Corynebacterium accolens Has Antimicrobial Activity against Staphylococcus aureus and Methicillin-Resistant S. aureus Pathogens Isolated from the Sinonasal Niche of Chronic Rhinosinusitis Patients
Abstract
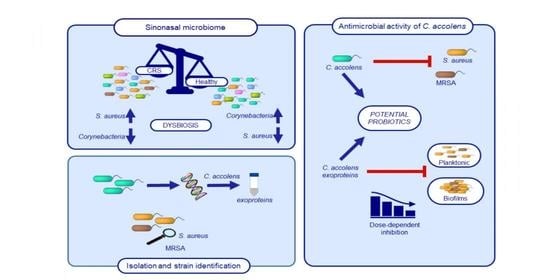
:1. Introduction
2. Results
2.1. Identification of C. accolens Isolates
2.2. Phylogenetic Relationship of the Strains
2.3. Spectrum of Antimicrobial Activity
2.4. Inhibitory Activity of C. accolens Concentrated Cell-Free Culture Supernatants (CFCSs)
2.5. Characterization of the Inhibitory Effect of CFCSs Produced by C. accolens Strains
2.5.1. Effect of Proteinase K and Heat Inactivation
2.5.2. Effect of Purified Protein Treatment
2.6. C. accolens CFCS Inhibits S. aureus and MRSA Biofilm Metabolic Activity
2.7. C. accolens CFCS Reduces S. aureus and MRSA Biofilm Biomass
3. Discussion
4. Materials and Methods
4.1. Collection of Clinical Isolates
4.2. C. accolens Genomic DNA (gDNA) Extraction and DNA Quality Control
4.3. Polymerase Chain Reaction (PCR) Amplification of Partial rpoB Gene
4.4. rpoB Gene Sequencing and Strain Identification of C. accolens
4.5. Phylogenetic Analysis
4.6. Nucleotide Sequence Accession Numbers
4.7. Deferred Growth Inhibition Assay
4.8. Preparation of Concentrated Cell-Free Culture Supernatants (CFCSs) from C. accolens Strains
4.9. Assessment of Anti-Bacterial Activity Using Concentrated CFCS and Minimum Inhibitory Treatment
4.10. Proteinase K and Heat Inactivation of CFCSs
4.11. Protein Clean-Up from CFCS and Detection of Anti-Bacterial Activity
4.12. Assessment of Anti-Biofilm Activity Using C. accolens CFCSs
4.12.1. Determination of Biofilm Metabolic Activity
4.12.2. Determination of Biofilm Biomass
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Riechelmann, H. Europäischen Akademie für Allergie und Klinische Immunologie (EAACI) un der European Rhinologic Society (ERS)—Chronic Rhinosinusitis. EPOS 2012 Part I]. Laryngorhinootologie 2013, 92, 193–201. [Google Scholar] [PubMed]
- Hoggard, M.; Biswas, K.; Zoing, M.; Mackenzie, B.W.; Taylor, M.W.; Douglas, R.G. Evidence of microbiota dysbiosis in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2017, 7, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Copeland, E.; Leonard, K.; Carney, R.; Kong, J.; Forer, M.; Naidoo, Y.; Oliver, B.G.G.; Seymour, J.R.; Woodcock, S.; Burke, C.M.; et al. Chronic rhinosinusitis: Potential role of microbial dysbiosis and recommendations for sampling sites. Front. Cell. Infect. Microbiol. 2018, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Paramasivan, S.; Bassiouni, A.; Shiffer, A.; Dillon, M.R.; Cope, E.K.; Cooksley, C.; Ramezanpour, M.; Moraitis, S.; Ali, M.J.; Bleier, B.; et al. The international sinonasal microbiome study: A multicentre, multinational characterization of sinonasal bacterial ecology. Allergy 2020, 75, 2037–2049. [Google Scholar] [CrossRef]
- Cleland, E.J.; Bassiouni, A.; Wormald, P.J. The bacteriology of chronic rhinosinusitis and the pre-eminence of Staphylococcus aureus in revision patients. Int. Forum Allergy Rhinol. 2013, 3, 862–866. [Google Scholar] [CrossRef]
- Vickery, T.W.; Ramakrishnan, V.R.; Suh, J.D. The role of Staphylococcus aureus in patients with chronic sinusitis and nasal polyposis. Curr. Allergy Asthma Rep. 2019, 19, 21. [Google Scholar] [CrossRef]
- Fokkens, W.J.; Lund, V.J.; Mullol, J.; Bachert, C.; Alobid, I.; Baroody, F.; Cohen, N.; Cervin, A.; Douglas, R.; Gevaert, P.; et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinology 2012, 23, 1–12. [Google Scholar]
- Foreman, A.; Wormald, P.J. Different biofilms, different disease? A clinical outcomes study. Laryngoscope 2010, 120, 1701–1706. [Google Scholar] [CrossRef]
- Stubbendieck, R.M.; Straight, P.D. Multifaceted interfaces of bacterial competition. J. Bacteriol. 2016, 198, 2145–2155. [Google Scholar] [CrossRef] [Green Version]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Microbiol. 2010, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Speert, D.P.; Wannamaker, L.W.; Gray, E.D.; Clawson, C.C. Bactericidal effect of oleic acid on group A streptococci: Mechanism of action. Infect. Immun. 1979, 26, 1202–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bomar, L.; Brugger, S.D.; Yost, B.H.; Davies, S.S.; Lemon, K.P. Corynebacterium accolens releases antipneumococcal free fatty acids from human nostril and skin surface triacylglycerols. MBio 2016, 7, e01725-15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: A review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef] [Green Version]
- Cleland, E.J.; Drilling, A.; Bassiouni, A.; James, C.; Vreugde, S.; Wormald, P.-J. Probiotic manipulation of the chronic rhinosinusitis microbiome. Int. Forum Allergy Rhinol. 2014, 309–314. [Google Scholar] [CrossRef]
- Abreu, N.A.; Nagalingam, N.A.; Song, Y.; Roediger, F.C.; Pletcher, S.D.; Goldberg, A.N.; Lynch, S.V. Sinus microbiome diversity depletion and Corynebacterium tuberculostearicum enrichment mediates rhinosinusitis. Sci. Transl. Med. 2012, 4, 151ra124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uehara, Y.; Nakama, H.; Agematsu, K.; Uchida, M.; Kawakami, Y.; Fattah, A.A.; Maruchi, N. Bacterial interference among nasal inhabitants: Eradication of Staphylococcus aureus from nasal cavities by artificial implantation of Corynebacterium sp. J. Hosp. Infect. 2000, 44, 127–133. [Google Scholar] [CrossRef]
- Khamis, A.D.; Raoult, D.; La Scola, B. rpoB gene sequencing for identification of Corynebacterium species. J. Clin. Microbiol. 2004, 42, 3925–3931. [Google Scholar] [CrossRef] [Green Version]
- Desrosiers, M.; Valera, F.C.P. Brave New (Microbial) World: Implications for nasal and sinus disorders. Braz. J. Otorhinolaryngol. 2019, 85, 675–677. [Google Scholar] [CrossRef]
- De Boeck, I.; Wittouck, S.; Martens, K.; Claes, J.; Jorissen, M.; Steelant, B.; van den Broek, M.F.L.; Seys, S.F.; Hellings, P.W.; Vanderveken, O.M.; et al. Anterior nares diversity and pathobionts represent sinus microbiome in chronic rhinosinusitis. MSphere 2019, 4. [Google Scholar] [CrossRef] [Green Version]
- Psaltis, A.J.; Wormald, P.-J. Therapy of sinonasal microbiome in CRS: A critical approach. Curr. Allergy Asthma Rep. 2017, 17, 59. [Google Scholar] [CrossRef]
- Khamis, A.D.; Raoult, D.; La Scola, B. Comparison between rpoB and 16S rRNA gene sequencing for molecular identification of 168 clinical isolates of Corynebacterium. J. Clin. Microbiol. 2005, 43, 1934–1936. [Google Scholar] [CrossRef] [Green Version]
- Wos-Oxley, M.L.; Plumeier, I.; Von Eiff, C.; Taudien, S.; Platzer, M.; Vilchez-Vargas, R.; Becker, K.; Pieper, D.H. A poke into the diversity and associations within human anterior nare microbial communities. ISME J. 2010, 4, 839–851. [Google Scholar] [CrossRef]
- Hardy, B.L.; Dickey, S.W.; Plaut, R.D.; Riggins, D.P.; Stibitz, S.; Otto, M.; Merrell, D.S. Corynebacterium pseudodiphtheriticum exploits Staphylococcus aureus virulence components in a novel polymicrobial defense strategy. MBio 2019, 10, e02491-18. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, M.M.; Freire, M.O.; Gabrilska, R.A.; Rumbaugh, K.P.; Lemon, K.P. Staphylococcus aureus shifts toward commensalism in response to Corynebacterium species. Front. Microbiol. 2016, 7, 1230. [Google Scholar] [CrossRef] [Green Version]
- Bitschar, K.; Sauer, B.; Focken, J.; Dehmer, H.; Moos, S.; Konnerth, M.; Schilling, N.A.; Grond, S.; Kalbacher, H.; Kurschus, F.C.; et al. Lugdunin amplifies innate immune responses in the skin in synergy with host-and microbiota-derived factors. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Thirumazhisi Sachithanandam, S. Rising methicillin-resistant Staphylococcus aureus infections in ear, nose, and throat diseases. Case Rep. Otolaryngol. 2014, 2014. [Google Scholar] [CrossRef] [Green Version]
- Psaltis, A.J.; Weitzel, E.K.; Ha, K.R.; Wormald, P.-J. The effect of bacterial biofilms on post-sinus surgical outcomes. Am. J. Rhinol. 2008, 22, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Mehta, R.; Agarwal, S.; Mishra, P. Bacterial biofilm on the sinus mucosa of healthy subjects and patients with chronic rhinosinusitis (with or without nasal polyposis). J. Laryngol. Otol. 2015, 129, 46–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, K. Tackling superbugs in their slime castles: Innovative approaches against antimicrobial-resistant biofilm infections. Microbiol. Aust. 2019, 40, 165–168. [Google Scholar] [CrossRef]
- Iwase, T.; Uehara, Y.; Shinji, H.; Tajima, A.; Seo, H.; Takada, K.; Agata, T.; Mizunoe, Y. Staphylococcus epidermidis Esp inhibits Staphylococcus aureus biofilm formation and nasal colonization. Nature 2010, 465, 346–349. [Google Scholar] [CrossRef]
- Dodémont, M.; Verhulst, C.; Nonhoff, C.; Nagant, C.; Denis, O.; Kluytmans, J. Prospective two-center comparison of three chromogenic agars for methicillin-resistant Staphylococcus aureus screening in hospitalized patients. J. Clin. Microbiol. 2015, 53, 3014–3016. [Google Scholar] [CrossRef] [Green Version]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Moran, J.C.; Crank, E.L.; Ghabban, H.A.; Horsburgh, M.J. Deferred growth inhibition assay to quantify the effect of bacteria-derived antimicrobials on competition. J. Vis. Exp. 2016, e54437. [Google Scholar] [CrossRef] [Green Version]
- Panchatcharam, B.S.; Cooksley, C.M.; Ramezanpour, M.; Vediappan, R.S.; Bassiouni, A.; Wormald, P.J.; Psaltis, A.J.; Vreugde, S. Staphylococcus aureus biofilm exoproteins are cytotoxic to human nasal epithelial barrier in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Inturri, R.; Trovato, L.; Volti, G.L.; Oliveri, S.; Blandino, G.; Trovato, L. In vitro inhibitory activity of Bifidobacterium longum BB536 and Lactobacillus rhamnosus HN001 alone or in combination against bacterial and Candida reference strains and clinical isolates. Heliyon 2019, 5, e02891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Akeel, R.; Mateen, A.; Alharbi, K.K.; Alyousef, A.A.; Al-Mandeel, H.M.; Syed, R. Purification and MIC analysis of antimicrobial proteins from Cucumis sativus L. seeds. BMC Complement. Altern. Med. 2018, 18, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Richter, K.; Ramezanpour, M.; Thomas, N.; Prestidge, C.A.; Wprmald, P.-J.; Vreugde, S. Mind “De GaPP”: In vitro efficacy of deferiprone and gallium-protoporphyrin against Staphylococcus aureus biofilms. Int. Forum Allergy Rhinol. 2016, 6, 737–743. [Google Scholar] [CrossRef] [PubMed]





| Characteristic | Non-CRS Controls, No. (%) | Patients with CRS, No. (%) |
|---|---|---|
| Number of subjects | 20 | 16 |
| Mean age (years) | 45.7 | 64.0 |
| Gender (M/F) | 8/12 | 8/8 |
| Active smoker | 0 (0) | 1 (6.3) |
| Asthma | 6 (30) | 8 (50) |
| Diabetes mellitus | 1 (5) | 0 (0) |
| Cystic fibrosis | 0 (0) | 0 (0) |
| GERD | 6 (30) | 3 (18.8) |
| Aspirin sensitivity | 0 (0) | 3 (18.8) |
| Tonsillitis in the past 6 months | 0 (0) | 0 (0) |
| Ear infection in the past 6months | 0 (0) | 0 (0) |
| Nasal polyposis | 0 (0) | 7 (43.8) |
| Isolate Code | API Coryne 20 Identification † (% Similarity) | rpoB Gene Sequence Identification | |||
|---|---|---|---|---|---|
| Strains | % Similarity | % Query Coverage | Accession Number | ||
| C778 | C. accolens (90.0) | C. accolens | 98.3 | 100 | MT856944 |
| C779 | C. accolens (95.6) | C. accolens | 96.0 | 100 | MT856945 |
| C780 | C. accolens (90.0) | C. accolens | 97.6 | 100 | MT856946 |
| C781 | C. accolens (99.4) | C. accolens | 98.7 | 100 | MT856947 |
| C782 | C. accolens (95.6) | C. accolens | 99.5 | 100 | MT856948 |
| C783 | C. accolens (90.0) | C. accolens | 98.2 | 99 | MT856949 |
| C784 | C. accolens (91.4) | C. accolens | 98.3 | 100 | MT856950 |
| C785 | C. accolens (90.0) | C. accolens | 96.6 | 100 | MT856951 |
| C786 | C. accolens (90.0) | C. accolens | 97.3 | 100 | MT856952 |
| C787 | C. accolens (90.0) | C. accolens | 96.4 | 100 | MT856953 |
| Tested Pathogens | Diameter of Growth Inhibition Zone (mm) † | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibitory Strains | |||||||||||
| C. accolens C778 | C. accolens C779 | C. accolens C780 | C. accolens C781 | C. accolens C782 | C. accolens C783 | C. accolens C784 | C. accolens C785 | C. accolens C786 | C. accolens C787 | C. accolens ATCC49726 | |
| MSSA | |||||||||||
| S. aureus C329 | − | + | ++ | + | − | ++ | ++ | − | + | +++ | + |
| S. aureus C262 | − | ++ | − | + | + | − | − | − | − | − | − |
| S. aureus C314 | − | − | ++ | ++ | − | + | − | − | ++ | − | ++ |
| S. aureus C124 | + | +++ | + | + | + | − | ++ | + | − | ++ | + |
| S. aureus C5 | + | +++ | + | + | − | − | + | ++ | ++ | ++ | + |
| S. aureus C26 | ++ | +++ | ++ | ++++ | + | +++ | ++ | + | + | ++++ | + |
| S. aureus C319 | − | + | − | ++ | + | − | + | ++ | − | + | − |
| S. aureus C71 | − | − | ++ | + | − | + | ++ | − | + | + | + |
| MSSA (% inhibition) | 3/8 (37.5%) | 6/8 (75.0%) | 6/8 (75.0%) | 8/8 (100%) | 4/8 (50.0%) | 4/8 (50.0%) | 6/8 (75.0%) | 4/8 (50.0%) | 5/8 (62.5%) | 6/8 (75.0%) | 6/8 (75.0%) |
| MRSA | |||||||||||
| S. aureus C300 | ++ | +++ | ++ | +++ | ++ | ++ | − | + | + | +++ | ++ |
| S. aureus C310 | + | − | + | + | − | ++ | ++ | + | + | ++ | + |
| S. aureus C292 | ++ | + | + | ++ | − | − | + | − | + | + | + |
| S. aureus C295 | − | − | − | − | − | − | − | − | − | − | − |
| S. aureus C261 | + | +++ | ++ | +++ | + | ++ | + | ++ | ++ | ++++ | ++ |
| S. aureus C24 | − | + | − | + | − | + | − | − | − | + | − |
| S. aureus C54 | + | − | − | + | − | − | − | − | − | − | − |
| S. aureus C38 | − | + | + | − | − | ++ | − | − | − | + | + |
| MRSA (% inhibition) | 5/8 (62.5%) | 5/8 (62.5%) | 5/8 (62.5%) | 6/8 (75.0%) | 2/8 (25.0%) | 5/8 (62.5%) | 3/8 (37.5%) | 3/8 (37.5%) | 4/8 (50.0%) | 6/8 (75.0%) | 5/8 (75.0%) |
| S. aureus ATCC25923 | + | + | − | + | + | − | + | + | + | + | + |
| Total (% inhibition) | 9/17 (52.9%) | 12/17 (70.6%) | 11/17 (64.7%) | 15/17 (88.2%) | 7/17 (41.2%) | 9/17 (52.9%) | 10/17 (58.8%) | 8/17 (47.1%) | 10/17 (58.8%) | 13/17 (76.5%) | 12/17 (70.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menberu, M.A.; Liu, S.; Cooksley, C.; Hayes, A.J.; Psaltis, A.J.; Wormald, P.-J.; Vreugde, S. Corynebacterium accolens Has Antimicrobial Activity against Staphylococcus aureus and Methicillin-Resistant S. aureus Pathogens Isolated from the Sinonasal Niche of Chronic Rhinosinusitis Patients. Pathogens 2021, 10, 207. https://doi.org/10.3390/pathogens10020207
Menberu MA, Liu S, Cooksley C, Hayes AJ, Psaltis AJ, Wormald P-J, Vreugde S. Corynebacterium accolens Has Antimicrobial Activity against Staphylococcus aureus and Methicillin-Resistant S. aureus Pathogens Isolated from the Sinonasal Niche of Chronic Rhinosinusitis Patients. Pathogens. 2021; 10(2):207. https://doi.org/10.3390/pathogens10020207
Chicago/Turabian StyleMenberu, Martha Alemayehu, Sha Liu, Clare Cooksley, Andrew James Hayes, Alkis James Psaltis, Peter-John Wormald, and Sarah Vreugde. 2021. "Corynebacterium accolens Has Antimicrobial Activity against Staphylococcus aureus and Methicillin-Resistant S. aureus Pathogens Isolated from the Sinonasal Niche of Chronic Rhinosinusitis Patients" Pathogens 10, no. 2: 207. https://doi.org/10.3390/pathogens10020207