 | Mucin-3 | CGTGGTCAACTGCGAGAATGG | CGGCTCTATCTCTACGCTCTCC |
| Occludin | Occludin | ACGGACCCTGACCACTATGA | TGGAGATGAGGCTTCTGCTT |
| ZO-1 | Zonula occludens-1 | GGGGCCTACACTGATCAAGA | TGGAGATGAGGCTTCTGCTT |
| IL-18 | Interleukin-18 | GACTCTTGCGTCAACTTCAAGG | CATGGACCGCTTCCCATA |
| IL-6 | Interleukin-6 | ACTTCACAAGTCCGGAGAGG | TTTCTGCAAGTGCATCATCG |
| VCAM-1 | Vascular cell adhesion molecule-1 | CTTCCAGAACCCTTCTCAG | GGGACCATTCCAGTCACACTT |
| FoxP3 | Forkhead box P3 | AGGCACTTCTCCAGGACAGA | CTGGACACCCATTCCAGACT |
| ROR-γ | Retinoid-related orphan receptor-gamma | GCCTACAATGCCAACAACCACACA | TGATGAGAACCAAGGCCGTGTAGA |
| TLR-7 | Toll-like receptor-7 | GCCATCCAGCTTACATCTTCT | TTTGACCCAGGTAGAGTGTTTC |
| TLR-9 | Toll-like receptor-9 | CTACAACAGCCAGCCCTTTA | GGACACACGGGTATGAATGT |
| CX3CR-1 | CX3C chemokine receptor 1 | TGAGTGACTGGCACTTCCTG | CGAGGACCACCAACAGATTT |
| CCR9 | C-C chemokine receptor type 9 | CCAGGAAATCTCTGGTCTGC | CTGTGGAAGCAGTGGAGTCA |
| Itga4 | Integrin alpha-4 | TGTGCAAATGTACACTCTCTTCCA | CTCCCTCAAGATGATAAGTTGTTCAA |
| RPL13 | Ribosomal protein L13 | CCTGCTGCTCTCAAGGTTGTT | TGGTTGTCACTGCCTGGTACTT |
| GAPDH | Glyceraldehyde-3-Phosphate Dehydrogenase | ACCACAGTCCATGCCATCAC | TCCACCACCCTGTTGCTGTA |
| Phylum | Ctrl (n = 8) | IMQ (n = 8) | IMQ + LC40 (n = 8) | IMQ + BFM (n = 8) |
|---|---|---|---|---|
| Tenericutes | 1.5 ± 0.2 | 0.3 ± 0.1 | 0.4 ± 0.2 | 0.9 ± 0.4 |
| Cyanobacteria | 0.5 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.7 ± 0.1 |
| Proteobacteria | 3.6 ± 0.3 | 4.4 ± 0.3 | 4.6 ± 0.8 | 3.7 ± 0.3 |
| Bacteroidetes | 49.9 ± 4.3 | 69.0 ± 3.9 **** | 63.0 ± 7.4 | 66.0 ± 5.9 |
| Firmicutes | 44.0 ± 4.5 | 24.9 ± 3.9 **** | 30.4 ± 6.8 | 28.0 ± 5.5 |
| Others | 0.6 ± 0.0 | 0.5 ± 0.1 | 0.8 ± 0.2 | 0.7 ± 0.1 |
| Variables | Ctrl (n = 8) | IMQ (n = 12) | IMQ + LC40 (n = 9) | IMQ + BFM (n = 10) |
|---|---|---|---|---|
| BW (g) | 21.4 ± 0.4 | 20.8 ± 0.6 | 19.5 ± 2.3 | 21.1 ± 0.7 |
| HW/TL (mg/cm) | 5.28 ± 0.16 | 5.58 ± 0.15 | 5.37 ± 0.14 | 5.43 ± 0.09 |
| LVW/TL (mg/cm) | 3.49 ± 0.12 | 3.87 ± 0.12 * | 3.44 ± 0.12 # | 3.49 ± 0.9 # |
| KW/TL (mg/cm) | 6.10 ± 0.19 | 7.50 ± 0.18 ** | 6.98 ± 0.10 # | 6.79 ± 0.21 # |
| LW/TL (mg/cm) | 53.77 ± 2.4 | 61.96 ± 4.12 | 67.58 ± 4.4 | 64.59 ± 3.7 |
| SW/TL (mg/cm) | 4.37 ± 0.43 | 27.97 ± 1.86 ** | 25.84 ± 1.09 | 19.19 ± 1.64 ## |
| CW/CL (mg/cm) | 21.53 ± 1.07 | 16.94 ± 0.64 ** | 17.34 ± 0.81 | 17.14 ± 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Visitación, N.; Robles-Vera, I.; Moleón-Moya, J.; Sánchez, M.; Jiménez, R.; Gómez-Guzmán, M.; González-Correa, C.; Olivares, M.; Toral, M.; Romero, M.; et al. Probiotics Prevent Hypertension in a Murine Model of Systemic Lupus Erythematosus Induced by Toll-Like Receptor 7 Activation. Nutrients 2021, 13, 2669. https://doi.org/10.3390/nu13082669
de la Visitación N, Robles-Vera I, Moleón-Moya J, Sánchez M, Jiménez R, Gómez-Guzmán M, González-Correa C, Olivares M, Toral M, Romero M, et al. Probiotics Prevent Hypertension in a Murine Model of Systemic Lupus Erythematosus Induced by Toll-Like Receptor 7 Activation. Nutrients. 2021; 13(8):2669. https://doi.org/10.3390/nu13082669
Chicago/Turabian Stylede la Visitación, Néstor, Iñaki Robles-Vera, Javier Moleón-Moya, Manuel Sánchez, Rosario Jiménez, Manuel Gómez-Guzmán, Cristina González-Correa, Mónica Olivares, Marta Toral, Miguel Romero, and et al. 2021. "Probiotics Prevent Hypertension in a Murine Model of Systemic Lupus Erythematosus Induced by Toll-Like Receptor 7 Activation" Nutrients 13, no. 8: 2669. https://doi.org/10.3390/nu13082669