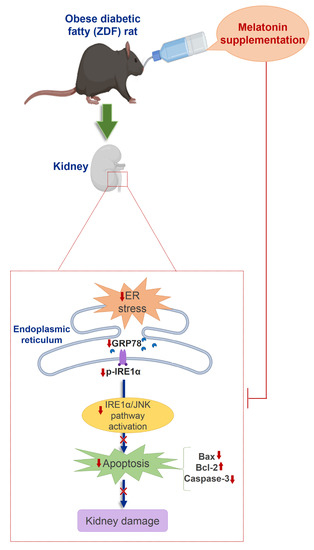
Melatonin Improves Endoplasmic Reticulum Stress-Mediated IRE1α Pathway in Zücker Diabetic Fatty Rat
Abstract
:1. Introduction
2. Results
2.1. Effects of Melatonin on Kidney ER Stress Response
2.2. Effects of Melatonin on Kidney ER Stress-Related Apopotosis Markers
2.3. Effects of Melatonin on Renal Tissu Morphology
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Animals and Experimental Protocol
4.3. Protein Extraction and Western Blotting
4.4. Microscopic Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, Z.; Cooper, M.E. Pathogenesis of diabetic nephropathy. J. Diabetes Investig. 2011, 2, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Ghemrawi, R.; Battaglia-Hsu, S.-F.; Arnold, C. Endoplasmic reticulum stress in metabolic disorders. Cells 2018, 7, 63. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cybulsky, A.V. Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat. Rev. Nephrol. 2017, 13, 681–696. [Google Scholar] [CrossRef] [PubMed]
- Cnop, M.; Foufelle, F.; Velloso, L.A. Endoplasmic reticulum stress, obesity and diabetes. Trends Mol. Med. 2012, 18, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Shu, S.; Guo, C.; Tang, C.; Dong, Z. Endoplasmic reticulum stress in ischemic and nephrotoxic acute kidney injury. Ann. Med. 2018, 50, 381–390. [Google Scholar] [CrossRef]
- Cunard, R. Endoplasmic reticulum stress in the diabetic kidney, the good, the bad and the ugly. J. Clin. Med. 2015, 4, 715–740. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wu, M.; Arvapalli, R.; Dai, X.; Mahmood, M.; Driscoll, H.; Rice, K.M.; Blough, E. Acetaminophen attenuates obesity-related renal injury through ER-mediated stress mechanisms. Cell. Physiol. Biochem. 2014, 33, 1139–1148. [Google Scholar] [CrossRef]
- Li, B.; Leung, J.C.K.; Chan, L.Y.Y.; Yiu, W.H.; Li, Y.; Lok, S.W.Y.; Liu, W.H.; Chan, K.W.; Tse, H.F.; Lai, K.N.; et al. Amelioration of Endoplasmic reticulum Stress by Mesenchymal Stem Cells via Hepatocyte Growth Factor/c-Met Signaling in Obesity-Associated Kidney Injury. Stem Cells Transl. Med. 2019, 8, 898–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Sun, Y.; Li, Z.; Song, T.; Wang, H.; Zhang, Y.; Ge, Z. Apoptosis induced by endoplasmic reticulum stress involved in diabetic kidney disease. Biochem. Biophys. Res. Commun. 2008, 370, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Morse, E.; Schroth, J.; You, N.H.; Pizzo, D.P.; Okada, S.; RamachandraRao, S.; Vallon, V.; Sharma, K.; Cunard, R. TRB3 is stimulated in diabetic kidneys, regulated by the ER stress marker CHOP, and is a suppressor of podocyte MCP-1. Am. J. Physiol. Ren. Physiol. 2010, 299. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Guo, Y.; Zeng, W.; Huang, L.; Pang, Q.; Nie, L.; Mu, J.; Yuan, F.; Feng, B. ER stress triggers MCP-1 expression through SET7/9-induced histone methylation in the kidneys of db/db mice. Am. J. Physiol. Ren. Physiol. 2014, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindenmeyer, M.T.; Rastaldi, M.P.; Ikehata, M.; Neusser, M.A.; Kretzler, M.; Cohen, C.D.; Schlöndorff, D. Proteinuria and hyperglycemia induce endoplasmic reticulum stress. J. Am. Soc. Nephrol. 2008, 19, 2225–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, D.W.; Jiang, Y.; Shalev, A.; Kowluru, R.; Crook, E.D.; Singh, L.P. An analysis of high glucose and glucosamine-induced gene expression and oxidative stress in renal mesangial cells. Arch. Physiol. Biochem. 2006, 112, 189–218. [Google Scholar] [CrossRef]
- Ohse, T.; Inagi, R.; Tanaka, T.; Ota, T.; Miyata, T.; Kojima, I.; Ingelfinger, J.R.; Ogawa, S.; Fujita, T.; Nangaku, M. Albumin induces endoplasmic reticulum stress and apoptosis in renal proximal tubular cells. Kidney Int. 2006, 70, 1447–1455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Promsan, S.; Lungkaphin, A. The roles of melatonin on kidney injury in obese and diabetic conditions. BioFactors 2020, 46, 531–549. [Google Scholar] [CrossRef]
- Navarro-Alarcón, M.; Ruiz-Ojeda, F.J.; Blanca-Herrera, R.M.; A-Serrano, M.M.; Acuña-Castroviejo, D.; Fernández-Vázquez, G.; Agil, A. Melatonin and metabolic regulation: A review. Food Funct. 2014, 5, 2806–2832. [Google Scholar] [CrossRef]
- Acuña-Castroviejo, D.; Escames, G.; Venegas, C.; Díaz-Casado, M.E.; Lima-Cabello, E.; López, L.C.; Rosales-Corral, S.; Tan, D.X.; Reiter, R.J. Extrapineal melatonin: Sources, regulation, and potential functions. Cell. Mol. Life Sci. 2014, 71, 2997–3025. [Google Scholar] [CrossRef]
- Sanchez-Hidalgo, M.; de la Lastra, C.A.; Carrascosa-Salmoral, M.P.; Naranjo, M.C.; Gomez-Corvera, A.; Caballero, B.; Guerrero, J.M. Age-related changes in melatonin synthesis in rat extrapineal tissues. Exp. Gerontol. 2009, 44, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Karamitri, A.; Jockers, R. Melatonin in type 2 diabetes mellitus and obesity. Nat. Rev. Endocrinol. 2019, 15, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Li, Y.; Li, S.; Zhou, Y.; Gan, R.Y.; Xu, D.P.; Li, H. Bin Dietary sources and bioactivities of melatonin. Nutrients 2017, 9, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cipolla-Neto, J.; Amaral, F.G.; Afeche, S.C.; Tan, D.X.; Reiter, R.J. Melatonin, energy metabolism, and obesity: A review. J. Pineal Res. 2014, 56, 371–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauriz, J.L.; Collado, P.S.; Veneroso, C.; Reiter, R.J.; González-Gallego, J. A review of the molecular aspects of melatonin’s anti-inflammatory actions: Recent insights and new perspectives. J. Pineal Res. 2013, 54, 1–14. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin as a natural ally against oxidative stress: A physicochemical examination. J. Pineal Res. 2011, 51, 1–16. [Google Scholar] [CrossRef]
- Ebaid, H.; Bashandy, S.A.E.; Abdel-Mageed, A.M.; Al-Tamimi, J.; Hassan, I.; Alhazza, I.M. Folic acid and melatonin mitigate diabetic nephropathy in rats via inhibition of oxidative stress. Nutr. Metab. 2020, 17. [Google Scholar] [CrossRef] [Green Version]
- Ko, J.W.; Shin, N.R.; Jung, T.Y.; Shin, I.S.; Moon, C.; Kim, S.H.; Lee, I.C.; Kim, S.H.; Yun, W.K.; Kim, H.C.; et al. Melatonin attenuates cisplatin-induced acute kidney injury in rats via induction of anti-aging protein, Klotho. Food Chem. Toxicol. 2019, 129, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Leibowitz, A.; Volkov, A.; Voloshin, K.; Shemesh, C.; Barshack, I.; Grossman, E. Melatonin prevents kidney injury in a high salt diet-induced hypertension model by decreasing oxidative stress. J. Pineal Res. 2016, 60, 48–54. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, J.; Wang, X.; Wang, H.; Ren, Q.; Li, Y. Effects of melatonin on diabetic nephropathy rats via Wnt/β-catenin signaling pathway and TGF-β-Smad signaling pathway. Int. J. Clin. Exp. Pathol. 2018, 11, 2488–2496. [Google Scholar] [PubMed]
- Kim, J.Y.; Leem, J.; Jeon, E.J. Protective effects of melatonin against aristolochic acid-induced nephropathy in mice. Biomolecules 2020, 10, 11. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Saha, S.; Mahalanobish, S.; Sadhukhan, P.; Sil, P.C. Melatonin attenuates arsenic induced nephropathy via the regulation of oxidative stress and inflammatory signaling cascades in mice. Food Chem. Toxicol. 2018, 118, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.K.; Ahmed, S.A.; Hamed, M.A.; El-Maraghy, S.A.; Aziz, W.M. Combination of melatonin and certain drugs for treatment of diabetic nephropathy in streptozotocin-induced diabetes in rats. Diabetol. Int. 2016, 7, 413–424. [Google Scholar] [CrossRef]
- Shi, S.; Lei, S.; Tang, C.; Wang, K.; Xia, Z. Melatonin attenuates acute kidney ischemia/reperfusion injury in diabetic rats by activation of the SIRT1/Nrf2/HO-1 signaling pathway. Biosci. Rep. 2019, 39. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.W.; Jo, J.; Kim, J.Y.; Choe, M.; Leem, J.; Park, J.H. Melatonin attenuates cisplatin-induced acute kidney injury through dual suppression of apoptosis and necroptosis. Biology 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potić, M.; Ignjatović, I.; Ničković, V.P.; Živković, J.B.; Krdžić, J.D.; Mitić, J.S.; Popović, D.; Ilić, I.R.; Stojanović, N.M.; Sokolović, D.T. Two different melatonin treatment regimens prevent an increase in kidney injury marker-1 induced by carbon tetrachloride in rat kidneys. Can. J. Physiol. Pharmacol. 2019, 97, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.Z.; He, T.; Gao, J.X.; Liu, Y.; Liu, J.Q.; Han, S.C.; Li, Y.; Shi, J.H.; Han, J.T.; Tao, K.; et al. Melatonin prevents acute kidney injury in severely burned rats via the activation of SIRT1. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Agil, A.; Chayah, M.; Visiedo, L.; Navarro-Alarcon, M.; Rodríguez Ferrer, J.M.; Tassi, M.; Reiter, R.J.; Fernández-Vázquez, G. Melatonin improves mitochondrial dynamics and function in the kidney of zücker diabetic fatty rats. J. Clin. Med. 2020, 9, 2916. [Google Scholar] [CrossRef]
- de Luxán-Delgado, B.; Potes, Y.; Rubio-González, A.; Caballero, B.; Solano, J.J.; Fernández-Fernández, M.; Bermúdez, M.; Rodrigues Moreira Guimarães, M.; Vega-Naredo, I.; Boga, J.A.; et al. Melatonin reduces endoplasmic reticulum stress and autophagy in liver of leptin-deficient mice. J. Pineal Res. 2016, 61, 108–123. [Google Scholar] [CrossRef]
- Kim, S.J.; Kang, H.S.; Lee, J.H.; Park, J.H.; Jung, C.H.; Bae, J.H.; Oh, B.C.; Song, D.K.; Baek, W.K.; Im, S.S. Melatonin ameliorates ER stress-mediated hepatic steatosis through miR-23a in the liver. Biochem. Biophys. Res. Commun. 2015, 458, 462–469. [Google Scholar] [CrossRef]
- Fernández, A.; Ordõñez, R.; Reiter, R.J.; González-Gallego, J.; Mauriz, J.L. Melatonin and endoplasmic reticulum stress: Relation to autophagy and apoptosis. J. Pineal Res. 2015, 59, 292–307. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; He, Z.; Li, B.; Liu, J.; Liu, L.; Liao, W.; Xiong, Y.; Song, C.; Yang, S.; Liu, Y. Melatonin inhibits oxalate-induced endoplasmic reticulum stress and apoptosis in HK-2 cells by activating the AMPK pathway. Cell Cycle 2020, 19, 2600–2610. [Google Scholar] [CrossRef] [PubMed]
- Hadj Ayed Tka, K.; Mahfoudh Boussaid, A.; Zaouali, M.A.; Kammoun, R.; Bejaoui, M.; Ghoul Mazgar, S.; Rosello Catafau, J.; Ben Abdennebi, H. Melatonin Modulates Endoplasmic Reticulum Stress and Akt/GSK3-Beta Signaling Pathway in a Rat Model of Renal Warm Ischemia Reperfusion. Anal. Cell. Pathol. 2015, 72, 6351. [Google Scholar] [CrossRef] [Green Version]
- Peterson, R.G.; Shaw, W.N.; Neel, M.-A.; Little, L.A.; Eichberg, J. Zucker diabetic fatty rat as a model for non-insulin-dependent diabetes mellitus. ILAR J. 1990, 32, 16–19. [Google Scholar] [CrossRef] [Green Version]
- Kasiske, B.L.; O’Donnell, M.P.; Keane, W.F. The zucker rat model of obesity, insulin resistance, hyperlipidemia, and renal injury. Hypertension 1992, 19, I-110–I-115. [Google Scholar] [CrossRef]
- Chander, P.N.; Gealekman, O.; Brodsky, S.V.; Elitok, S.; Tojo, A.; Crabtree, M.; Gross, S.S.; Goligorsky, M.S. Nephropathy in Zucker diabetic fat rat is associated with oxidative and nitrosative stress: Prevention by chronic therapy with a peroxynitrite scavenger ebselen. J. Am. Soc. Nephrol. 2004, 15, 2391–2403. [Google Scholar] [CrossRef] [Green Version]
- Ionescu, E.; Sauter, J.F.; Jeanrenaud, B. Abnormal oral glucose tolerance in genetically obese (fa/fa) rats. Am. J. Physiol. Endocrinol. Metab. 1985, 248, E500–E506. [Google Scholar] [CrossRef]
- Coimbra, T.M.; Janssen, U.; Gröne, H.J.; Ostendorf, T.; Kunter, U.; Schmidt, H.; Brabant, G.; Floege, J. Early events leading to renal injury in obese Zucker (fatty) rats with type II diabetes. Kidney Int. 2000, 57, 167–182. [Google Scholar] [CrossRef] [Green Version]
- Binukumar, B.K. Crosstalk Between the unfolded protein response, micrornas, and insulin signaling pathways: In search of biomarkers for the diagnosis and treatment of type 2 diabetes. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Sankrityayan, H.; Oza, M.J.; Kulkarni, Y.A.; Mulay, S.R.; Gaikwad, A.B. ER stress response mediates diabetic microvascular complications. Drug Discov. Today 2019, 24, 2247–2257. [Google Scholar] [CrossRef] [PubMed]
- Agil, A.; Reiter, R.J.; Jiménez-Aranda, A.; Ibán-Arias, R.; Navarro-Alarcón, M.; Marchal, J.A.; Adem, A.; Fernández-Vázquez, G. Melatonin ameliorates low-grade inflammation and oxidative stress in young Zucker diabetic fatty rats. J. Pineal Res. 2013, 54, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, K.R.; Chaudhary, M.; Kim, H.; Chae, H. Endoplasmic reticulum (ER) stress response failure in diseases trends in cell biology. Trends Cell Biol. 2020, 30, 672–675. [Google Scholar] [CrossRef]
- Grynberg, K.; Ma, F.Y.; Nikolic-Paterson, D.J. The JNK signaling pathway in renal fibrosis. Front. Physiol. 2017, 8, 829. [Google Scholar] [CrossRef]
- Nishiyama, A.; Yoshizumi, M.; Hitomi, H.; Kagami, S.; Kondo, S.; Miyatake, A.; Fukunaga, M.; Tamaki, T.; Kiyomoto, H.; Kohno, M.; et al. The SOD mimetic tempol ameliorates glomerular injury and reduces mitogen-activated protein kinase activity in dahl salt-sensitive rats. J. Am. Soc. Nephrol. 2004, 15, 306–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, S.J.; Jeong, K.S. Cell-type-specific activation of mitogen-activated protein kinases in PAN-induced progressive renal disease in rats. Biochem. Biophys. Res. Commun. 2004, 323, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Flanc, R.S.; Ma, F.Y.; Tesch, G.H.; Han, Y.; Atkins, R.C.; Bennett, B.L.; Friedman, G.C.; Fan, J.H.; Nikolic-Paterson, D.J. A pathogenic role for JNK signaling in experimental anti-GBM glomerulonephritis. Kidney Int. 2007, 72, 698–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, A.K.H.; Ma, F.Y.; Nikolic-Paterson, D.J.; Ozols, E.; Young, M.J.; Bennet, B.L.; Friedman, G.C.; Tesh, G.H. Evaluation of JNK blockade as an early intervention treatment for type 1 diabetic nephropathy in hypertensive rats. Am. J. Nephrol. 2011, 34, 337–346. [Google Scholar] [CrossRef]
- Nakagawa, N.; Barron, L.; Gomez, I.G.; Johnson, B.G.; Roach, A.M.; Kameoka, S.; Jack, R.M.; Lupher, M.L.; Gharib, S.A.; Duffield, J.S. Pentraxin-2 suppresses c-Jun/AP-1 signaling to inhibit progressive fibrotic disease. JCI Insight 2016, 1, e87446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Jung, K.J.; Yu, B.P.; Cho, C.G.; Chung, H.Y. Influence of aging and calorie restriction on MAPKs activity in rat kidney. Exp. Gerontol. 2002, 37, 1041–1053. [Google Scholar] [CrossRef]
- Bilbault, P.; Lavaux, T.; Launoy, A.; Gaub, M.P.; Meyer, N.; Oudet, P.; Pottecher, T.; Jaeger, A.; Schneider, F. Influence of drotrecogin alpha (activated) infusion on the variation of Bax/Bcl-2 and Bax/Bcl-xl ratios in circulating mononuclear cells: A cohort study in septic shock patients. Crit. Care Med. 2007, 35, 69–75. [Google Scholar] [CrossRef]
- Manucha, W.; Vallés, P.G. Apoptosis modulated by oxidative stress and inflammation during obstructive nephropathy. Inflamm. Allergy Drug Targets 2012, 11, 303–312. [Google Scholar] [CrossRef]
- Radogna, F.; Cristofanon, S.; Paternoster, L.; D’Alessio, M.; De Nicola, M.; Cerella, C.; Dicato, M.; Diederich, M.; Ghibelli, L. Melatonin antagonizes the intrinsic pathway of apoptosis via mitochondrial targeting of Bcl-2. J. Pineal Res. 2008, 44, 316–325. [Google Scholar] [CrossRef]
- Juknat, A.A.; del Valle Armanino Méndez, M.; Quaglino, A.; Fameli, C.I.; Mena, M.; Kotler, M.L. Melatonin prevents hydrogen peroxide-induced Bax expression in cultured rat astrocytes. J. Pineal Res. 2005, 38, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Xiong, F.Y.; Tang, S.T.; Su, H.; Tang, H.Q.; Jiang, P.; Zhou, Q.; Wang, Y.; Zhu, H.Q. Melatonin ameliorates myocardial apoptosis by suppressing endoplasmic reticulum stress in rats with long-term diabetic cardiomyopathy. Mol. Med. Rep. 2018, 17, 374–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, D.; Wang, B.; Wang, L.; Abraham, N.; Tao, K.; Huang, L.; Shi, W.; Dong, Y.; Qu, Y. Pre-ischemia melatonin treatment alleviated acute neuronal injury after ischemic stroke by inhibiting endoplasmic reticulum stress-dependent autophagy via PERK and IRE1 signalings. J. Pineal Res. 2017, 62, e12395. [Google Scholar] [CrossRef]
- Lehmann, R.; Schleicher, E.D. Molecular mechanism of diabetic nephropathy. Clin. Chim. Acta 2000, 297, 135–144. [Google Scholar] [CrossRef]
- Raptis, A.E.; Viberti, G. Pathogenesis of diabetic nephropathy. Exp. Clin. Endocrinol. Diabetes 2001, 109, S424–S437. [Google Scholar] [CrossRef]
- De Borst, M.H.; Prakash, J.; Melenhorst, W.B.W.H.; Van Den Heuvel, M.C.; Kok, R.J.; Navis, G.; Van Goor, H. Glomerular and tubular induction of the transcription factor c-Jun in human renal disease. J. Pathol. 2007, 213, 219–228. [Google Scholar] [CrossRef]
- Maric-Bilkan, C. Obesity and Diabetic Kidney Disease. Med. Clin. 2013, 97, 59–74. [Google Scholar] [CrossRef] [Green Version]
- Agil, A.; Navarro-Alarcõn, M.; Ruiz, R.; Abuhamadah, S.; El-Mir, M.Y.; Vázquez, G.F. Beneficial effects of melatonin on obesity and lipid profile in young Zucker diabetic fatty rats. J. Pineal Res. 2011, 50, 207–212. [Google Scholar] [CrossRef]
- Agil, A.; Rosado, I.; Ruiz, R.; Figueroa, A.; Zen, N.; Fernández-Vázquez, G. Melatonin improves glucose homeostasis in young Zucker diabetic fatty rats. J. Pineal Res. 2012, 52, 203–210. [Google Scholar] [CrossRef]
- Jiménez-Aranda, A.; Fernández-Vázquez, G.; Campos, D.; Tassi, M.; Velasco-Perez, L.; Tan, D.X.; Reiter, R.J.; Agil, A. Melatonin induces browning of inguinal white adipose tissue in Zucker diabetic fatty rats. J. Pineal Res. 2013, 55, 416–423. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aouichat, S.; Navarro-Alarcon, M.; Alarcón-Guijo, P.; Salagre, D.; Ncir, M.; Zourgui, L.; Agil, A. Melatonin Improves Endoplasmic Reticulum Stress-Mediated IRE1α Pathway in Zücker Diabetic Fatty Rat. Pharmaceuticals 2021, 14, 232. https://doi.org/10.3390/ph14030232
Aouichat S, Navarro-Alarcon M, Alarcón-Guijo P, Salagre D, Ncir M, Zourgui L, Agil A. Melatonin Improves Endoplasmic Reticulum Stress-Mediated IRE1α Pathway in Zücker Diabetic Fatty Rat. Pharmaceuticals. 2021; 14(3):232. https://doi.org/10.3390/ph14030232
Chicago/Turabian StyleAouichat, Samira, Miguel Navarro-Alarcon, Pablo Alarcón-Guijo, Diego Salagre, Marwa Ncir, Lazhar Zourgui, and Ahmad Agil. 2021. "Melatonin Improves Endoplasmic Reticulum Stress-Mediated IRE1α Pathway in Zücker Diabetic Fatty Rat" Pharmaceuticals 14, no. 3: 232. https://doi.org/10.3390/ph14030232