Biomimetic Magnetoliposomes as Oxaliplatin Nanocarriers: In Vitro Study for Potential Application in Colon Cancer
Abstract
:1. Introduction
2. Materials and Methods
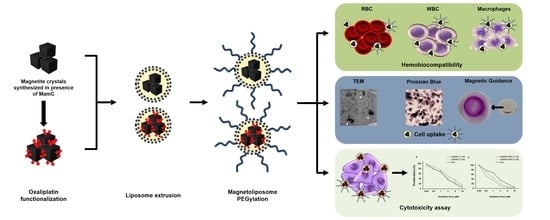
2.1. Preparation of Oxa-BMNP Nanoassemblies
2.2. Preparation of BMLs and Oxa-BMLs
2.3. Characterization of Magnetoliposomes
2.4. Cell Culturing
2.5. Blood Cells Compatibility of Liposomes and Magnetoliposomes
2.5.1. Red Blood Cells Assay
2.5.2. White Blood Cells Proliferation Assay
2.5.3. Cell Cytotoxicity in RAW 264.7
2.6. In Vitro Proliferation Assays
2.7. Cell Uptake and Intracellular Location of BMLs and IMLs
2.7.1. Cell Migration Assay
2.7.2. Cell Staining for Iron Determination
2.7.3. Transmission Electron Microscopy Assays
2.8. Statistical Analysis
3. Results
3.1. Characterization of the Nanoformulations
3.2. BMLs and IMLs Biocompatibility in Blood Cells
3.3. In Vitro Proliferation Assays
3.4. Cell Migration under a Magnetic Field In Vitro
3.5. BMLs Internalization in Colon Cell Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bazylinski, D.A.; Frankel, R.B. Magnetosome formation in prokaryotes. Nat. Rev. Microbiol. 2004, 2, 217–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prozorov, T.; Bazylinski, D.A.; Mallapragada, S.K.; Prozorov, R. Novel magnetic nanomaterials inspired by magnetotactic bacteria: Topical review. Mater. Sci. Eng. R Rep. 2013, 74, 133–172. [Google Scholar] [CrossRef]
- Peigneux, A.; Valverde-Tercedor, C.; Lopez-Moreno, R.; Perez-Gonzalez, T.; Fernandez-Vivas, M.A.; Jimenez-Lopez, C. Learning from magnetotactic bacteria: A review on the synthesis of biomimetic nanoparticles mediated by magnetosome-associated proteins. J. Struct. Biol. 2016, 196, 75–84. [Google Scholar] [CrossRef]
- Valverde-Tercedor, C.; Montalbán-López, M.; Perez-Gonzalez, T.; Sanchez-Quesada, M.S.; Prozorov, T.; Pineda-Molina, E.; Fernandez-Vivas, M.A.; Rodriguez-Navarro, A.B.; Trubitsyn, D.; Bazylinski, D.A.; et al. Size control of in vitro synthesized magnetite crystals by the MamC protein of Magnetococcus marinus strain MC-1. Appl. Microbiol. Biotechnol. 2015, 99, 5109–5121. [Google Scholar] [CrossRef]
- García Rubia, G.; Peigneux, A.; Jabalera, Y.; Puerma, J.; Oltolina, F.; Elert, K.; Colangelo, D.; Gómez Morales, J.; Prat, M.; Jimenez-Lopez, C. PH-Dependent Adsorption Release of Doxorubicin on MamC-Biomimetic Magnetite Nanoparticles. Langmuir 2018, 34, 13713–13724. [Google Scholar] [CrossRef] [PubMed]
- Peigneux, A.; Oltolina, F.; Colangelo, D.; Iglesias, G.R.; Delgado, A.V.; Prat, M.; Jimenez-Lopez, C. Functionalized Biomimetic Magnetic Nanoparticles as Effective Nanocarriers for Targeted Chemotherapy. Part. Part. Syst. Charact. 2019, 36, 1900057. [Google Scholar] [CrossRef]
- Amemiya, Y.; Arakaki, A.; Staniland, S.S.; Tanaka, T.; Matsunaga, T. Controlled formation of magnetite crystal by partial oxidation of ferrous hydroxide in the presence of recombinant magnetotactic bacterial protein Mms6. Biomaterials 2007, 28, 5381–5389. [Google Scholar] [CrossRef]
- Arakaki, A.; Yamagishi, A.; Matsunaga, T. Protein-mediated Morphological Regulation of Magnetite Crystal in Magnetotactic Bacteria. J. Japan Soc. Powder Powder Metall. 2014, 61, S99–S103. [Google Scholar] [CrossRef] [Green Version]
- Bird, S.M.; Rawlings, A.E.; Galloway, J.M.; Staniland, S.S. Using a biomimetic membrane surface experiment to investigate the activity of the magnetite biomineralisation protein Mms6. RSC Adv. 2016, 6, 7356–7363. [Google Scholar] [CrossRef] [Green Version]
- Galloway, J.M.; Staniland, S.S. Protein and peptide biotemplated metal and metal oxide nanoparticles and their patterning onto surfaces. J. Mater. Chem. 2012, 22, 12423. [Google Scholar] [CrossRef]
- Prozorov, T.; Mallapragada, S.K.; Narasimhan, B.; Wang, L.; Palo, P.; Nilsen-Hamilton, M.; Williams, T.J.; Bazylinski, D.A.; Prozorov, R.; Canfield, P.C. Protein-mediated synthesis of uniform superparamagnetic magnetite nanocrystals. Adv. Funct. Mater. 2007, 17, 951–957. [Google Scholar] [CrossRef]
- Arakaki, A.; Masuda, F.; Amemiya, Y.; Tanaka, T.; Matsunaga, T. Control of the morphology and size of magnetite particles with peptides mimicking the Mms6 protein from magnetotactic bacteria. J. Colloid Interface Sci. 2010, 343, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, A.E.; Bramble, J.P.; Walker, R.; Bain, J.; Galloway, J.M.; Staniland, S.S. Self-assembled MmsF proteinosomes control magnetite nanoparticle formation in vitro. Proc. Natl. Acad. Sci. USA 2014, 111, 16094–16099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabalera, Y.; Garcia-Pinel, B.; Ortiz, R.; Iglesias, G.; Cabeza, L.; Prados, J.; Jimenez-Lopez, C.; Melguizo, C. Oxaliplatin–Biomimetic Magnetic Nanoparticle Assemblies for Colon Cancer-Targeted Chemotherapy: An In Vitro Study. Pharmaceutics 2019, 11, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabalera, Y.; Sola-Leyva, A.; Peigneux, A.; Vurro, F.; Iglesias, G.R.; Vilchez-Garcia, J.; Pérez-Prieto, I.; Aguilar-Troyano, F.J.; López-Cara, L.C.; Carrasco-Jiménez, M.P.; et al. Biomimetic magnetic nanocarriers drive choline kinase alpha inhibitor inside cancer cells for combined chemo-hyperthermia therapy. Pharmaceutics 2019, 11, 408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglesias, G.R.; Jabalera, Y.; Peigneux, A.; Fernández, B.L.C.; Delgado, Á.V.; Jimenez-Lopez, C. Enhancement of magnetic hyperthermia by mixing synthetic inorganic and biomimetic magnetic nanoparticles. Pharmaceutics 2019, 11, 273. [Google Scholar] [CrossRef] [Green Version]
- Jabalera, Y.; Fernández-Vivas, A.; Iglesias, G.R.; Delgado, Á.V.; Jimenez-Lopez, C. Magnetoliposomes of mixed biomimetic and inorganic magnetic nanoparticles as enhanced hyperthermia agents. Colloids Surf. B Biointerfaces 2019, 183, 110435. [Google Scholar] [CrossRef]
- Lopez-Moreno, R.; Fernández-Vivas, A.; Valverde-Tercedor, C.; Azuaga Fortes, A.I.; Casares Atienza, S.; Rodriguez-Navarro, A.B.; Zarivach, R.; Jimenez-Lopez, C. Magnetite Nanoparticles Biomineralization in the Presence of the Magnetosome Membrane Protein MamC: Effect of Protein Aggregation and Protein Structure on Magnetite Formation. Cryst. Growth Des. 2017, 17, 1620–1629. [Google Scholar] [CrossRef]
- Bender, U.; Rho, Y.S.; Barrera, I.; Aghajanyan, S.; Acoba, J.; Kavan, P. Adjuvant therapy for stages II and III colon cancer: Risk stratification, treatment duration, and future directions. Curr. Oncol. 2019, 26, S43–S52. [Google Scholar] [CrossRef] [Green Version]
- Chan, G.H.J.; Chee, C.E. Making sense of adjuvant chemotherapy in colorectal cancer. J. Gastrointest. Oncol. 2019, 10, 1183–1192. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Cederquist, L.; Chen, Y.-J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; et al. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J. Natl. Compr. Cancer Netw. 2018, 16, 359–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyne, D.J.; O’Sullivan, D.E.; Heer, E.V.; Hilsden, R.J.; Sajobi, T.T.; Cheung, W.Y.; Brenner, D.R.; Friedenreich, C.M. Prognostic factors of adjuvant chemotherapy discontinuation among stage III colon cancer patients: A survey of medical oncologists and a systematic review and meta-analysis. Cancer Med. 2020, 9, 1613–1627. [Google Scholar] [CrossRef] [PubMed]
- Harada, D.; Kozuki, T.; Nogami, N.; Bessho, A.; Hosokawa, S.; Fukamatsu, N.; Hotta, K.; Ohashi, K.; Kubo, T.; Yoshioka, H.; et al. A phase I/II trial of weekly nab-paclitaxel for pretreated non-small-cell lung cancer patients without epidermal growth factor receptor mutations and anaplastic lymphoma kinase rearrangement. Asia Pac. J. Clin. Oncol. 2019, 15, 250–256. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Mukai, H.; Saeki, T.; Ro, J.; Lin, Y.-C.; Nagai, S.E.; Lee, K.S.; Watanabe, J.; Ohtani, S.; Kim, S.B.; et al. A multi-national, randomised, open-label, parallel, phase III non-inferiority study comparing NK105 and paclitaxel in metastatic or recurrent breast cancer patients. Br. J. Cancer 2019, 120, 475–480. [Google Scholar] [CrossRef] [Green Version]
- Sanoff, H.K.; Moon, D.H.; Moore, D.T.; Boles, J.; Bui, C.; Blackstock, W.; O’Neil, B.H.; Subramaniam, S.; McRee, A.J.; Carlson, C.; et al. Phase I/II trial of nano-camptothecin CRLX101 with capecitabine and radiotherapy as neoadjuvant treatment for locally advanced rectal cancer. Nanomed. Nanotechnol. Biol. Med. 2019, 18, 189–195. [Google Scholar] [CrossRef]
- Pelzer, U.; Blanc, J.-F.; Melisi, D.; Cubillo, A.; Von Hoff, D.D.; Wang-Gillam, A.; Chen, L.-T.; Siveke, J.T.; Wan, Y.; Solem, C.T.; et al. Quality-adjusted survival with combination nal-IRI+5-FU/LV vs 5-FU/LV alone in metastatic pancreatic cancer patients previously treated with gemcitabine-based therapy: A Q-TWiST analysis. Br. J. Cancer 2017, 116, 1247–1253. [Google Scholar] [CrossRef] [Green Version]
- Bakhshi, P.K.; Bain, J.; Gul, M.O.; Stride, E.; Edirisinghe, M.; Staniland, S.S. Manufacturing Man-Made Magnetosomes: High-Throughput In Situ Synthesis of Biomimetic Magnetite Loaded Nanovesicles. Macromol. Biosci. 2016, 16, 1555–1561. [Google Scholar] [CrossRef]
- Bain, J.; Ruiz-Pérez, L.; Kennerley, A.J.; Muench, S.P.; Thompson, R.; Battaglia, G.; Staniland, S.S. In situ formation of magnetopolymersomes via electroporation for MRI. Sci. Rep. 2015, 5, 14311. [Google Scholar] [CrossRef] [Green Version]
- Bain, J.; Legge, C.J.; Beattie, D.L.; Sahota, A.; Dirks, C.; Lovett, J.R.; Staniland, S.S. A biomimetic magnetosome: Formation of iron oxide within carboxylic acid terminated polymersomes. Nanoscale 2019, 11, 11617–11625. [Google Scholar] [CrossRef] [Green Version]
- Perez-Gonzalez, T.; Rodriguez-Navarro, A.; Jimenez-Lopez, C. Inorganic magnetite precipitation at 25 °C: A low-cost inorganic coprecipitation method. J. Supercond. Nov. Magn. 2011, 24, 549–557. [Google Scholar] [CrossRef]
- Eugenia Fortes Brollo, M.; Herná ndez Flores, P.; Gutié rrez, L.; Johansson, C.; Francisco Barber, D.; del Puerto Morales, M. Magnetic properties of nanoparticles as a function of their spatial distribution on liposomes and cells †. Phys. Chem. Chem. Phys. 2018, 20, 17829. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bose, A.; Bothun, G.D. Controlled release from bilayer-decorated magnetoliposomes via electromagnetic heating. ACS Nano 2010, 4, 3215–3221. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, R.; Cabeza, L.; Arias, J.L.; Melguizo, C.; Álvarez, P.J.; Vélez, C.; Clares, B.; Áranega, A.; Prados, J. Poly(butylcyanoacrylate) and poly(ε-caprolactone) nanoparticles loaded with 5-fluorouracil increase the cytotoxic effect of the drug in experimental colon cancer. AAPS J. 2015, 17, 918–929. [Google Scholar] [CrossRef] [Green Version]
- Lorente, C.; Cabeza, L.; Clares, B.; Ortiz, R.; Halbaut, L.; Delgado, Á.V.; Perazzoli, G.; Prados, J.; Arias, J.L.; Melguizo, C. Formulation and in vitro evaluation of magnetoliposomes as a potential nanotool in colorectal cancer therapy. Colloids Surf. B Biointerfaces 2018, 171, 553–565. [Google Scholar] [CrossRef]
- Clares, B.; Biedma-Ortiz, R.A.; Sáez-Fernández, E.; Prados, J.C.; Melguizo, C.; Cabeza, L.; Ortiz, R.; Arias, J.L. Nano-engineering of 5-fluorouracil-loaded magnetoliposomes for combined hyperthermia and chemotherapy against colon cancer. Eur. J. Pharm. Biopharm. 2013, 85, 329–338. [Google Scholar] [CrossRef]
- Song, Y.; Sheng, Z.; Xu, Y.; Dong, L.; Xu, W.; Li, F.; Wang, J.; Wu, Z.; Yang, Y.; Su, Y.; et al. Magnetic liposomal emodin composite with enhanced killing efficiency against breast cancer. Biomater. Sci. 2019, 7, 867–875. [Google Scholar] [CrossRef]
- Naumowicz, M. Cyclic voltammetry and chronoamperometry techniques in description of the surface-active phospholipid bilayer relative to acid-base equilibria. J. Electrochem. Soc. 2016, 163, H750–H756. [Google Scholar] [CrossRef]
- Schlenk, F.; Werner, S.; Rabel, M.; Jacobs, F.; Bergemann, C.; Clement, J.H.; Fischer, D. Comprehensive analysis of the in vitro and ex ovo hemocompatibility of surface engineered iron oxide nanoparticles for biomedical applications. Arch. Toxicol. 2017, 91, 3271–3286. [Google Scholar] [CrossRef]
- Bozzuto, G.; Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 2015, 10, 975–999. [Google Scholar] [CrossRef] [Green Version]
- Manna, P.T.; Obado, S.O.; Boehm, C.; Gadelha, C.; Sali, A.; Chait, B.T.; Rout, M.P.; Field, M.C. Lineage-specific proteins essential for endocytosis in trypanosomes. J. Cell Sci. 2017, 130, 1379–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercanti, V.; Marchetti, A.; Lelong, E.; Perez, F.; Orci, L.; Cosson, P. Transmembrane domains control exclusion of membrane proteins from clathrin-coated pits. J. Cell Sci. 2010, 123, 3329–3335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soenen, S.J.H.; Hodenius, M.; De Cuyper, M. Magnetoliposomes: Versatile innovative nanocolloids for use in biotechnology and biomedicine. Nanomedicine 2009, 4, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Hoekstra, D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef]
- Guo, M.; Yan, Y.; Zhang, H.; Yan, H.; Cao, Y.; Liu, K.; Wan, S.; Huang, J.; Yue, W. Magnetic and pH-responsive nanocarriers with multilayer core-shell architecture for anticancer drug delivery. J. Mater. Chem. 2008, 18, 5104–5112. [Google Scholar] [CrossRef]
- Geisow, M.J.; Evans, W.H. pH in the endosome. Measurements during pinocytosis and receptor-mediated endocytosis. Exp. Cell Res. 1984, 150, 36–46. [Google Scholar] [CrossRef]
- Raguraman, V.; Suthindhiran, K. Comparative ecotoxicity assessment of magnetosomes and magnetite nanoparticles. Int. J. Environ. Health Res. 2020, 30, 13–25. [Google Scholar] [CrossRef]
- Stepien, G.; Moros, M.; Pérez-Hernández, M.; Monge, M.; Gutiérrez, L.; Fratila, R.M.; Las Heras, M.d.; Menao Guillén, S.; Puente Lanzarote, J.J.; Solans, C.; et al. Effect of Surface Chemistry and Associated Protein Corona on the Long-Term Biodegradation of Iron Oxide Nanoparticles In Vivo. ACS Appl. Mater. Interfaces 2018, 10, 4548–4560. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Tang, M.; Yao, Y.; Ma, Y.; Pu, Y. MWCNT interactions with protein: Surface-induced changes in protein adsorption and the impact of protein corona on cellular uptake and cytotoxicity. Int. J. Nanomed. 2019, 14, 993–1009. [Google Scholar] [CrossRef] [Green Version]
- Chiu, C.-Y.; Chung, T.-W.; Chen, S.-Y.; Ma, Y.-H. Effects of PEGylation on capture of dextran-coated magnetic nanoparticles in microcirculation. Int. J. Nanomed. 2019, 14, 4767–4780. [Google Scholar] [CrossRef] [Green Version]
- Orlando, A.; Colombo, M.; Prosperi, D.; Gregori, M.; Panariti, A.; Rivolta, I.; Masserini, M.; Cazzaniga, E. Iron oxide nanoparticles surface coating and cell uptake affect biocompatibility and inflammatory responses of endothelial cells and macrophages. J. Nanopart. Res. 2015, 17, 351. [Google Scholar] [CrossRef]
- Kurtz-Chalot, A.; Villiers, C.; Pourchez, J.; Boudard, D.; Martini, M.; Marche, P.N.; Cottier, M.; Forest, V. Impact of silica nanoparticle surface chemistry on protein corona formation and consequential interactions with biological cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, L.; Romero, S.; da Silva, G.B.; Costo, R.; Vargas, M.D.; Ronconi, C.M.; Serna, C.J.; Veintemillas-Verdaguer, S.; Del Puerto Morales, M. Degradation of magnetic nanoparticles mimicking lysosomal conditions followed by {AC} susceptibility. Biomed. Tech. 2015, 60, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Lartigue, L.; Alloyeau, D.; Kolosnjaj-Tabi, J.; Javed, Y.; Guardia, P.; Riedinger, A.; Péchoux, C.; Pellegrino, T.; Wilhelm, C.; Gazeau, F. Biodegradation of iron oxide nanocubes: High-resolution in situ monitoring. ACS Nano 2013, 7, 3939–3952. [Google Scholar] [CrossRef]
- Kono, Y.; Gogatsubo, S.; Ohba, T.; Fujita, T. Enhanced macrophage delivery to the colon using magnetic lipoplexes with a magnetic field. Drug Deliv. 2019, 26, 935–943. [Google Scholar] [CrossRef]
- Park, E.-J.; Choi, D.-H.; Kim, Y.; Lee, E.-W.; Song, J.; Cho, M.-H.; Kim, J.-H.; Kim, S.-W. Magnetic iron oxide nanoparticles induce autophagy preceding apoptosis through mitochondrial damage and {ER} stress in {RAW264}.7 cells. Toxicol. In Vitro 2014, 28, 1402–1412. [Google Scholar] [CrossRef]
- Zhang, Q.; Rajan, S.S.; Tyner, K.M.; Casey, B.J.; Dugard, C.K.; Jones, Y.; Paredes, A.M.; Clingman, C.S.; Howard, P.C.; Goering, P.L. Effects of iron oxide nanoparticles on biological responses and MR imaging properties in human mammary healthy and breast cancer epithelial cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 104, 1032–1042. [Google Scholar] [CrossRef]
- Estelrich, J.; Busquets, M.A.; del Carmen Morán, M. Effect of PEGylation on Ligand-Targeted Magnetoliposomes: A Missed Goal. ACS Omega 2017, 2, 6544–6555. [Google Scholar] [CrossRef]
- Matha, K.; Lollo, G.; Taurino, G.; Respaud, R.; Marigo, I.; Shariati, M.; Bussolati, O.; Vermeulen, A.; Remaut, K.; Benoit, J.-P. Bioinspired hyaluronic acid and polyarginine nanoparticles for DACHPt delivery. Eur. J. Pharm. Biopharm. 2020, 150, 1–13. [Google Scholar] [CrossRef]
- Tummala, S.; Gowthamarajan, K.; Satish Kumar, M.N.; Wadhwani, A. Oxaliplatin immuno hybrid nanoparticles for active targeting: An approach for enhanced apoptotic activity and drug delivery to colorectal tumors. Drug Deliv. 2016, 23, 1773–1787. [Google Scholar] [CrossRef] [Green Version]
- Han, G.; Zhang, B.; Zhang, H.; Han, D.; Tan, J.; Yang, B. The synthesis and characterization of glutathione-modified superparamagnetic iron oxide nanoparticles and their distribution in rat brains after injection in substantia nigra. J. Mater. Sci. Mater. Med. 2019, 30, 5. [Google Scholar] [CrossRef] [PubMed]
- Iacovita, C.; Florea, A.; Dudric, R.; Pall, E.; Moldovan, A.; Tetean, R.; Stiufiuc, R.; Lucaciu, C. Small versus Large Iron Oxide Magnetic Nanoparticles: Hyperthermia and Cell Uptake Properties. Molecules 2016, 21, 1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Jiang, C.; Wu, L.; Bai, X.; Zhai, S. Cytotoxicity-Related Bioeffects Induced by Nanoparticles: The Role of Surface Chemistry. Front. Bioeng. Biotechnol. 2019, 7, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Abbreviation | Composition |
|---|---|
| LIP | Empty liposome (without magnetite) |
| LIP-PEG | Empty pegylated liposome (without magnetite) |
| IMLs | Magnetoliposome containing MNPs |
| IMLs-PEG | Pegylated magnetoliposome containing MNPs |
| BMLs | Magnetoliposome containing BMNPs |
| BMLs-PEG | Pegylated magnetoliposome containing BMNPs |
| Oxa-BMLs | Magnetoliposome containing BMNPs loaded with oxaliplatin (Oxa-BMNPs) |
| Oxa-BMLs-PEG | Pegylated magnetoliposome containing Oxa-BMNPs |
| BMNPs | Biomimetic magnetic nanoparticles |
| All liposomes and magnetoliposomes were synthesized at 100 and 200 nm. BMNPs had an average size of 34 nm. | |
| Cell Line | OXA | Oxa-BMLs | Oxa-BMLs-PEG | ||
|---|---|---|---|---|---|
| 100 nm | 200 nm | 100 nm | 200 nm | ||
| T84 | 3.21 ± 0.21 | 3.95 ± 0.25 | 2.71 ± 0.29 | 2.60 ± 0.33 | 2.71 ± 0.27 |
| CCD18 | 1.09 ± 0.17 | 0.87 ± 0.16 | 1.20 ± 0.25 | 0.81 ± 0.22 | 0.84 ± 0.15 |
| SW480 | 1.81 ± 0.12 | 2.15 ± 0.16 | 1.35 ± 0.10 | 1.36 ± 0.12 | 2.14 ± 0.46 |
| HCT15 | 1.60 ± 0.09 | 2.80 ± 0.23 | 2.31 ± 0.22 | 0.96 ± 0.09 | 1.33 ± 0.10 |
| HT29 | 4.02 ± 0.74 | 5.57 ± 0.63 | 4.64 ± 0.72 | 2.54 ± 0.68 | 2.19 ± 0.75 |
| MC38 | 1.67 ± 0.12 | 2.83 ± 0.17 | 1.88 ± 0.17 | 0.60 ± 0.04 | 0.93 ± 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Pinel, B.; Jabalera, Y.; Ortiz, R.; Cabeza, L.; Jimenez-Lopez, C.; Melguizo, C.; Prados, J. Biomimetic Magnetoliposomes as Oxaliplatin Nanocarriers: In Vitro Study for Potential Application in Colon Cancer. Pharmaceutics 2020, 12, 589. https://doi.org/10.3390/pharmaceutics12060589
Garcia-Pinel B, Jabalera Y, Ortiz R, Cabeza L, Jimenez-Lopez C, Melguizo C, Prados J. Biomimetic Magnetoliposomes as Oxaliplatin Nanocarriers: In Vitro Study for Potential Application in Colon Cancer. Pharmaceutics. 2020; 12(6):589. https://doi.org/10.3390/pharmaceutics12060589
Chicago/Turabian StyleGarcia-Pinel, Beatriz, Ylenia Jabalera, Raul Ortiz, Laura Cabeza, Concepción Jimenez-Lopez, Consolación Melguizo, and Jose Prados. 2020. "Biomimetic Magnetoliposomes as Oxaliplatin Nanocarriers: In Vitro Study for Potential Application in Colon Cancer" Pharmaceutics 12, no. 6: 589. https://doi.org/10.3390/pharmaceutics12060589