Impact of Dietary Patterns on H. pylori Infection and the Modulation of Microbiota to Counteract Its Effect. A Narrative Review
Abstract
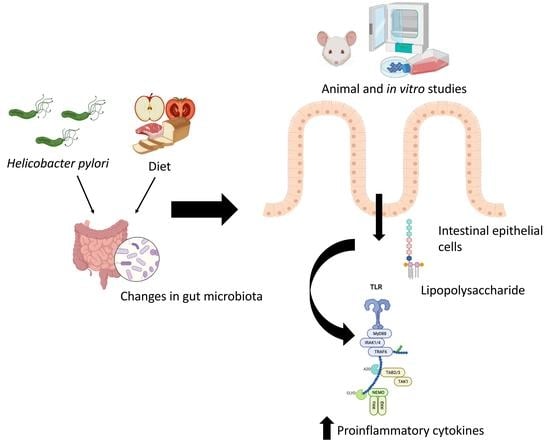
:1. Introduction
2. The Impact of Diet Patterns in Helicobacter pylori Infection
Diet, Helicobacter pylori, and the Gut Microbiota
3. Management of Helicobacter pylori Infection
3.1. Current Standard Treatments
3.2. New Antibiotics
3.3. Nutraceutical Approaches
3.3.1. Extracts and Compounds Isolated from Food
3.3.2. Traditional Plants or Herbs
3.3.3. Vitamin D3
3.3.4. Probiotics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Conti, L.; Annibale, B.; Lahner, E. Autoimmune Gastritis and Gastric Microbiota. Microorganisms 2020, 8, 1827. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chiang, T.H.; Chiu, H.M.; Wu, M.S.; Yeh, Y.P.; Chen, T.H.H. Community-based Gastric Cancer Screening Coupled with a National Colorectal Cancer Screening Program: Baseline Results. Gastroenterology 2021, 21, 67–76. [Google Scholar] [CrossRef]
- Haile, K.; Yemane, T.; Tesfaye, G.; Wolde, D.; Timerga, A.; Haile, A. Anemia and its association with Helicobacter pylori infection among adult dyspeptic patients attending Wachemo University Nigist Eleni Mohammad Memorial Referral Hospital, Southwest Ethiopia: A cross-sectional study. PLoS ONE 2021, 16, e0245168. [Google Scholar] [CrossRef]
- Zamani, M.; Ebrahimtabar, F.; Zamani, V.; Miller, W.H.; Alizadeh-Navaei, R.; Shokri-Shirvani, J.; Derakhshan, M.H. Systematic review with meta-analysis: The worldwide prevalence of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2018, 47, 868–876. [Google Scholar] [CrossRef] [Green Version]
- Feili, O.; Bakhti, S.Z.; Latifi-Navid, S.; Zahri, S.; Yazdanbod, A. Contrasting association of Helicobacter pylori oipA genotype with risk of peptic ulceration and gastric cancer. Infect. Genet. Evol. 2021, 89, 104720. [Google Scholar] [CrossRef] [PubMed]
- Nejati, S.; Karkhah, A.; Darvish, H.; Validi, M.; Ebrahimpour, S.; Nouri, H.R. Influence of Helicobacter pylori virulence factors CagA and VacA on pathogenesis of gastrointestinal disorders. Microb. Pathog. 2018, 117, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Yong, X.; Tang, B.; Li, B.S.; Xie, R.; Hu, C.J.; Luo, G.; Qin, Y.; Dong, H.; Yang, S.M. Helicobacter pylori virulence factor CagA promotes tumorigenesis of gastric cancer via multiple signaling pathways. Cell Commun. Signal. 2015, 13, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, P.; Luo, Z.Q.; Blanke, S.R. Helicobacter pylori vacuolating cytotoxin A (VacA) engages the mitochondrial fission machinery to induce host cell death. Proc. Natl. Acad. Sci. USA 2011, 108, 16032–16037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, P.; Xue, J.; Zhang, Z.J.; Jia, Y.P.; Tong, Y.N.; Han, D.; Li, Q.; Xiang, Y.; Mao, X.H.; Tang, B. Helicobacter pylori VacA induces autophagic cell death in gastric epithelial cells via the endoplasmic reticulum stress pathway. Cell Death Dis. 2017, 8, 3207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caron, T.J.; Scott, K.E.; Fox, J.G.; Hagen, S.J. Tight junction disruption: Helicobacter pylori and dysregulation of the gastric mucosal barrier. World J. Gastroenterol. 2015, 21, 11411–11427. [Google Scholar] [CrossRef]
- Djekic, A.; Muller, A. The Immunomodulator VacA Promotes Immune Tolerance and Persistent Helicobacter pylori Infection through Its Activities on T-Cells and Antigen-Presenting Cells. Toxins 2016, 8, 187. [Google Scholar] [CrossRef]
- Kim, I.J.; Lee, J.; Oh, S.J.; Yoon, M.S.; Jang, S.S.; Holland, R.L.; Reno, M.L.; Hamad, M.N.; Maeda, T.; Chung, H.J.; et al. Helicobacter pylori Infection Modulates Host Cell Metabolism through VacA-Dependent Inhibition of mTORC1. Cell Host Microbe 2018, 23, 583–593 e588. [Google Scholar] [CrossRef] [Green Version]
- Dzierzanowska-Fangrat, K.; Dzierzanowska, D. Helicobacter pylori: Microbiology and interactions with gastrointestinal microflora. J. Physiol. Pharmacol. 2006, 57 (Suppl. 3), 5–14. [Google Scholar]
- Stordal, K.; Kahrs, C.; Tapia, G.; Agardh, D.; Kurppa, K.; Stene, L.C. Review article: Exposure to microbes and risk of coeliac disease. Aliment. Pharmacol. Ther. 2021, 53, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Boltin, D.; Beniashvili, Z.; Lahat, A.; Hirsch, J.; Nyssen, O.P.; Megraud, F.; O′Morain, C.; Gisbert, J.P.; Niv, Y. European Registry on Helicobacter pylori management (Hp-EuReg): First-line Therapy in Israel. Isr. Med. Assoc. J. 2021, 23, 38–42. [Google Scholar] [PubMed]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Schistosomes, Liver Flukes and Helicobacter pylori; International Agency for Research on Cancer: Lyon, France, 1994; Volume 61, pp. 1–241. [Google Scholar]
- Machlowska, J.; Baj, J.; Sitarz, M.; Maciejewski, R.; Sitarz, R. Gastric Cancer: Epidemiology, Risk Factors, Classification, Genomic Characteristics and Treatment Strategies. Int. J. Mol. Sci. 2020, 21, 4012. [Google Scholar] [CrossRef] [PubMed]
- Haley, K.P.; Gaddy, J.A. Nutrition and Helicobacter pylori: Host Diet and Nutritional Immunity Influence Bacterial Virulence and Disease Outcome. Gastroenterol. Res. Pract. 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Annibale, B.; Capurso, G.; Delle Fave, G. Consequences of Helicobacter pylori infection on the absorption of micronutrients. Dig. Liver Dis. 2002, 34 (Suppl. 2), S72–S77. [Google Scholar] [CrossRef]
- Mohammadi, S.O.; Yadegar, A.; Kargar, M.; Mirjalali, H.; Kafilzadeh, F. The impact of Helicobacter pylori infection on gut microbiota-endocrine system axis; modulation of metabolic hormone levels and energy homeostasis. J. Diabetes Metab. Disord. 2020, 1855–1861. [Google Scholar] [CrossRef] [PubMed]
- Isomoto, H.; Nishi, Y.; Ohnita, K.; Mizuta, Y.; Kohno, S.; Ueno, H.; Nakazato, M. The Relationship between Plasma and Gastric Ghrelin Levels and Strain Diversity in Helicobacter pylori Virulence. Am. J. Gastroenterol. 2005, 100, 1425–1427. [Google Scholar] [CrossRef]
- Nweneka, C.V.; Prentice, A.M. Helicobacter pylori infection and circulating ghrelin levels—A systematic review. BMC Gastroenterol. 2011, 11, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capurso, G.; Ricci, R.; Panzuto, F.; Baccini, F.; Passi, S.; Di Giulio, E.; Delle Fave, G.; Annibale, B. Intragastric ascorbic but not uric acid is depleted in relation with the increased pH in patients with atrophic body gastritis and H. pylori gastritis. Helicobacter 2003, 8, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Holubiuk, L.; Imiela, J. Diet and Helicobacter pylori infection. Prz. Gastroenterol. 2016, 11, 150–154. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Cho, H.J.; Moon, S.; Choi, J.; Lee, S.; Ahn, C.; Yoo, K.Y.; Kim, I.; Ko, K.P.; Lee, J.E.; et al. Pickled Vegetable and Salted Fish Intake and the Risk of Gastric Cancer: Two Prospective Cohort Studies and a Meta-Analysis. Cancers 2020, 12, 996. [Google Scholar] [CrossRef]
- Bergin, I.L.; Sheppard, B.J.; Fox, J.G. Helicobacter pylori infection and high dietary salt independently induce atrophic gastritis and intestinal metaplasia in commercially available outbred Mongolian gerbils. Dig. Dis. Sci. 2003, 48, 475–485. [Google Scholar] [CrossRef]
- Gaddy, J.A.; Radin, J.N.; Loh, J.T.; Zhang, F.; Washington, M.K.; Peek, R.M., Jr.; Algood, H.M.; Cover, T.L. High dietary salt intake exacerbates Helicobacter pylori-induced gastric carcinogenesis. Infect. Immun. 2013, 81, 2258–2267. [Google Scholar] [CrossRef] [Green Version]
- Loh, J.T.; Gaddy, J.A.; Algood, H.M.; Gaudieri, S.; Mallal, S.; Cover, T.L. Helicobacter pylori adaptation in vivo in response to a high-salt diet. Infect. Immun. 2015, 83, 4871–4883. [Google Scholar] [CrossRef] [Green Version]
- Loh, J.T.; Beckett, A.C.; Scholz, M.B.; Cover, T.L. High-Salt Conditions Alter Transcription of Helicobacter pylori Genes Encoding Outer Membrane Proteins. Infect. Immun. 2018, 86, e00626-17. [Google Scholar] [CrossRef] [Green Version]
- Voss, B.J.; Loh, J.T.; Hill, S.; Rose, K.L.; McDonald, W.H.; Cover, T.L. Alteration of the Helicobacter pylori membrane proteome in response to changes in environmental salt concentration. Proteom. Clin. Appl. 2015, 9, 1021–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanaka, A.; Fahey, J.W.; Fukumoto, A.; Nakayama, M.; Inoue, S.; Zhang, S.; Tauchi, M.; Suzuki, H.; Hyodo, I.; Yamamoto, M. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res. 2009, 2, 353–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caston, R.R.; Loh, J.T.; Voss, B.J.; McDonald, W.H.; Scholz, M.B.; McClain, M.S.; Cover, T.L. Effect of environmental salt concentration on the Helicobacter pylori exoproteome. J. Proteom. 2019, 202, 103374. [Google Scholar] [CrossRef]
- Beckett, A.C.; Piazuelo, M.B.; Noto, J.M.; Peek, R.M., Jr.; Washington, M.K.; Algood, H.M.; Cover, T.L. Dietary Composition Influences Incidence of Helicobacter pylori-Induced Iron Deficiency Anemia and Gastric Ulceration. Infect. Immun. 2016, 84, 3338–3349. [Google Scholar] [CrossRef] [Green Version]
- Park, J.M.; Han, Y.M.; Park, Y.J.; Hahm, K.B. Dietary intake of walnut prevented Helicobacter pylori-associated gastric cancer through rejuvenation of chronic atrophic gastritis. J. Clin. Biochem. Nutr. 2021, 68, 37–50. [Google Scholar] [CrossRef]
- Jeong, M.; Park, J.M.; Han, Y.M.; Kangwan, N.; Kwon, S.O.; Kim, B.N.; Kim, W.H.; Hahm, K.B. Dietary Intervention of Artemisia and Green Tea Extracts to Rejuvenate Helicobacter pylori-Associated Chronic Atrophic Gastritis and to Prevent Tumorigenesis. Helicobacter 2016, 21, 40–59. [Google Scholar] [CrossRef]
- Pich, O.Q.; Merrell, D.S. The ferric uptake regulator of Helicobacter pylori: A critical player in the battle for iron and colonization of the stomach. Future Microbiol. 2013, 8, 725–738. [Google Scholar] [CrossRef] [Green Version]
- Akcam, M.; Ozdem, S.; Yilmaz, A.; Gultekin, M.; Artan, R. Serum ferritin, vitamin B(12), folate, and zinc levels in children infected with Helicobacter pylori. Dig. Dis. Sci. 2007, 52, 405–410. [Google Scholar] [CrossRef]
- Capurso, G.; Marignani, M.; Delle Fave, G.; Annibale, B. Iron-deficiency anemia in premenopausal women: Why not consider atrophic body gastritis and Helicobacter pylori role? Am. J. Gastroenterol. 1999, 94, 3084–3085. [Google Scholar] [CrossRef]
- Annibale, B.; Capurso, G.; Lahner, E.; Passi, S.; Ricci, R.; Maggio, F.; Delle Fave, G. Concomitant alterations in intragastric pH and ascorbic acid concentration in patients with Helicobacter pylori gastritis and associated iron deficiency anaemia. Gut 2003, 52, 496–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capurso, G.; Lahner, E.; Marcheggiano, A.; Caruana, P.; Carnuccio, A.; Bordi, C.; Delle Fave, G.; Annibale, B. Involvement of the corporal mucosa and related changes in gastric acid secretion characterize patients with iron deficiency anaemia associated with Helicobacter pylori infection. Aliment. Pharmacol. Ther. 2001, 15, 1753–1761. [Google Scholar] [CrossRef]
- Noto, J.M.; Gaddy, J.A.; Lee, J.Y.; Piazuelo, M.B.; Friedman, D.B.; Colvin, D.C.; Romero-Gallo, J.; Suarez, G.; Loh, J.; Slaughter, J.C.; et al. Iron deficiency accelerates Helicobacter pylori-induced carcinogenesis in rodents and humans. J. Clin. Investig. 2013, 123, 479–492. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.R.; Kim, K.; Lee, S.A.; Kwon, S.O.; Lee, J.K.; Keum, N.; Park, S.M. Effect of Red, Processed, and White Meat Consumption on the Risk of Gastric Cancer: An Overall and Dose(-)Response Meta-Analysis. Nutrients 2019, 11, 826. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, A. Effect of Food on Causation and Prevention of Gastric Cancer. J. Cancer Prev. Curr. Res. 2017, 8, 289. [Google Scholar] [CrossRef] [Green Version]
- Van Hecke, T.; Van Camp, J.; De Smet, S. Oxidation During Digestion of Meat: Interactions with the Diet and Helicobacter pylori Gastritis, and Implications on Human Health. Compr. Rev. Food Sci. Food Saf. 2017, 16, 214–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Jiang, A.; Qi, B.; Ma, Z.; Xiong, Y.; Dou, J.; Wang, J. Resveratrol Protects against Helicobacter pylori-Associated Gastritis by Combating Oxidative Stress. Int. J. Mol. Sci. 2015, 16, 27757–27769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Merrell, D.S.; Semino-Mora, C.; Goldman, M.; Rahman, A.; Mog, S.; Dubois, A. Diet synergistically affects helicobacter pylori-induced gastric carcinogenesis in nonhuman primates. Gastroenterology 2009, 137, 1367–1379.e1361–e1366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyriazanos, I.D.; Sfiniadakis, I.; Gizaris, V.; Hountis, P.; Hatziveis, K.; Dafnopoulou, A.; Datsakis, K. The incidence of Helicobacter pylori infection is not increased among obese young individuals in Greece. J. Clin. Gastroenterol. 2002, 34, 541–546. [Google Scholar] [CrossRef]
- Xia, Y.; Meng, G.; Zhang, Q.; Liu, L.; Wu, H.; Shi, H.; Bao, X.; Su, Q.; Gu, Y.; Fang, L.; et al. Dietary Patterns are Associated with Helicobacter Pylori Infection in Chinese Adults: A Cross-Sectional Study. Sci. Rep. 2016, 6, 32334. [Google Scholar] [CrossRef] [Green Version]
- Shu, L.; Zheng, P.F.; Zhang, X.Y.; Feng, Y.L. Dietary patterns and Helicobacter pylori infection in a group of Chinese adults ages between 45 and 59 years old: An observational study. Medicine 2019, 98, e14113. [Google Scholar] [CrossRef]
- Jeong, M.; Park, J.M.; Han, Y.M.; Park, K.Y.; Lee, D.H.; Yoo, J.H.; Cho, J.Y.; Hahm, K.B. Dietary prevention of Helicobacter pylori-associated gastric cancer with kimchi. Oncotarget 2015, 6, 29513–29526. [Google Scholar] [CrossRef] [Green Version]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Maslowski, K.M.; Mackay, C.R. Diet, gut microbiota and immune responses. Nat. Immunol. 2011, 12, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Solis-Urra, P.; Aragon-Vela, J.; Rodriguez-Rodriguez, F.; Olivares-Arancibia, J.; Alvarez-Mercado, A.I. Insights into the Impact of Microbiota in the Treatment of NAFLD/NASH and Its Potential as a Biomarker for Prognosis and Diagnosis. Biomedicines 2021, 9, 145. [Google Scholar] [CrossRef]
- Pastor-Villaescusa, B.; Plaza-Diaz, J.; Egea-Zorrilla, A.; Leis, R.; Bueno, G.; Hoyos, R.; Vazquez-Cobela, R.; Latorre, M.; Canete, M.D.; Caballero-Villarraso, J.; et al. Evaluation of the gut microbiota after metformin intervention in children with obesity: A metagenomic study of a randomized controlled trial. Biomed. Pharmacother. 2021, 134, 111117. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Bernal, M.J.; Schutte, S.; Chenoll, E.; Genoves, S.; Codoner, F.M.; Gil, A.; Sanchez-Siles, L.M. Effects of Whole-Grain and Sugar Content in Infant Cereals on Gut Microbiota at Weaning: A Randomized Trial. Nutrients 2021, 13, 1496. [Google Scholar] [CrossRef]
- Li, T.H.; Qin, Y.; Sham, P.C.; Lau, K.S.; Chu, K.M.; Leung, W.K. Alterations in Gastric Microbiota After H. Pylori Eradication and in Different Histological Stages of Gastric Carcinogenesis. Sci. Rep. 2017, 7, 44935. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Gerhard, M.; Gao, J.J.; Mejias-Luque, R.; Zhang, L.; Vieth, M.; Ma, J.L.; Bajbouj, M.; Suchanek, S.; et al. Effect of Helicobacter pylori on gastrointestinal microbiota: A population-based study in Linqu, a high-risk area of gastric cancer. Gut 2020, 69, 1598–1607. [Google Scholar] [CrossRef] [Green Version]
- Pero, R.; Brancaccio, M.; Laneri, S.; Biasi, M.G.; Lombardo, B.; Scudiero, O. A Novel View of Human Helicobacter pylori Infections: Interplay between Microbiota and Beta-Defensins. Biomolecules 2019, 9, 237. [Google Scholar] [CrossRef] [Green Version]
- Maldonado-Contreras, A.; Goldfarb, K.C.; Godoy-Vitorino, F.; Karaoz, U.; Contreras, M.; Blaser, M.J.; Brodie, E.L.; Dominguez-Bello, M.G. Structure of the human gastric bacterial community in relation to Helicobacter pylori status. ISME J. 2011, 5, 574–579. [Google Scholar] [CrossRef]
- Dong, Q.; Xin, Y.; Wang, L.; Meng, X.; Yu, X.; Lu, L.; Xuan, S. Characterization of gastric microbiota in twins. Curr. Microbiol. 2017, 74, 224–229. [Google Scholar] [CrossRef]
- Brawner, K.M.; Kumar, R.; Serrano, C.A.; Ptacek, T.; Lefkowitz, E.; Morrow, C.D.; Zhi, D.; Kyanam-Kabir-Baig, K.R.; Smythies, L.E.; Harris, P.R.; et al. Helicobacter pylori infection is associated with an altered gastric microbiota in children. Mucosal Immunol. 2017, 10, 1169–1177. [Google Scholar] [CrossRef] [Green Version]
- Dash, N.R.; Khoder, G.; Nada, A.M.; Al Bataineh, M.T. Exploring the impact of Helicobacter pylori on gut microbiome composition. PLoS ONE 2019, 14, e0218274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llorca, L.; Perez-Perez, G.; Urruzuno, P.; Martinez, M.J.; Iizumi, T.; Gao, Z.; Sohn, J.; Chung, J.; Cox, L.; Simon-Soro, A.; et al. Characterization of the Gastric Microbiota in a Pediatric Population According to Helicobacter pylori Status. Pediatr. Infect. Dis. J. 2017, 36, 173–178. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Cilleros, D.; Ramos, S.; Lopez-Oliva, M.E.; Escriva, F.; Alvarez, C.; Fernandez-Millan, E.; Martin, M.A. Cocoa diet modulates gut microbiota composition and improves intestinal health in Zucker diabetic rats. Food Res. Int. 2020, 132, 109058. [Google Scholar] [CrossRef]
- Sen, T.; Cawthon, C.R.; Ihde, B.T.; Hajnal, A.; DiLorenzo, P.M.; de La Serre, C.B.; Czaja, K. Diet-driven microbiota dysbiosis is associated with vagal remodeling and obesity. Physiol. Behav. 2017, 173, 305–317. [Google Scholar] [CrossRef] [Green Version]
- Krznaric, Z.; Vranesic Bender, D.; Mestrovic, T. The Mediterranean diet and its association with selected gut bacteria. Curr. Opin. Clin. Nutr. Metab. Care 2019, 22, 401–406. [Google Scholar] [CrossRef]
- Zmora, N.; Suez, J.; Elinav, E. You are what you eat: Diet, health and the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 35–56. [Google Scholar] [CrossRef]
- Zhong, X.; Harrington, J.M.; Millar, S.R.; Perry, I.J.; O’Toole, P.W.; Phillips, C.M. Gut Microbiota Associations with Metabolic Health and Obesity Status in Older Adults. Nutrients 2020, 12, 2364. [Google Scholar] [CrossRef]
- Li, H.; Fang, Q.; Nie, Q.; Hu, J.; Yang, C.; Huang, T.; Li, H.; Nie, S. Hypoglycemic and Hypolipidemic Mechanism of Tea Polysaccharides on Type 2 Diabetic Rats via Gut Microbiota and Metabolism Alteration. J. Agric. Food Chem. 2020, 68, 10015–10028. [Google Scholar] [CrossRef]
- Kim, J.; Choi, J.H.; Oh, T.; Ahn, B.; Unno, T. Codium fragile Ameliorates High-Fat Diet-Induced Metabolism by Modulating the Gut Microbiota in Mice. Nutrients 2020, 12, 1848. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, Y.; Seow, S.W.; Amoyo, A.A.; Chiow, K.H.; Tan, T.L.; Wong, W.Y.; Poh, Q.H.; Sentosa, I.M.; Bunte, R.M.; Pettersson, S.; et al. Helicobacter pylori infection can affect energy modulating hormones and body weight in germ free mice. Sci. Rep. 2015, 5, 8731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, C.; Yang, Z.; Cheng, D.; Xie, C.; Zhu, Y.; Ge, Z.; Luo, Z.; Lu, N. Helicobacter pylori Infection Aggravates Diet-induced Insulin Resistance in Association With Gut Microbiota of Mice. EBioMedicine 2016, 12, 247–254. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Xu, X.; Li, Y.; Li, X.; Yang, X.; Chen, H.; Zhu, Y.; Lu, N.; He, C. Sex-specific association between the gut microbiome and high-fat diet-induced metabolic disorders in mice. Biol. Sex. Differ. 2020, 11, 5. [Google Scholar] [CrossRef] [Green Version]
- Macpherson, M.E.; Hov, J.R.; Ueland, T.; Dahl, T.B.; Kummen, M.; Otterdal, K.; Holm, K.; Berge, R.K.; Mollnes, T.E.; Troseid, M.; et al. Gut Microbiota-Dependent Trimethylamine N-Oxide Associates With Inflammation in Common Variable Immunodeficiency. Front. Immunol. 2020, 11, 2217. [Google Scholar] [CrossRef]
- Hamaya, R.; Ivey, K.L.; Lee, D.H.; Wang, M.; Li, J.; Franke, A.; Sun, Q.; Rimm, E.B. Association of diet with circulating trimethylamine-N-oxide concentration. Am. J. Clin. Nutr. 2020, 112, 1448–1455. [Google Scholar] [CrossRef]
- Barrea, L.; Annunziata, G.; Muscogiuri, G.; Laudisio, D.; Di Somma, C.; Maisto, M.; Tenore, G.C.; Colao, A.; Savastano, S. Trimethylamine N-oxide, Mediterranean diet, and nutrition in healthy, normal-weight adults: Also a matter of sex? Nutrition 2019, 62, 7–17. [Google Scholar] [CrossRef]
- Wu, D.; Cao, M.; Li, N.; Zhang, A.; Yu, Z.; Cheng, J.; Xie, X.; Wang, Z.; Lu, S.; Yan, S.; et al. Effect of trimethylamine N-oxide on inflammation and the gut microbiota in Helicobacter pylori-infected mice. Int. Immunopharmacol. 2020, 81, 106026. [Google Scholar] [CrossRef]
- Li, S.; Wu, D.; Cao, M.; Yu, Z.; Wu, M.; Liu, Y.; Zhou, J.; Yan, S.; Chen, J.; Huang, M.; et al. Effects of choline supplementation on liver biology, gut microbiota, and inflammation in Helicobacter pylori-infected mice. Life. Sci. 2020, 259, 118200. [Google Scholar] [CrossRef]
- Kienesberger, S.; Cox, L.M.; Livanos, A.; Zhang, X.S.; Chung, J.; Perez-Perez, G.I.; Gorkiewicz, G.; Zechner, E.L.; Blaser, M.J. Gastric Helicobacter pylori Infection Affects Local and Distant Microbial Populations and Host Responses. Cell Rep. 2016, 14, 1395–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espinoza, J.L.; Matsumoto, A.; Tanaka, H.; Matsumura, I. Gastric microbiota: An emerging player in Helicobacter pylori-induced gastric malignancies. Cancer Lett. 2018, 414, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Chua, E.G.; Chong, J.Y.; Lamichhane, B.; Webberley, K.M.; Marshall, B.J.; Wise, M.J.; Tay, C.Y. Gastric Helicobacter pylori infection perturbs human oral microbiota. PeerJ 2019, 7, e6336. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhang, J.; Xu, J.; Wei, X.; Yang, J.; Liu, Y.; Li, H.; Zhao, C.; Wang, Y.; Zhang, L.; et al. Helicobacter pylori Infection Aggravates Dysbiosis of Gut Microbiome in Children With Gastritis. Front. Cell Infect. Microbiol. 2019, 9, 375. [Google Scholar] [CrossRef] [Green Version]
- Li, X.X.; Wong, G.L.; To, K.F.; Wong, V.W.; Lai, L.H.; Chow, D.K.; Lau, J.Y.; Sung, J.J.; Ding, C. Bacterial microbiota profiling in gastritis without Helicobacter pylori infection or non-steroidal anti-inflammatory drug use. PLoS ONE 2009, 4, e7985. [Google Scholar] [CrossRef]
- Peng, C.; Xu, X.; He, Z.; Li, N.; Ouyang, Y.; Zhu, Y.; Lu, N.; He, C. Helicobacter pylori infection worsens impaired glucose regulation in high-fat diet mice in association with an altered gut microbiome and metabolome. Appl. Microbiol. Biotechnol. 2021, 105, 2081–2095. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [Green Version]
- Liou, J.M.; Malfertheiner, P.; Lee, Y.C.; Sheu, B.S.; Sugano, K.; Cheng, H.C.; Yeoh, K.G.; Hsu, P.I.; Goh, K.L.; Mahachai, V.; et al. Screening and eradication of Helicobacter pylori for gastric cancer prevention: The Taipei global consensus. Gut 2020, 69, 2093–2112. [Google Scholar] [CrossRef] [PubMed]
- De Brito, B.B.; da Silva, F.A.F.; Soares, A.S.; Pereira, V.A.; Santos, M.L.C.; Sampaio, M.M.; Neves, P.H.M.; de Melo, F.F. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J. Gastroenterol. 2019, 25, 5578–5589. [Google Scholar] [CrossRef] [PubMed]
- Diaconu, S.; Predescu, A.; Moldoveanu, A.; Pop, C.S.; Fierbinteanu-Braticevici, C. Helicobacter pylori infection: Old and new. J. Med. Life. 2017, 10, 112–117. [Google Scholar] [PubMed]
- Georgopoulos, S.; Papastergiou, V. An update on current and advancing pharmacotherapy options for the treatment of H. pylori infection. Expert Opin. Pharmacother. 2020, 12, 1–13. [Google Scholar] [CrossRef]
- Shi, J.; Jiang, Y.; Zhao, Y. Promising in vitro and in vivo inhibition of multidrug-resistant Helicobacter pylori by linezolid and novel oxazolidinone analogues. J. Glob. Antimicrob. Resist. 2016, 7, 106–109. [Google Scholar] [CrossRef]
- Jeong, S.J.; Lee, K.H.; Kim, J.H.; Park, S.Y.; Song, Y.G. Efficacy and Gut Dysbiosis of Gentamicin-Intercalated Smectite as a New Therapeutic Agent against Helicobacter pylori in a Mouse Model. Antibiotics 2020, 9, 502. [Google Scholar] [CrossRef]
- Jeong, S.J.; Kim, J.H.; Jung, D.H.; Lee, K.H.; Park, S.Y.; Song, Y.; Kang, I.M.; Song, Y.G. Gentamicin-intercalated smectite as a new therapeutic option for Helicobacter pylori eradication. J. Antimicrob. Chemother. 2018, 73, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Quinn, L.; Sheh, A.; Ellis, J.L.; Smith, D.E.; Booth, S.L.; Fu, X.; Muthupalani, S.; Ge, Z.; Puglisi, D.A.; Wang, T.C.; et al. Helicobacter pylori antibiotic eradication coupled with a chemically defined diet in INS-GAS mice triggers dysbiosis and vitamin K deficiency resulting in gastric hemorrhage. Gut Microbes 2020, 11, 820–841. [Google Scholar] [CrossRef] [PubMed]
- Angsantikul, P.; Thamphiwatana, S.; Zhang, Q.; Spiekermann, K.; Zhuang, J.; Fang, R.H.; Gao, W.; Obonyo, M.; Zhang, L. Coating nanoparticles with gastric epithelial cell membrane for targeted antibiotic delivery against Helicobacter pylori infection. Adv. Ther. 2018, 1, 1800016. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Sarkar, A.; Ghosh, P.; Ganguly, M.; Karmakar, B.C.; Saha, D.R.; Halder, A.; Chowdhury, A.; Mukhopadhyay, A.K. Antimicrobial activity of ellagic acid against Helicobacter pylori isolates from India and during infections in mice. J. Antimicrob. Chemother. 2018, 73, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Takahashi, T.; Murayama, S.Y.; Kawaguchi, M.; Matsuo, K.; Nakamura, M. Protective efficacy of a hydroxy fatty acid against gastric Helicobacter infections. Helicobacter 2017, 22, e12430. [Google Scholar] [CrossRef]
- Yulizal, O.K.; Lelo, A.; Ilyas, S.; Kusumawati, R.L. The effect of snakehead fish extract supplementation to first-line eradication regimen on macrophage migration inhibitory factor (MIF) expression in rats induced by Helicobacter pylori infection. J. Adv. Vet. Anim. Res. 2020, 7, 209–217. [Google Scholar] [CrossRef]
- Yulizal, O.K.; Lelo, A.; Ilyas, S.; Kusumawati, R.L. The effect of Channa striata extract and standard eradication regimen on asymmetric dimethylarginine in Helicobacter pylori gastritis rat model. Vet. World. 2020, 13, 1605–1612. [Google Scholar] [CrossRef]
- Davinelli, S.; Melvang, H.M.; Andersen, L.P.; Scapagnini, G.; Nielsen, M.E. Astaxanthin from Shrimp Cephalothorax Stimulates the Immune Response by Enhancing IFN-gamma, IL-10, and IL-2 Secretion in Splenocytes of Helicobacter Pylori-Infected Mice. Mar. Drugs. 2019, 17, 382. [Google Scholar] [CrossRef] [Green Version]
- Park, H.S.; Wijerathne, C.U.B.; Jeong, H.Y.; Seo, C.S.; Ha, H.; Kwun, H.J. Gastroprotective effects of Hwanglyeonhaedok-tang against Helicobacter pylori-induced gastric cell injury. J. Ethnopharmacol. 2018, 216, 239–250. [Google Scholar] [CrossRef]
- So, H.M.; Yu, J.S.; Khan, Z.; Subedi, L.; Ko, Y.J.; Lee, I.K.; Park, W.S.; Chung, S.J.; Ahn, M.J.; Kim, S.Y.; et al. Chemical constituents of the root bark of Ulmus davidiana var. japonica and their potential biological activities. Bioorg. Chem. 2019, 91, 103145. [Google Scholar] [CrossRef]
- Rong, Q.; Xu, M.; Dong, Q.; Zhang, Y.; Li, Y.; Ye, G.; Zhao, L. In vitro and in vivo bactericidal activity of Tinospora sagittata (Oliv.) Gagnep. var. craveniana (S.Y.Hu) Lo and its main effective component, palmatine, against porcine Helicobacter pylori. BMC Complement Altern. Med. 2016, 16, 331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Zhang, J.; Wang, R.; Liu, H.; Bao, C.; Wu, S.; Wen, J.; Yang, T.; Wei, Y.; Ren, S.; et al. UPLC-Q-TOF/MS-Based Serum and Urine Metabonomics Study on the Ameliorative Effects of Palmatine on Helicobacter pylori-Induced Chronic Atrophic Gastritis. Front. Pharmacol. 2020, 11, 586954. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.K.; Xu, Y.F.; Wei, W.H.; Huang, P.; Lian, D.W.; Fu, L.J.; Yang, X.F.; Chen, F.J.; Wang, J.; Cao, H.Y.; et al. Effect of patchouli alcohol on Helicobacter pylori-induced neutrophil recruitment and activation. Int. Immunopharmacol. 2019, 68, 7–16. [Google Scholar] [CrossRef]
- Lian, D.W.; Xu, Y.F.; Deng, Q.H.; Lin, X.M.; Huang, B.; Xian, S.X.; Huang, P. Effect of patchouli alcohol on macrophage mediated Helicobacter pylori digestion based on intracellular urease inhibition. Phytomedicine 2019, 65, 153097. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Wang, R.; Zhang, J.; Bao, C.; Zhang, J.; Li, R.; Chen, X.; Wu, S.; Wen, J.; Wei, S.; et al. Mechanism of berberine in treating Helicobacter pylori induced chronic atrophic gastritis through IRF8-IFN-gamma signaling axis suppressing. Life Sci. 2020, 248, 117456. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Dang, Z.; Jia, Y. Berberine demonstrates anti-inflammatory properties in Helicobacter pylori-infected mice with chronic gastritis by attenuating the Th17 response triggered by the B cell-activating factor. J. Cell Biochem. 2018, 119, 5373–5381. [Google Scholar] [CrossRef]
- Minozzo, B.R.; Lemes, B.M.; Justo, A.D.S.; Lara, J.E.; Petry, V.E.K.; Fernandes, D.; Bello, C.; Vellosa, J.C.R.; Campagnoli, E.B.; Nunes, O.C.; et al. Anti-ulcer mechanisms of polyphenols extract of Euphorbia umbellata (Pax) Bruyns (Euphorbiaceae). J. Ethnopharmacol. 2016, 191, 29–40. [Google Scholar] [CrossRef]
- Da Silva Junior, I.F.; Balogun, S.O.; de Oliveira, R.G.; Damazo, A.S.; Martins, D.T.O. Piper umbellatum L.: A medicinal plant with gastric-ulcer protective and ulcer healing effects in experimental rodent models. J. Ethnopharmacol. 2016, 192, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zhou, A.; Li, L.; Zhao, G.; Min, L.; Liu, S.; Zhu, S.; Guo, Q.; Liu, C.; Zhang, S.; Li, P. Vitamin D3 Inhibits Helicobacter pylori Infection by Activating the VitD3/VDR-CAMP Pathway in Mice. Front. Cell Infect. Microbiol. 2020, 10, 566730. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, L.; Li, M.X.; Shen, J.; Liu, X.D.; Xiao, Z.G.; Wu, D.L.; Ho, I.H.T.; Wu, J.C.Y.; Cheung, C.K.Y.; et al. Vitamin D3 activates the autolysosomal degradation function against Helicobacter pylori through the PDIA3 receptor in gastric epithelial cells. Autophagy 2019, 15, 707–725. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.C.; Huang, W.C.; Su, C.H.; Lin, W.D.; Wu, W.T.; Yu, B.; Hsu, Y.M. Effects of Multi-Strain Probiotics on Immune Responses and Metabolic Balance in Helicobacter pylori-Infected Mice. Nutrients 2020, 12, 2476. [Google Scholar] [CrossRef]
- De Klerk, N.; Maudsdotter, L.; Gebreegziabher, H.; Saroj, S.D.; Eriksson, B.; Eriksson, O.S.; Roos, S.; Linden, S.; Sjolinder, H.; Jonsson, A.B. Lactobacilli Reduce Helicobacter pylori Attachment to Host Gastric Epithelial Cells by Inhibiting Adhesion Gene Expression. Infect. Immun. 2016, 84, 1526–1535. [Google Scholar] [CrossRef] [Green Version]
- Yarmohammadi, M.; Yadegar, A.; Ebrahimi, M.T.; Zali, M.R. Effects of a Potential Probiotic Strain Lactobacillus gasseri ATCC 33323 on Helicobacter pylori-Induced Inflammatory Response and Gene Expression in Coinfected Gastric Epithelial Cells. Probiotics Antimicrob. Proteins 2020, 18. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Tsai, W.H.; Wu, H.Y.; Chen, C.Y.; Yeh, W.L.; Chen, Y.H.; Hsu, H.Y.; Chen, W.W.; Chen, Y.W.; Chang, W.W.; et al. Probiotic Lactobacillus spp. act Against Helicobacter pylori-induced Inflammation. J. Clin. Med. 2019, 8, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, S.S.; Lee, H.A.; Kim, J.Y.; Jeong, J.W.; Shim, J.J.; Lee, J.L.; Sim, J.H.; Chung, Y.; Kim, O. In vitro and in vivo inhibition of Helicobacter pylori by Lactobacilllus paracasei HP7. Lab. Anim. Res. 2018, 34, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.E.; Su, C.H.; Yang, J.S.; Lu, C.C.; Hou, Y.C.; Wu, J.B.; Hsu, Y.M. Baicalin, Baicalein, and Lactobacillus Rhamnosus JB3 Alleviated Helicobacter pylori Infections in Vitro and in Vivo. J. Food Sci. 2018, 83, 3118–3125. [Google Scholar] [CrossRef]
- Asgari, B.; Kermanian, F.; Derakhshan, N.; Asna-Ashari, M.; Sadat, Z.R.N.; Yaslianifard, S. Honey-Derived Lactobacillus Rhamnosus Alleviates Helicobacter Pylori-Induced Gastro-Intestinal Infection and Gastric Inflammation in C57bl/6 Mice: An Immuno-Histologic Study. Arq. Gastroenterol. 2018, 55, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Cho, D.; Huang, E.; Seo, J.Y.; Kim, W.G.; Todorov, S.D.; Ji, Y.; Holzapfel, W.H. Amelioration of Alcohol Induced Gastric Ulcers Through the Administration of Lactobacillus plantarum APSulloc 331261 Isolated From Green Tea. Front. Microbiol. 2020, 11, 420. [Google Scholar] [CrossRef] [Green Version]
- Pan, M.; Wan, C.; Xie, Q.; Huang, R.; Tao, X.; Shah, N.P.; Wei, H. Changes in gastric microbiota induced by Helicobacter pylori infection and preventive effects of Lactobacillus plantarum ZDY 2013 against such infection. J. Dairy Sci. 2016, 99, 970–981. [Google Scholar] [CrossRef]
- Aiba, Y.; Ishikawa, H.; Tokunaga, M.; Komatsu, Y. Anti-Helicobacter pylori activity of non-living, heat-killed form of lactobacilli including Lactobacillus johnsonii No.1088. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef]
- Kamiya, S.; Backert, S. Advances in Microbiology, Infectious Diseases and Public Health. In Helicobacter pylori in Human Diseases; Springer International Publishing: Cham, Switzerland, 2019; Volume 11. [Google Scholar]
- Ozbey, G.; Sproston, E.; Hanafiah, A. Helicobacter pylori Infection and Gastric Microbiota. Euroasian J. Hepatogastroenterol. 2020, 10, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Garza-Gonzalez, E.; Perez-Perez, G.I.; Maldonado-Garza, H.J.; Bosques-Padilla, F.J. A review of Helicobacter pylori diagnosis, treatment, and methods to detect eradication. World J. Gastroenterol. 2014, 20, 1438–1449. [Google Scholar] [CrossRef] [PubMed]

| Dietary Pattern | Animal Model (n) | Effects | Main Effects in Microbiota | Reference |
|---|---|---|---|---|
| (1) Excessive salt intake (2) High-salt and low-iron diet | (1) Mongolian gerbil n =108 (2) Mongolian gerbil model n = 96 | ↑ Colonization by H. pylori (1) ↑ Risk of gastric cancer, hypochlorhydria and epithelial damage, Loss of parietal cells, and intestinal metaplasia (2) ↓ Hemoglobin levels | NA | [18,27,33] |
| Cured, pickled, and smoked foods | Rhesus monkey n= 23 | Induction of intestinal metaplasia and intraepithelial neoplasia ↑ Expression of oncogenes | NA | [46] |
| Diet poor in iron | Mongolian gerbil n = 10 | ↑ Infiltration of immune cells at the site of infection ↑ Onset of gastritis ↑ Rate of cancer development | NA | [41] |
| High-fat diet | C57BL/6 mice n = 10 | Intestinal microbiota alteration | ↑ Firmicutes and Proteobacteria ↓ Bacteroidetes and Verrucomicrobia | [72] |
| High-fat diet | Specific-pathogen-free C57BL/6 mice n = 10 | H. pylori infection and high-fat diet promote dysbiosis intestinal microbiota | ↑ Firmicutes/Bacteroidetes (F/B) ratio ↑ Prevotellaceae-UCG-001, Helicobacter, and Rikenella ↓ Blautia, Lactobacillus, and Lachnoclostridium | [84] |
| Meat, fish, eggs, and dairy products | BALB/c female mice n = 40 | TMA and TMAO taken by diet induced: ↑ H. pylori development ↓ Intestinal microbiota richness and diversity | ↓ Anaerovorax, Rikenellaceae RC9 gut group, Lachnospraceae UCG 008 and Parabacteroides ↑ Escherichia/Shigella | [77] |
| Nutraceutical Tested | Type and Nature of Strain Analyzed | Sensitivity Profile | Animal Model (n) | Effects | Main Effects in Microbiota | References |
|---|---|---|---|---|---|---|
| Extracts and compounds isolated from food | ||||||
| Ellagic acid | Sydney Strain 1 isolated from gastroduodenal patients | In vitro minimum inhibitory concentration 15 mg/L ellagic acid, 0.015 mg/L clarithromycin, 0.5 mg/L amoxicillin, 0.25 mg/L metronidazole (resistant) | C57BL/6 mice n = 6 | Elimination of H. pylori in cultures and mice Reversion on H. pylori-induced gastric mucosa injury in mice | NA | [95] |
| Linoleic acid | The nature of the strain: Not indicated. Sydney Strain 1 and TN2GF4 strains | Not indicated | C57BL/6NCrl mice n = 6 | Inhibition of H. pylori growth | NA | [96] |
| Snakehead fish extract | The nature of the strain: Gastric biopsy specimens of duodenal ulcer patients. Strain: unknown | Not indicated | Albino rats n = 7 | Elimination of H. pylori | NA | [97] |
| Channa striata fish extract | The nature of the strain: Gastric biopsy specimens of duodenal ulcer patients. Strain: unknown | Not indicated | Albino rats n = 7 | In combination with standard triple therapy, reversion of gastritis | NA | [98] |
| Astaxanthin from shrimp cephalothorax | The nature of the strain: Gastric biopsy specimens of duodenal ulcer patients. J99 strain | Not indicated | BALB/c mice n = 40 | ↑synthesis of IFN-γ, IL-2 and IL-10 in splenocytes in infected mice | NA | [99] |
| Traditional plants or herbs | ||||||
| Hwanglyeonhaedok-tang | The nature of the strain: Not indicated. Sydney Strain 1 | In vitro minimum inhibitory concentration: 400 to 1600 μg/mL Hwanglyeonhaedok-tang; 0.00396 ~ 0.125 µg/mL amoxicillin; 0.001953 ~ 32 µg/mL clarithromycin | C57BL/6 mice n = 7 | Elimination of H. pylori cultures and mice ↓ H. pylori-induced inflammation in cultures and mice | NA | [100] |
| Yugeunpi | The nature of the strain: Not indicated. 51 and 43,504 strains | In vitro minimum inhibitory concentration: 25 and 50 µM bioactive compounds of Yugeunpi: (2R,3S)-2-ethoxychroman-3,5,7-triol-7-O-β-d-apiofuranoside, fraxetin, 4-O-β-d-glucopyranosyl vanillic acid, syringic acid | In vitro Murine microglia BV-2 cell line | Elimination of H. pylori in cultures ↓ H. pylori-induced inflammation in cultures | NA | [101] |
| Palmatine | The nature of the strain: (1): not indicated; (2): Chronic atrophic gastritis. (1): Sydney Strain 1; (2): SCYA201401 and Sydney Strain 1 strains | (2) In vitro minimum inhibitory concentration: strain SCYA201401 6.25 μg /mL Palmitine; Strain Sydney Strain 1, 3.12 μg/mL Palmitine | (1) Male Sprague-Dawley rats n = 6, (2) C57BL/6 mice n = 8 | ↓ H. pylori-induced chronic atrophic gastritis in rats Elimination of H. pylori in cultures and mice | NA | [102,103] |
| Patchouli alcohol | The nature of the strain: (1): not indicated; (2): not indicated. (1): NCTC11637; (2): ATCC43504 | Not indicated | Male Sprague-Dawley rats n = 6 | (1) Inhibition of H. pylori urease activity (2) ↑ macrophage digestive activity in cultures ↓ H. pylori-promoted recruitment and activation of neutrophils in cultures and rats | NA | [104,105] |
| Berberine | The nature of the strain: (1): not indicated; (2): stomach tissue of a male chronic atrophic gastritis patient No. ZCDC111001. (1): CagA+/VacA+ H. pylori strain 342 | Not indicated | (1) C57Bl/6 mice, n = not indicated (2) Sprague-Dawley rats n = 6 | Attenuates H. pylori-induced inflammation and chronic gastritis in mice and rats | NA | [106,107] |
| Euphorbia umbellata | The nature of the strain: not indicated | Not indicated | Wistar rats n = 7 | Heals H. pylori-caused ulcers in rats | NA | [108] |
| Piper umbellatum L. | The nature of the strain: not indicated. ATCC 43,504 (vacA and cagA positives) strain | Not indicated | Swiss-Webster mice, n = 8 | Heals H. pylori-caused ulcers in mice | NA | [109] |
| Vitamin D3 | The nature of the strain: (1), (2): not indicated. (1) Sydney Strain 1 (2) Sydney Strain (SS)1 (VacA+ and CagA+) strain | Not indicated | (1) C57BL/6 mice n = 8 (2) C57BL/6 mice n = 5 | Elimination of H. pylori | NA | [110,111] |
| Probiotics | ||||||
| L. fermentum, L. casei and L. rhamnosus | The nature of the strain: gastritis patient. 26,695 (ATCC 700392) strain | Not indicated | C57BL/6 mice n = 10 | Elimination of H. pylori Restoration of H. pylori-induced metabolic imbalance | NA | [112] |
| L. gasseri and L. brevis | The nature of the strain: not indicated. Sydney Strain 1 | Not indicated | hCD46Ge transgenic mouse line (CD46+/+) n = 6 | Inhibit H. pylori attachment in cultures Prevent H. pylori infection in mice | NA | [113] |
| L. gasseri | The nature of the strain: isolated from individual infected patients with chronic gastritis and peptic ulcer disease. Strains: OC168, OC235, OC250, OC824, OC912, OC562, OC576, OC722, OC803, OC805 | Not indicated | In vitro. Human gastric adenocarcinoma epithelial AGS cell line | ↓H. pylori-induced inflammation | NA | [114] |
| L. rhamnosus and L. acidophilus | The nature of the strain: not indicated. 26695 (ATCC 700392) strain | Not indicated | BALB/c mice n = 4–5 | Inhibition of growth, adhesion and invasion of H. pylori in cultures ↓ H. pylori-induced IL-8 production in cultures ↓ H. pylori colonization and H. pylori-induced inflammation in mice | L. rhamnosus associated ↑ Bifidobacterium, Proteobacteria, and A. muciniphila. L. acidophilus associated ↓ Actinobacteria and E. coli, ↑ Proteobacteria and A. muciniphila | [115] |
| L. paracasei | The nature of the strain: not indicated. Sydney Strain 1 strain | Not indicated | C57BL/6 mice n = 10 | ↓H. pylori adhesion and H. pylori-related inflammation (IL-8 expression) in cultures and mice. ↓ epithelial lesions in the stomach of mice | NA | [116] |
| L. rhamnosus | The nature of the strain: not indicated. (1) ATCC43504 strain. (2) 26695 ATCC 700392 strain | Not indicated | (1) C57BL/6 mice n = 8, (2) C57BL/6 mice n = 10 | Elimination of H. pylori ↓H. pylori-caused gastritis | The treatment maintains the balance lactic acid bacteria/coliform bacteria | [117,118] |
| L. plantarum | The nature of the strain: not indicated. (1) Sydney Strain 1 strain (2) Sydney Strain 1 (HpKTCC) strain | Not indicated | (1) (2) C57BL/6J mice n = 8 H. pylori and ethanol treatment | ↓inflammation ↓gastric ulcers in mice treated with H. pylori and ethanol Prevents H. pylori-induced gastric mucosa inflammation and gastric dysbiosis in mice | ↑ Bifidobacterium spp. and Clostridium butyricum. Prevention of the decrease in Shannon’s diversity index and Simpson’s diversity index | [119,120] |
| Dead L. johnsonii | The nature of the strain: not indicated. H. pylori No.130 strain | Not indicated | Germ-free Balb/c mice n = 10 | Elimination of H. pylori in cultures and mice | NA | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rueda-Robles, A.; Rubio-Tomás, T.; Plaza-Diaz, J.; Álvarez-Mercado, A.I. Impact of Dietary Patterns on H. pylori Infection and the Modulation of Microbiota to Counteract Its Effect. A Narrative Review. Pathogens 2021, 10, 875. https://doi.org/10.3390/pathogens10070875
Rueda-Robles A, Rubio-Tomás T, Plaza-Diaz J, Álvarez-Mercado AI. Impact of Dietary Patterns on H. pylori Infection and the Modulation of Microbiota to Counteract Its Effect. A Narrative Review. Pathogens. 2021; 10(7):875. https://doi.org/10.3390/pathogens10070875
Chicago/Turabian StyleRueda-Robles, Ascensión, Teresa Rubio-Tomás, Julio Plaza-Diaz, and Ana I. Álvarez-Mercado. 2021. "Impact of Dietary Patterns on H. pylori Infection and the Modulation of Microbiota to Counteract Its Effect. A Narrative Review" Pathogens 10, no. 7: 875. https://doi.org/10.3390/pathogens10070875