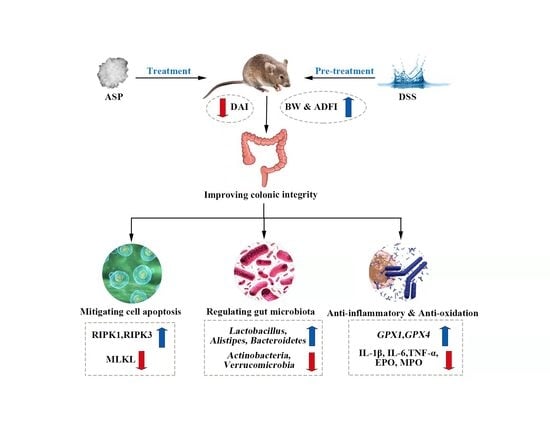
Improvement of Ulcerative Colitis by Aspartate via RIPK Pathway Modulation and Gut Microbiota Composition in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Histological Analysis
2.3. Quantification of Myeloperoxidase and Eosinophil Peroxidase Activity and Inflammatory Cytokines Contents in the Colon
2.4. RT-qPCR Analysis
2.5. Protein Qualification Using the Wes Simple Western System
2.6. Terminal Deoxynucleotidyl Transferase Mediated dUTP Nick End Labelling (TUNEL) Assay
2.7. Transmission Electron Microscopy (TEM)
2.8. Taxonomic Analyses of the Gut Microbiota
2.9. Statistical Analysis
3. Results
3.1. Effects of Asp on Growth Status, Colonic Morphology, and Pathology in DSS-Induced Mice
3.2. Effects of Asp on Concentration of Inflammatory Cytokines and Expression of Inflammation Related-Genes in the Colon of DSS-Induced Mice
3.3. Effects of Asp on Intestinal Mitochondrial Ultrastructure and Antioxidant Indexes in the Colon ff DSS-Induced Mice
3.4. Effects of Asp on Cell Apoptosis and Expression of RIPK Pathway in the Colon of DSS-Induced Mice
3.5. Effects of Asp on Colonic Microbes in DSS-Induced Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Okobi, O.E.; Udoete, I.O.; Fasehun, O.O.; Okobi, T.; Evbayekha, E.O.; Ekabua, J.J.; Elukeme, H.; Ebong, I.L.; Ajayi, O.O.; Olateju, I.V.; et al. A Review of Four Practice Guidelines of Inflammatory Bowel Disease. Cureus 2021, 13, e16859. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, P.; An, Y.; Ren, J.; Yan, D.; Cui, J.; Li, D.; Li, M.; Wang, M.; Zhong, G. Phloretin ameliorates dextran sulfate sodium-induced ulcerative colitis in mice by regulating the gut microbiota. Pharmacol. Res. 2019, 150, 104489. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Gao, X.; Nie, L.; Xie, J.; Sheng, J. Astragalin Attenuates Dextran Sulfate Sodium (DSS)-Induced Acute Experimental Colitis by Alleviating Gut Microbiota Dysbiosis and Inhibiting NF-κB Activation in Mice. Front. Immunol. 2020, 11, 2058. [Google Scholar] [PubMed]
- Yan, H.; Wang, H.; Zhang, X.; Li, X.; Yu, J. Ascorbic acid ameliorates oxidative stress and inflammation in dextran sulfate sodium-induced ulcerative colitis in mice. Int. J. Clin. Exp. Med. 2015, 8, 20245–20253. [Google Scholar]
- Yousef, M.; Pichyangkura, R.; Soodvilai, S.; Chatsudthipong, V.; Muanprasat, C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: Therapeutic efficacy and possible mechanisms of action. Pharmacol. Res. 2012, 66, 66–79. [Google Scholar]
- Goh, J.; O’Morain, C.A. Review article: Nutrition and adult inflammatory bowel disease. Aliment. Pharmacol. Ther. 2003, 17, 307–320. [Google Scholar]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Shi, H.; Wang, X.; Zhu, H.; Pi, D.; Leng, W.; Li, S. Aspartate attenuates intestinal injury and inhibits TLR4 and NODs/NF-kappaB and p38 signaling in weaned pigs after LPS challenge. Eur. J. Nutr. 2017, 56, 1433–1443. [Google Scholar] [CrossRef]
- Sivakumar, R.; Babu, P.V.; Shyamaladevi, C.S. Aspartate and glutamate prevents isoproterenol-induced cardiac toxicity by alleviating oxidative stress in rats. Exp. Toxicol. Pathol. 2011, 63, 137–142. [Google Scholar] [CrossRef]
- Xu, H.; Liu, G.; Gu, H.; Wang, J.; Li, Y. Glutamine protects intestine against ischemia-reperfusion injury by alleviating endoplasmic reticulum stress induced apoptosis in rats. Acta Cir. Bras. 2020, 35, e202000104. [Google Scholar] [CrossRef]
- Takahashi, N.; Vereecke, L.; Bertrand, M.J.; Duprez, L.; Berger, S.B.; Divert, T.; Goncalves, A.; Sze, M.; Gilbert, B.; Kourula, S.; et al. RIPK1 ensures intestinal homeostasis by protecting the epithelium against apoptosis. Nature 2014, 513, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Weinlich, R.; Oberst, A.; Beere, H.M.; Green, D.R. Necroptosis in development, inflammation and disease. Nat. Rev. Mol. Cell Biol. 2017, 18, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Liu, J.; Shen, P.; Cai, J.; Han, Y.; Zhu, K.; Fu, Y.; Zhang, N.; Zhang, Z.; Cao, Y. Protective Effect of Naringin on DSS-Induced Ulcerative Colitis in Mice. J. Agric. Food Chem. 2018, 66, 13133–13140. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Duan, X.Y.; Chen, Q.Y.; Fan, H.; Hong, Z.C.; Deng, S.J.; Nan, Z.; Wu, H.; Dong, Y.L.; Liu, Y.J.; et al. Effect of compound sophorae decoction on dextran sodium sulfate (DSS)-induced colitis in mice by regulating Th17/Treg cell balance. Biomed. Pharm. 2019, 109, 2396–2408. [Google Scholar]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15.25.11–15.25.14. [Google Scholar] [CrossRef]
- Shi, H.; Liu, Y.; Li, S.; Zhu, H.; Chen, F.; Hou, Y.; Ding, B.; Pi, D.; Leng, W. Effect of Aspartic Acid on Growth Performance, Blood Cell Differential Count, and Blood Biochemical Measurements of Weaned Piglets after Lipopolysaccharide Challenge. J. Chin. J. Anim. Sci. 2013, 49, 38–43. [Google Scholar]
- Wang, Y.; Liu, J.; Huang, Z.; Li, Y.; Liang, Y.; Luo, C.; Ni, C.; Xie, J.; Su, Z.; Chen, J.; et al. Coptisine ameliorates DSS-induced ulcerative colitis via improving intestinal barrier dysfunction and suppressing inflammatory response. Eur. J. Pharmacol. 2021, 896, 173912. [Google Scholar] [CrossRef]
- Calder, P.C. Branched-chain amino acids and immunity. J. Nutr. 2006, 136, 288S–293S. [Google Scholar] [CrossRef]
- Pi, D.; Liu, Y.; Shi, H.; Li, S.; Odle, J.; Lin, X.; Zhu, H.; Chen, F.; Hou, Y.; Leng, W. Dietary supplementation of aspartate enhances intestinal integrity and energy status in weanling piglets after lipopolysaccharide challenge. J. Nutr. Biochem. 2014, 25, 456–462. [Google Scholar]
- Tang, W.; Wu, J.; Jin, S.; He, L.; Lin, Q.; Luo, F.; He, X.; Feng, Y.; He, B.; Bing, P.; et al. Glutamate and aspartate alleviate testicular/epididymal oxidative stress by supporting antioxidant enzymes and immune defense systems in boars. Sci. China Life Sci. 2020, 63, 116–124. [Google Scholar] [CrossRef]
- Li, Q.; Chen, C.; Liu, C.; Sun, W.; Liu, X.; Ci, Y.; Song, Y. The Effects of Cellulose on AOM/DSS-Treated C57BL/6 Colorectal Cancer Mice by Changing Intestinal Flora Composition and Inflammatory Factors. Nutr. Cancer 2021, 73, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, Y.; Feng, L.; Jiang, W.D.; Kuang, S.Y.; Jiang, J.; Li, S.H.; Tang, L.; Zhou, X.Q. Effects of dietary arginine supplementation on growth performance, flesh quality, muscle antioxidant capacity and antioxidant-related signalling molecule expression in young grass carp (Ctenopharyngodon idella). Food Chem. 2015, 167, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Luo, Q.; Cui, H.; Deng, H.; Kuang, P.; Liu, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; et al. Sodium fluoride causes oxidative stress and apoptosis in the mouse live. Aging 2017, 9, 1623–1639. [Google Scholar] [CrossRef] [PubMed]
- Kearney, C.J.; Cullen, S.P.; Tynan, G.A.; Henry, C.M.; Clancy, D.; Lavelle, E.C.; Martin, S.J. Necroptosis suppresses inflammation via termination of TNF- or LPS-induced cytokine and chemokine production. Cell Death Differ. 2015, 22, 1313–1327. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Kumari, S.; Kim, C.; Van, T.M.; Wachsmuth, L.; Polykratis, A.; Pasparakis, M. RIPK1 counteracts ZBP1-mediated necroptosis to inhibit inflammation. Nature 2016, 540, 124–128. [Google Scholar] [CrossRef]
- Tian, M.; Ma, P.; Zhang, Y.; Mi, Y.; Fan, D. Ginsenoside Rk3 alleviated DSS-induced ulcerative colitis by protecting colon barrier and inhibiting NLRP3 inflammasome pathway. Int. Immunopharmacol. 2020, 85, 106645. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, J.L.; Jian, X.; Leip, D.D.; Chen, C.H.; Westover, B.P.; Weatherford, J.; Buhler, J.D.; Gordon, J.I. Glycan Foraging in Vivo by an Intestine-Adapted Bacterial Symbiont. Science 2005, 307, 1955–1959. [Google Scholar]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Derrien, M.; Collado, M.C.; Ben-Amor, K.; Salminen, S.; de Vos, W.M. The Mucin Degrader Akkermansia muciniphila Is an Abundant Resident of the Human Intestinal Tract. Appl. Environ. Microbiol. 2008, 74, 1646–1648. [Google Scholar] [CrossRef]
- Reunanen, J.; Kainulainen, V.; Huuskonen, L.; Ottman, N.; Belzer, C.; Huhtinen, H.; De Vos, W.M.; Satokari, R.; Goodrich-Blair, H. Akkermansia muciniphila Adheres to Enterocytes and Strengthens the Integrity of the Epithelial Cell Layer. Appl. Environ. Microbiol. 2015, 81, 3655–3662. [Google Scholar] [CrossRef]
- Koliada, A.; Syzenko, G.; Moseiko, V.; Budovska, L.; Puchkov, K.; Perederiy, V.; Gavalko, Y.; Dorofeyev, A.; Romanenko, M.; Tkach, S.; et al. Association between body mass index and Firmicutes/Bacteroidetes ratio in an adult Ukrainian population. BMC Microbiol. 2017, 17, 120. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Score | Stool Consistency | Bleeding | Weight Loss (%) |
|---|---|---|---|
| 0 | Normal | Normal | 0 |
| 1 | Softer stool/sticks to cage wall | Weak hemoccult-positive spots in stool | 1–5 |
| 2 | Moderate diarrhea/unformed stool | Visual blood in stool/strongly hemoccult-positive stool | 6–10 |
| 3 | Diarrhea (watery stool) | Fresh rectal bleeding | 10–15 |
| 4 | - | - | >15 |
| Score | Severity of Inflammation | Crypt Damage | Ulceration |
|---|---|---|---|
| 0 | Rare inflammatory cells in the lamina propria | Intact crypt and goblet cell | 0 foci of ulceration |
| 1 | Increased numbers of granulocytes in the lamina propria | Loss of basal 1/3 of crypt and depletion of goblet cells | 1–2 foci of ulceration |
| 2 | Confluent inflammatory cells extended to submucosa | Loss of basal 2/3 of crypt and depletion of goblet cells | 3–4 foci of ulceration |
| 3 | Transmural extension of the inflammatory infiltration | Loss of entire crypt and depletion of goblet cells | Confluent or extensive ulceration |
| 4 | - | Change in epithelial surface caused by erosion | - |
| 5 | - | Confluent erosion | - |
| Genes | Primer Sequences (5′-3′) | Serial Number | Product Length/bp |
|---|---|---|---|
| GAPDH | F: GCACAGTCAAGGCCGAGAAT R: GCCTTCTCCATGGTGGTGAA | XM_017321385.2 | 151 |
| GPX1 | F: GGTTCGAGCCCAATTTTACA R: CCCACCAGGAACTTCTCAAA | XM_021172037.2 | 199 |
| GPX4 | F: CTCCATGCACGAATTCTCAG R: ACGTCAGTTTTGCCTCATTG | NM_001367995.1 | 117 |
| TLR4 | F: TTCAGAACTTCAGTGGCTGGATT R: CCATGCCTTGTCTTCAATTGTTT | NM_021297.3 | 64 |
| MYD88 | F: GCATGGTGGTGGTTGTTTCTG R: GAATCAGTCGCTTCTGTTGG | NM_010851.3 | 108 |
| RIPK3 | F: GCCTTCCTCTCAGTCCACAC R: ACGCACCAGTAGGCCATAAC | NM_019955.2 | 127 |
| RIPK1 | F: GCTGTCATCTAGCGGGAGGT R: TCCGCTGTCTAGGTCTGTCT | NM_009068.3 | 197 |
| MLKL | F: GATTGCCCTGAGTTGTTGCG R: CTCTCCAAGATTCCGTCCACA | XM_030243820.2 | 89 |
| IL-6 | F: AGTTGCCTTCTTGGGACTGA R: TCCACGATTTCCCAGAGAAC | NM_001314054.1 | 159 |
| TNF-α | F: CTGGGACAGTGACCTGGACT R: GCACCTCAGGGAAGAGTCTG | NM_001278601.1 | 204 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; He, X.; Peng, C.; He, Y.; Wang, C.; Tang, W.; Chen, H.; Feng, Y.; Liu, D.; Li, T.; et al. Improvement of Ulcerative Colitis by Aspartate via RIPK Pathway Modulation and Gut Microbiota Composition in Mice. Nutrients 2022, 14, 3707. https://doi.org/10.3390/nu14183707
Hu X, He X, Peng C, He Y, Wang C, Tang W, Chen H, Feng Y, Liu D, Li T, et al. Improvement of Ulcerative Colitis by Aspartate via RIPK Pathway Modulation and Gut Microbiota Composition in Mice. Nutrients. 2022; 14(18):3707. https://doi.org/10.3390/nu14183707
Chicago/Turabian StyleHu, Xian, Xinmiao He, Can Peng, Yiwen He, Chenyu Wang, Wenjie Tang, Heshu Chen, Yanzhong Feng, Di Liu, Tiejun Li, and et al. 2022. "Improvement of Ulcerative Colitis by Aspartate via RIPK Pathway Modulation and Gut Microbiota Composition in Mice" Nutrients 14, no. 18: 3707. https://doi.org/10.3390/nu14183707