Effects of Beetroot Juice on Recovery of Muscle Function and Performance between Bouts of Repeated Sprint Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
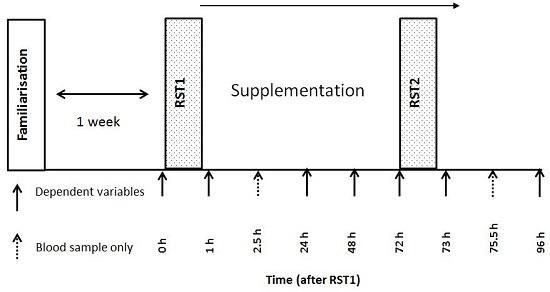
2.2. Experimental Design
2.3. Repeated Sprint Test
2.4. Maximal Isometric Voluntary Contractions
2.5. Counter Movement Jump
2.6. Reactive Strength Index
2.7. Treatments and Dietary Control
2.8. Muscle Soreness
2.9. Blood Sampling
2.10. Biochemical Analysis
2.11. Data Analysis
3. Results
3.1. Repeated Sprints
3.2. Functional Measures
3.3. Biochemical Indices
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Girard, O.; Mendez-Villanueva, A.; Bishop, D. Repeated-sprint ability—Part I: Factors contributing to fatigue. Sports Med. 2011, 41, 673–694. [Google Scholar] [CrossRef] [PubMed]
- Bogdanis, G.C.; Stavrinou, P.; Fatouros, I.G.; Philippou, A.; Chatzinikolaou, A.; Draganidis, D.; Ermidis, G.; Maridaki, M. Short-term high-intensity interval exercise training attenuates oxidative stress responses and improves antioxidant status in healthy humans. Food Chem. Toxicol. 2013, 61, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; Milak, A. Exercise-induced muscle damage following a bout of sport specific repeated sprints. J. Strength Cond. Res. 2009, 23, 2419–2424. [Google Scholar] [CrossRef] [PubMed]
- Duffield, R.; Cannon, J.; King, M. The effects of compression garments on recovery of muscle performance following high-intensity sprint and plyometric exercise. J. Sci. Med. Sport 2010, 13, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; van Someren, K.A. The prevention and treatment of exercise-induced muscle damage. Sports Med. 2008, 38, 483–503. [Google Scholar] [CrossRef] [PubMed]
- Mohr, M.; Draganidis, D.; Chatzinikolaou, A.; Barbero-Alvarez, J.C.; Castagna, C.; Douroudos, I.; Avloniti, A.; Margeli, A.; Papassotiriou, I.; Flouris, A.D.; et al. Muscle damage, inflammatory, immune and performance responses to three football games in 1 week in competitive male players. Eur. J. Appl. Physiol. 2016, 116, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Barnett, A. Using recovery modalities between training sessions in elite athletes—Does it help? Sports Med. 2006, 36, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Nedelec, M.; McCall, A.; Carling, C.; Legall, F.; Berthoin, S.; Dupont, G. Recovery in soccer: Part II—Ecovery strategies. Sports Med. 2013, 43, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81, S52–S69. [Google Scholar] [CrossRef] [PubMed]
- Hyldahl, R.D.; Hubal, M.J. Lengthening our perspective: Morphological, cellular, and molecular responses to eccentric exercise. Muscle Nerve 2014, 49, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Brickson, S.; Ji, L.L.; Schell, K.; Olabisi, R.; St Pierre Schneider, B.; Best, T.M. M1/70 attenuates blood-borne neutrophil oxidants, activation, and myofiber damage following stretch injury. J. Appl. Physiol. 2003, 95, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Pizza, F.X.; Peterson, J.M.; Baas, J.H.; Koh, T.J. Neutrophils contribute to muscle injury and impair its resolution after lengthening contractions in mice. J. Physiol. 2005, 562, 899–913. [Google Scholar] [CrossRef] [PubMed]
- Toumi, H.; Best, T.M. The inflammatory response: Friend or enemy for muscle injury? Br. J. Sports Med. 2003, 37, 284–286. [Google Scholar] [CrossRef] [PubMed]
- Jowko, E.; Dlugolecka, B.; Makaruk, B.; Cieslinski, I. The effect of green tea extract supplementation on exercise-induced oxidative stress parameters in male sprinters. Eur. J. Nutr. 2015, 54, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.M.; Williams, C.; Betts, J.A.; Thompson, D.; Hurst, T.L. Oxidative stress, inflammation and recovery of muscle function after damaging exercise: Effect of 6-week mixed antioxidant supplementation. Eur. J. Appl. Physiol. 2011, 111, 925–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolaidis, M.G.; Kerksick, C.M.; Lamprecht, M.; McAnulty, S.R. Does vitamin C and E supplementation impair the favorable adaptations of regular exercise? Oxid. Med. Cell. Longev. 2012, 2012, 707941. [Google Scholar] [CrossRef] [PubMed]
- Close, G.L.; Ashton, T.; Cable, T.; Doran, D.; Holloway, C.; McArdle, F.; MacLaren, D.P. Ascorbic acid supplementation does not attenuate post-exercise muscle soreness following muscle-damaging exercise but may delay the recovery process. Br. J. Nutr. 2006, 95, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.; Teixeira, V.H.; Soares, J. Dietary strategies to recover from exercise-induced muscle damage. Int. J. Food Sci. Nutr. 2014, 65, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Bell, O.; West, D.J.; Howatson, G.; Stevenson, E.J. The effects of beetroot juice supplementation on indices of muscle damage following eccentric exercise. Eur. J. Appl. Physiol. 2016, 116, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Clifford, T.; Constantinou, C.M.; Keane, K.M.; West, D.J.; Howatson, G.; Stevenson, E.J. The plasma bioavailability of nitrate and betanin from beta vulgaris rubra in humans. Eur. J. Nutr. 2016. [Google Scholar] [CrossRef] [PubMed]
- Wootton-Beard, P.C.; Moran, A.; Ryan, L. Stability of the total antioxidant capacity and total polyphenol content of 23 commercially available vegetable juices before and after in vitro digestion measured by frap, dpph, abts and folin–ciocalteu methods. Food Res. Int. 2011, 44, 217–224. [Google Scholar] [CrossRef]
- Ryan, L.; Prescott, S.L. Stability of the antioxidant capacity of twenty-five commercially available fruit juices subjected to an in vitro digestion. Int. J. Food Sci. Technol. 2010, 45, 1191–1197. [Google Scholar] [CrossRef]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- El Gamal, A.A.; AlSaid, M.S.; Raish, M.; Al-Sohaibani, M.; Al-Massarani, S.M.; Ahmad, A.; Hefnawy, M.; Al-Yahya, M.; Basoudan, O.A.; Rafatullah, S. Beetroot (Beta vulgaris L.) extract ameliorates Gentamicin-induced nephrotoxicity associated oxidative stress, inflammation, and apoptosis in rodent model. Mediat. Inflamm. 2014, 2014, 983952. [Google Scholar] [CrossRef] [PubMed]
- Pietrzkowski, Z.; Nemzer, B.; Spórna, A.; Stalica, P.; Tresher, W.; Keller, R.; Jimenez, R.; Michałowski, T.; Wybraniec, S. Influence of betalain-rich extract on reduction of discomfort associated with osteoarthritis. New Med. 2010, 1, 12–17. [Google Scholar]
- Vulic, J.J.; Cebovic, T.N.; Canadanovic, V.M.; Cetkovic, G.S.; Djilas, S.M.; Canadanovic-Brunet, J.M.; Velicanski, A.S.; Cvetkovic, D.D.; Tumbas, V.T. Antiradical, antimicrobial and cytotoxic activities of commercial beetroot pomace. Food Funct. 2013, 4, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Wootton-Beard, P.C.; Ryan, L. A beetroot juice shot is a significant and convenient source of bioaccessible antioxidants. J. Funct. Foods 2011, 3, 329–334. [Google Scholar] [CrossRef]
- Esatbeyoglu, T.; Wagner, A.E.; Motafakkerazad, R.; Nakajima, Y.; Matsugo, S.; Rimbach, G. Free radical scavenging and antioxidant activity of betanin: Electron spin resonance spectroscopy studies and studies in cultured cells. Food Chem. Toxicol. 2014, 73, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Wagner, A.E.; Schini-Kerth, V.B.; Rimbach, G. Betanin-a food colorant with biological activity. Mol. Nutr. Food Res. 2015, 59, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Krajka-Kuzniak, V.; Paluszczak, J.; Szaefer, H.; Baer-Dubowska, W. Betanin, a beetroot component, induces nuclear factor erythroid-2-related factor 2-mediated expression of detoxifying/antioxidant enzymes in human liver cell lines. Br. J. Nutr. 2013, 110, 2138–2149. [Google Scholar] [CrossRef] [PubMed]
- Szaefer, H.; Krajka-Kuzniak, V.; Ignatowicz, E.; Adamska, T.; Baer-Dubowska, W. Evaluation of the effect of beetroot juice on DMBA-induced damage in liver and mammary gland of female sprague-dawley rats. Phytother. Res. 2014, 28, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Jadert, C.; Petersson, J.; Massena, S.; Ahl, D.; Grapensparr, L.; Holm, L.; Lundberg, J.O.; Phillipson, M. Decreased leukocyte recruitment by inorganic nitrate and nitrite in microvascular inflammation and nsaid-induced intestinal injury. Free Radic. Biol. Med. 2012, 52, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.G.; Jamurtas, A.Z.; Paschalis, V.; Fatouros, I.G.; Koutedakis, Y.; Kouretas, D. The effect of muscle-damaging exercise on blood and skeletal muscle oxidative stress: Magnitude and time-course considerations. Sports Med. 2008, 38, 579–606. [Google Scholar] [CrossRef] [PubMed]
- Gathercole, R.J.; Sporer, B.C.; Stellingwerff, T.; Sleivert, G.G. Comparison of the capacity of different jump and sprint field tests to detect neuromuscular fatigue. J. Strength Cond. Res. 2015, 29, 2522–2531. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.M.; Salicki, R.; Goodall, S.; Thomas, K.; Howatson, G. Muscle damage response in female collegiate athletes after repeated sprint activity. J. Strength Cond. Res. 2015, 29, 2802–2807. [Google Scholar] [CrossRef] [PubMed]
- Lakomy, J.; Haydon, D.T. The effects of enforced, rapid deceleration on performance in a multiple sprint test. J. Strength Cond. Res. 2004, 18, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Nicholas, C.W.; Williams, C. Muscular soreness following prolonged intermittent high-intensity shuttle running. J. Sports Sci. 1999, 17, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Andersson, H.; Raastad, T.; Nilsson, J.; Paulsen, G.; Garthe, I.; Kadi, F. Neuromuscular fatigue and recovery in elite female soccer: Effects of active recovery. Med. Sci. Sports Exerc. 2008, 40, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Cockburn, E.; Robson-Ansley, P.; Hayes, P.R.; Stevenson, E. Effect of volume of milk consumed on the attenuation of exercise-induced muscle damage. Eur. J. Appl. Physiol. 2012, 112, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Howatson, G.; Keane, K.; Stevenson, E.J. Exercise-induced muscle damage following dance and sprint specific exercise in females. J. Sports Med. Phys. Fit. 2015, in press. [Google Scholar]
- Woolley, B.P.; Jakeman, J.R.; Faulkner, J.A. Multiple sprint exercise with a short deceleration induces muscle damage and performance impairment in young, physically active males. J. Athl. Enhanc. 2014, 3. [Google Scholar] [CrossRef]
- Semark, A.; Noakes, T.D.; St Clair Gibson, A.; Lambert, M.I. The effect of a prophylactic dose of flurbiprofen on muscle soreness and sprinting performance in trained subjects. J. Sports Sci. 1999, 17, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Close, G.L.; Ashton, T.; Cable, T.; Doran, D.; MacLaren, D.P. Eccentric exercise, isokinetic muscle torque and delayed onset muscle soreness: The role of reactive oxygen species. Eur. J. Appl. Physiol. 2004, 91, 615–621. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.; Howatson, G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients 2014, 6, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb, A.H.; Garten, R.S.; Cho, C.; Chee, P.D.; Chambers, L.A. Effects of a fruit/berry/vegetable supplement on muscle function and oxidative stress. Med. Sci. Sports Exerc. 2011, 43, 501–508. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R.; Jurkiewicz, B.A. Ascorbate free-radical as a marker of oxidative stress—An EPR study. Free Radic. Biol. Med. 1993, 14, 49–55. [Google Scholar] [CrossRef]
- Pietri, S.; Seguin, J.R.; Darbigny, P.; Culcasi, M. Ascorbyl free-radical—A noninvasive marker of oxidative stress in human open-heart-surgery. Free Radic. Biol. Med. 1994, 16, 523–528. [Google Scholar] [CrossRef]
- Jackson, M.J.; Vasilaki, A.; McArdle, A. Cellular mechanisms underlying oxidative stress in human exercise. Free Radic. Biol. Med. 2016, 98, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Belcastro, A.N.; Shewchuk, L.D.; Raj, D.A. Exercise-induced muscle injury: A calpain hypothesis. Mol. Cell. Biochem. 1998, 179, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.R.; Benestad, H.B.; Strom-Gundersen, I.; Morkrid, L.; Lappegard, K.T.; Raastad, T. Delayed leukocytosis and cytokine response to high-force eccentric exercise. Med. Sci. Sports Exerc. 2005, 37, 1877–1883. [Google Scholar] [CrossRef] [PubMed]
- Warren, G.L.; Ingalls, C.P.; Lowe, D.A.; Armstrong, R.B. What mechanisms contribute to the strength loss that occurs during and in the recovery from skeletal muscle injury? J. Orthop. Sports Phys. Ther. 2002, 32, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Rigamonti, E.; Touvier, T.; Clementi, E.; Manfredi, A.A.; Brunelli, S.; Rovere-Querini, P. Requirement of inducible nitric oxide synthase for skeletal muscle regeneration after acute damage. J. Immunol. 2013, 190, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Kashimura, O.; Kano, Y.; Ohno, H.; Ji, L.L.; Izawa, T.; Best, T.M. Role of nitric oxide in muscle regeneration following eccentric muscle contractions in rat skeletal muscle. J. Physiol. Sci. 2013, 63, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Kruger, M.J.; Smith, C. Postcontusion polyphenol treatment alters inflammation and muscle regeneration. Med. Sci. Sports Exerc. 2012, 44, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Lomonosova, Y.N.; Shenkman, B.S.; Kalamkarov, G.R.; Kostrominova, T.Y.; Nemirovskaya, T.L. l-arginine supplementation protects exercise performance and structural integrity of muscle fibers after a single bout of eccentric exercise in rats. PLoS ONE 2014, 9, e94448. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.K.; Holdsworth, C.T.; Wright, J.L.; Fees, A.J.; Allen, J.D.; Jones, A.M.; Musch, T.I.; Poole, D.C. Microvascular oxygen pressures in muscles comprised of different fiber types: Impact of dietary nitrate supplementation. Nitric Oxide 2015, 48, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.K.; Hirai, D.M.; Copp, S.W.; Holdsworth, C.T.; Allen, J.D.; Jones, A.M.; Musch, T.I.; Poole, D.C. Effects of nitrate supplementation via beetroot juice on contracting rat skeletal muscle microvascular oxygen pressure dynamics. Respir. Physiol. Neurobiol. 2013, 187, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.; Schiffer, T.A.; Ivarsson, N.; Cheng, A.J.; Bruton, J.D.; Lundberg, J.O.; Weitzberg, E.; Westerblad, H. Dietary nitrate increases tetanic [ca2+]i and contractile force in mouse fast-twitch muscle. J. Physiol. 2012, 590, 3575–3583. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Leibowitz, J.L.; Kadkhodayan, A.; Thomas, D.P.; Ramamurthy, S.; Spearie, C.A.; Waller, S.; Farmer, M.; Peterson, L.R. Effect of acute dietary nitrate intake on maximal knee extensor speed and power in healthy men and women. Nitric Oxide 2015, 48, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.A.; McHugh, M.P.; Padilla-Zakour, O.I.; Carlson, L.; Sayers, S.P. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br. J. Sports Med. 2006, 40, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Izquierdo, T.; Pietrzkowski, Z.; Argumedo, R.; Shu, C.; Nemzer, B.; Wybraniec, S. Betalain-rich red beet concentrate improves reduced knee discomfort and joint function: A double blind, placebo-controlled pilot clinical study. Nutr. Diet. Suppl. 2014, 2014, 9–10. [Google Scholar] [CrossRef]
- Crameri, R.M.; Aagaard, P.; Qvortrup, K.; Langberg, H.; Olesen, J.; Kjaer, M. Myofibre damage in human skeletal muscle: Effects of electrical stimulation versus voluntary contraction. J. Physiol. 2007, 583, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Murase, S.; Terazawa, E.; Queme, F.; Ota, H.; Matsuda, T.; Hirate, K.; Kozaki, Y.; Katanosaka, K.; Taguchi, T.; Urai, H.; et al. Bradykinin and nerve growth factor play pivotal roles in muscular mechanical hyperalgesia after exercise (delayed-onset muscle soreness). J. Neurosci. 2010, 30, 3752–3761. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.B. Ginger (zingiber officinale) as an analgesic and ergogenic aid in sport: A systemic review. J. Strength Cond. Res. 2015, 29, 2980–2995. [Google Scholar] [CrossRef] [PubMed]
- Drobnic, F.; Riera, J.; Appendino, G.; Togni, S.; Franceschi, F.; Valle, X.; Pons, A.; Tur, J. Reduction of delayed onset muscle soreness by a novel curcumin delivery system (meriva®): A randomised, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2014, 11, 31. [Google Scholar] [CrossRef] [PubMed]




| Group | Age (Years) | Height (m) | Mass (kg) |
|---|---|---|---|
| BTJ | 23 ± 3 | 1.83 ± 0.90 | 76.8 ± 9.5 |
| PLA | 21 ± 2 | 1.77 ± 0.51 | 73.4 ± 12.4 |
| Treatment | BTJ | PLA |
|---|---|---|
| Energy (Kcals) | 81 | 77 |
| Volume (mL) | 250 | 250 |
| Carbohydrate (g) | 16.4 | 16.4 |
| Protein (g) | 2.8 | 2.8 |
| Fat (g) | 0.4 | Trace |
| Nitrate (mg) | ≥143 | Trace |
| TEAC * (mmol∙L−1) | 11.4 ± 0.2 | 0.25 ± 0.02 |
| Mean Dietary Intake (5 Days) | ||
|---|---|---|
| BTJ | PLA | |
| Energy (Kcal) | 2554 ± 682 | 2448 ± 390 |
| Carbohydrates (%) | 41 ± 5 | 43 ± 6 |
| Protein (%) | 21 ± 3 | 24 ± 7 |
| Fat (%) | 38 ± 7 | 33 ± 9 |
| Group | Average Sprint Time (s) | Fastest Sprint Time (s) | Fatigue Index (%) | RPE |
|---|---|---|---|---|
| BTJ | ||||
| RST1 | 4.65 ± 0.25 | 4.41 ± 0.23 | 5.60 ± 2.13 | 15 ± 1 |
| RST2 | 4.66 ± 0.24 | 4.38 ± 0.17 | 6.48 ± 2.66 | 15 ± 1 |
| PLA | ||||
| RST1 | 4.70 ± 0.15 | 4.48 ± 0.14 | 4.91 ± 1.51 | 14 ± 2 |
| RST2 | 4.77 ± 0.20 | 4.53 ± 0.15 | 5.19 ± 3.21 | 14 ± 2 |
| 0 h | 1 h | 2.5 h | 24 h | 48 h | 72 h | 73 h | 75.5 h | 96 h | |
|---|---|---|---|---|---|---|---|---|---|
| MIVC (N) * | |||||||||
| BTJ | 601 ± 89 | 554 ± 75 | 545 ± 92 | 558 ± 94 | 579 ± 121 | 516 ± 101 | 558 ± 75 | ||
| PLA | 590 ± 123 | 541 ± 128 | 535 ± 120 | 544 ± 130 | 540 ± 126 | 509 ± 124 | 537 ± 122 | ||
| hsCRP (mg∙L−1) | |||||||||
| BTJ | 0.6 ± 0.72 | 0.61 ± 0.75 | 0.57 ± 0.69 | 0.64 ± 0.56 | 0.64 ± 0.58 | 0.52 ± 0.52 | 0.52 ± 0.49 | 0.46 ± 0.50 | 0.48 ± 0.50 |
| PLA | 0.44 ± 0.39 | 0.46 ± 0.45 | 0.44 ± 0.42 | 0.52 ± 0.52 | 0.4 ± 0.25 | 0.34 ± 0.21 | 0.35 ± 0.21 | 0.38 ± 0.22 | 0.38 ± 0.24 |
| CK (IU∙L−1) * | |||||||||
| BTJ | 188 ± 62 | 219 ± 68 | 383 ± 197 | 542 ± 461 | 406 ± 252 | 310 ± 145 | 349 ± 163 | 474 ± 246 | 516 ± 210 |
| PLA | 318 ± 145 | 362 ± 154 | 518 ± 274 | 592 ± 321 | 435 ± 255 | 387 ± 273 | 433 ± 290 | 623 ± 423 | 749 ± 423 |
| LOOH (mmol∙mL−1) * | |||||||||
| BTJ | 1.49 ± 0.25 | 1.68 ± 0.23 | 1.69 ± 0.37 | 1.33 ± 0.36 | 1.46 ± 0.19 | 1.47 ± 0.17 | 1.53 ± 0.40 | 1.74 ± 0.31 | 1.44 ± 0.21 |
| PLA | 1.53 ± 0.14 | 1.79 ± 0.23 | 1.77 ± 0.40 | 1.54 ± 0.16 | 1.57 ± 0.14 | 1.53 ± 0.12 | 1.69 ± 0.23 | 1.94 ± 0.75 | 1.44 ± 0.14 |
| PC (μmol∙L−1) | |||||||||
| BTJ | 14 ± 6 | 16 ± 0 | 15 ± 6 | 17 ± 5 | 18 ± 7 | 14 ± 5 | 16 ± 5 | 17 ± 10 | 15 ± 6 |
| PLA | 16 ± 8 | 19 ± 6 | 14 ± 6 | 13 ± 5 | 14 ± 5 | 18 ± 9 | 16 ± 7 | 15 ± 5 | 15 ± 4 |
| A•− (AU) | |||||||||
| BTJ | 5567 ± 1898 | 6111 ± 2145 | 5883 ± 2044 | 6247 ± 1846 | 6202 ± 1911 | 5809 ± 2238 | 5752 ± 1854 | 5422 ± 2002 | 5862 ± 1802 |
| PLA | 6372 ± 1454 | 6730 ± 1337 | 6559 ± 2027 | 5674 ± 1716 | 5225 ± 1088 | 6013 ± 671 | 5433 ± 1521 | 6354 ± 1315 | 5819 ± 975 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clifford, T.; Berntzen, B.; Davison, G.W.; West, D.J.; Howatson, G.; Stevenson, E.J. Effects of Beetroot Juice on Recovery of Muscle Function and Performance between Bouts of Repeated Sprint Exercise. Nutrients 2016, 8, 506. https://doi.org/10.3390/nu8080506
Clifford T, Berntzen B, Davison GW, West DJ, Howatson G, Stevenson EJ. Effects of Beetroot Juice on Recovery of Muscle Function and Performance between Bouts of Repeated Sprint Exercise. Nutrients. 2016; 8(8):506. https://doi.org/10.3390/nu8080506
Chicago/Turabian StyleClifford, Tom, Bram Berntzen, Gareth W. Davison, Daniel J. West, Glyn Howatson, and Emma J. Stevenson. 2016. "Effects of Beetroot Juice on Recovery of Muscle Function and Performance between Bouts of Repeated Sprint Exercise" Nutrients 8, no. 8: 506. https://doi.org/10.3390/nu8080506