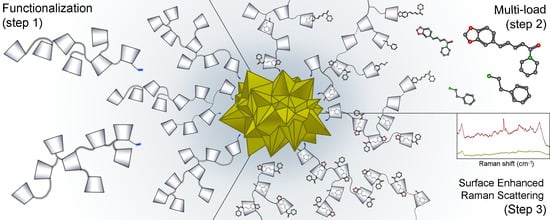
Functionalization of Gold Nanostars with Cationic β-Cyclodextrin-Based Polymer for Drug Co-Loading and SERS Monitoring
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Gold Nanostars
2.3. Synthesis of Cationic β-Cyclodextrin-Based Polymer
2.4. Inclusion of Phenylethylamine and Piperine in Cationic β-Cyclodextrin-Based Polymers
2.5. Functionalization of Gold Nanostars with Cationic β-Cyclodextrin-Based Polymer Loaded with Phenylethylamine and Piperine
2.6. Characterization of the Systems
2.7. Raman and Surface-Enhanced Raman Scattering Measurements
2.8. Molecular Model, Methods, and Calculations for Theoretical Raman Spectra
3. Results and Discussion
3.1. Preparation and Stabilization of Gold Nanostars
3.2. Preparation of Cationic β-Cyclodextrin-Based Polymers and Loading with Phenylethylamine and Piperine
3.3. Stabilization of Gold Nanostars with Cationic β-Cyclodextrin-Based Polymer and with Cationic β-Cyclodextrin-Based Polymer Including Phenylethylamine and Piperine
3.4. SERS Measurements of Phenylethylamine and Piperine Included on the Surface of Cationic β-Cyclodextrin-Based Polymer-Functionalized Gold Nanostars
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tapia-Arellano, A.; Gallardo-Toledo, E.; Ortiz, C.; Henríquez, J.; Feijóo, C.G.; Araya, E.; Sierpe, R.; Kogan, M.J. Functionalization with PEG/Angiopep-2 peptide to improve the delivery of gold nanoprisms to central nervous system: In vitro and in vivo studies. Mater. Sci. Eng. C 2021, 121, 111785. [Google Scholar] [CrossRef]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871–1880. [Google Scholar] [CrossRef]
- Daniel, M.C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef]
- Kumar, A.; Mazinder Boruah, B.; Liang, X.J. Gold nanoparticles: Promising nanomaterials for the diagnosis of cancer and HIV/AIDS. J. Nanomater. 2011, 2011, 17. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Rahme, K.; He, Y.; Li, L.L.; Holmes, J.D.; O’Driscoll, C.M. Gold nanoparticles enlighten the future of cancer theranostics. Int. J. Nanomed. 2017, 12, 6131–6152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, C.J.; Gole, A.M.; Stone, J.W.; Sisco, P.N.; Alkilany, A.M.; Goldsmith, E.C.; Baxter, S.C. Gold nanoparticles in biology: Beyond toxicity to cellular imaging. Acc. Chem. Res. 2008, 41, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Jara-Guajardo, P.; Cabrera, P.; Celis, F.; Soler, M.; Berlanga, I.; Parra-Muñoz, N.; Acosta, G.; Albericio, F.; Guzman, F.; Campos, M.; et al. Gold nanoparticles mediate improved detection of β-amyloid aggregates by fluorescence. Nanomaterials 2020, 10, 690. [Google Scholar] [CrossRef] [Green Version]
- Morales-Zavala, F.; Arriagada, H.; Hassan, N.; Velasco, C.; Riveros, A.; Álvarez, A.R.; Minniti, A.N.; Rojas-Silva, X.; Muñoz, L.L.; Vasquez, R.; et al. Peptide multifunctionalized gold nanorods decrease toxicity of β-amyloid peptide in a Caenorhabditis elegans model of Alzheimer’s disease. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2341–2350. [Google Scholar] [CrossRef]
- Hassan, N.; Cordero, M.L.; Sierpe, R.; Almada, M.; Juárez, J.; Valdez, M.; Riveros, A.; Vargas, E.; Abou-Hassan, A.; Ruso, J.M.; et al. Peptide functionalized magneto-plasmonic nanoparticles obtained by microfluidics for inhibition of β-amyloid aggregation. J. Mater. Chem. B 2018, 6, 5091–5099. [Google Scholar] [CrossRef]
- Bao, C.; Conde, J.; Pan, F.; Li, C.; Zhang, C.; Tian, F.; Liang, S.; de la Fuente, J.M.; Cui, D. Gold nanoprisms as a hybrid in vivo cancer theranostic platform for in situ photoacoustic imaging, angiography, and localized hyperthermia. Nano Res. 2016, 9, 1043–1056. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.Y.; Wang, J.; Kong, L.T.; Zheng, G.C.; Guo, Z.; Liu, J.H. SERS detection of explosive agent by macrocyclic compound functionalized triangular gold nanoprisms. J. Raman Spectrosc. 2011, 42, 1728–1735. [Google Scholar] [CrossRef]
- Wu, X.; Ming, T.; Wang, X.; Wang, P.; Wang, J.; Chen, J. High-photoluminescence-yield gold nanocubes: For cell imaging and photothermal therapy. ACS Nano 2010. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhang, F.; Chen, B.B.; Li, J.J.; Zhao, J.W. Tuning the shell thickness-dependent plasmonic absorption of Ag coated Au nanocubes: The effect of synthesis temperature. Mater. Sci. Eng. B Solid State Mater. Adv. Technol. 2015, 199, 113–120. [Google Scholar] [CrossRef]
- Liu, Y.; Ashton, J.R.; Moding, E.J.; Yuan, H.; Register, J.K.; Fales, A.M.; Choi, J.; Whitley, M.J.; Zhao, X.; Qi, Y.; et al. A plasmonic gold nanostar theranostic probe for in vivo tumor imaging and photothermal therapy. Theranostics 2015, 5, 946–960. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhang, X.; Dai, S.; Ma, Y.; Cui, S.; Achilefu, S.; Gu, Y. Multifunctional gold nanostar conjugates for tumor imaging and combined photothermal and chemo-therapy. Theranostics 2013, 3, 633–649. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.M.; Zarei, M.; Hashemi, S.A.; Ramakrishna, S.; Chiang, W.H.; Lai, C.W.; Gholami, A. Gold nanostars-diagnosis, bioimaging and biomedical applications. Drug Metab. Rev. 2020, 52, 299–318. [Google Scholar] [CrossRef]
- del Valle, A.C.; Su, C.-K.; Sun, Y.-C.; Huang, Y.-F. NIR-cleavable drug adducts of gold nanostars for overcoming multidrug-resistant tumors. Biomater. Sci. 2020, 8, 1934–1950. [Google Scholar] [CrossRef]
- Liu, X.L.; Wang, J.H.; Liang, S.; Yang, D.J.; Nan, F.; Ding, S.J.; Zhou, L.; Hao, Z.H.; Wang, Q.Q. Tuning plasmon resonance of gold nanostars for enhancements of nonlinear optical response and raman scattering. J. Phys. Chem. C 2014, 118, 9659–9664. [Google Scholar] [CrossRef]
- Barbosa, S.; Agrawal, A.; Rodríguez-Lorenzo, L.; Pastoriza-Santos, I.; Alvarez-Puebla, R.A.; Kornowski, A.; Weller, H.; Liz-Marzán, L.M. Tuning size and sensing properties in colloidal gold nanostars. Langmuir 2010, 26, 14943–14950. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold nanostars: Surfactant-free synthesis, 3D modelling, and two-photon photoluminescence imaging. Nanotechnology 2012, 23, 075102. [Google Scholar] [CrossRef] [Green Version]
- Serrano-Montes, A.B.; Langer, J.; Henriksen-Lacey, M.; Jimenez De Aberasturi, D.; Solís, D.M.; Taboada, J.M.; Obelleiro, F.; Sentosun, K.; Bals, S.; Bekdemir, A.; et al. Gold Nanostar-Coated Polystyrene Beads as Multifunctional Nanoprobes for SERS Bioimaging. J. Phys. Chem. C 2016, 120, 20860–20868. [Google Scholar] [CrossRef] [Green Version]
- Moram, S.S.B.; Byram, C.; Soma, V.R. Gold-nanoparticle- and nanostar-loaded paper-based SERS substrates for sensing nanogram-level Picric acid with a portable Raman spectrometer. Bull. Mater. Sci. 2020, 43. [Google Scholar] [CrossRef]
- Meng, X.; Dyer, J.; Huo, Y.; Jiang, C. Greater SERS Activity of Ligand-Stabilized Gold Nanostars with Sharp Branches. Langmuir 2020, 36, 3558–3564. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, I.G.; Ruenraroengsak, P.; Gonzalez-Carter, D.A.; Jiang, Q.; Yagüe, E.; Aboagye, E.O.; Coombes, R.C.; Porter, A.E.; Ryan, M.P.; Xie, F. Towards multiplexed near-infrared cellular imaging using gold nanostar arrays with tunable fluorescence enhancement. Nanoscale 2019, 11, 2079–2088. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Z.; Zhang, F.; Xu, H.; Chen, W.; Jiang, T. A novel nanocomposite based on fluorescent turn-on gold nanostars for near-infrared photothermal therapy and self-theranostic caspase-3 imaging of glioblastoma tumor cell. Colloids Surf. B Biointerfaces 2018, 170, 303–311. [Google Scholar] [CrossRef]
- Wang, Y.; Serrano, A.B.; Sentosun, K.; Bals, S.; Liz-Marzán, L.M. Stabilization and Encapsulation of Gold Nanostars Mediated by Dithiols. Small 2015, 11, 4314–4320. [Google Scholar] [CrossRef]
- Vega, M.M.; Bonifacio, A.; Lughi, V.; Marsi, S.; Carrato, S.; Sergo, V. Long-term stability of surfactant-free gold nanostars. J. Nanopart. Res. 2014, 16, 2729. [Google Scholar] [CrossRef]
- Alinejad, Z.; Mahdavian, A.R. Polymerization induced shape-tuning and multi-triggered switchability of gold nanostructures. Polymer 2018, 138, 302–306. [Google Scholar] [CrossRef]
- Borzenkov, M.; Chirico, G.; D’Alfonso, L.; Sironi, L.; Collini, M.; Cabrini, E.; Dacarro, G.; Milanese, C.; Pallavicini, P.; Taglietti, A.; et al. Thermal and Chemical Stability of Thiol Bonding on Gold Nanostars. Langmuir 2015, 31, 8081–8091. [Google Scholar] [CrossRef] [PubMed]
- Loudy, C.M.; Chasvised, S.; Paybou, C.; Courrèges, C.; Allouche, J.; Martinez, H.; Bousquet, A.; Billon, L. Revealing surface functionalities via microwave for the para-fluoro-Thiol click reaction. Polymer 2020, 202, 122675. [Google Scholar] [CrossRef]
- Yuan, H.; Fales, A.M.; Vo-Dinh, T. TAT Peptide-Functionalized Gold Nanostars: Enhanced Intracellular Delivery and Efficient NIR Photothermal Therapy Using Ultralow Irradiance. J. Am. Chem. Soc. 2012, 134, 11358–11361. [Google Scholar] [CrossRef] [Green Version]
- Sasidharan, S.; Bahadur, D.; Srivastava, R. Rapid, One-Pot, Protein-Mediated Green Synthesis of Gold Nanostars for Computed Tomographic Imaging and Photothermal Therapy of Cancer. ACS Sustain. Chem. Eng. 2017. [Google Scholar] [CrossRef]
- Jana, D.; Matti, C.; He, J.; Sagle, L. Capping Agent-Free Gold Nanostars Show Greatly Increased Versatility and Sensitivity for Biosensing. Anal. Chem. 2015, 87, 3964–3972. [Google Scholar] [CrossRef]
- Rotz, M.W.; Culver, K.S.B.; Parigi, G.; Macrenaris, K.W.; Luchinat, C.; Odom, T.W.; Meade, T.J. High relaxivity Gd(III)-DNA gold nanostars: Investigation of shape effects on proton relaxation. ACS Nano 2015, 9, 3385–3396. [Google Scholar] [CrossRef] [Green Version]
- Mariani, S.; Scarano, S.; Spadavecchia, J.; Minunni, M. A reusable optical biosensor for the ultrasensitive and selective detection of unamplified human genomic DNA with gold nanostars. Biosens. Bioelectron. 2015, 74, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Li, C.; Zhang, C.; Chen, Y.; Xu, L.; Bao, C.; Wang, X.; Liu, G.; Zhang, F.; Cui, D. CD44v6 monoclonal antibody-conjugated gold nanostars for targeted photoacoustic imaging and plasmonic photothermal therapy of gastric cancer stem-like cells. Theranostics 2015, 5, 970–984. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.P.; Perner, S.; Bankfalvi, A.; Schlücker, S. Effect of Antigen Retrieval Methods on Nonspecific Binding of Antibody-Metal Nanoparticle Conjugates on Formalin-Fixed Paraffin-Embedded Tissue. Anal. Chem. 2018, 90, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhi, X.; Yang, M.; Zhang, J.; Lin, L.; Zhao, X.; Hou, W.; Zhang, C.; Zhang, Q.; Pan, F.; et al. Tumor-triggered drug release from calcium carbonate-encapsulated gold nanostars for near-infrared photodynamic/photothermal combination antitumor therapy. Theranostics 2017, 7, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Conde, J.; Bao, C.; Chen, Y.; Curtin, J.; Cui, D. Gold nanostars for efficient in vitro and in vivo real-time SERS detection and drug delivery via plasmonic-tunable Raman/FTIR imaging. Biomaterials 2016, 106, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhai, M.; Xu, H. Controllable synthesis of sea urchin-like gold nanoparticles and their optical characteristics. Appl. Surf. Sci. 2019, 498, 143864. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.; Song, H. Synergistic effects of photonic crystal and gold nanostars for quantitative SERS detection of 3-Phenoxybenzoic acid. Appl. Surf. Sci. 2019, 476, 587–593. [Google Scholar] [CrossRef]
- Theodorou, I.G.; Jawad, Z.A.R.; Jiang, Q.; Aboagye, E.O.; Porter, A.E.; Ryan, M.P.; Xie, F. Gold Nanostar Substrates for Metal-Enhanced Fluorescence through the First and Second Near-Infrared Windows. Chem. Mater. 2017, 29, 6916–6926. [Google Scholar] [CrossRef] [Green Version]
- Haleem, A.; Chen, J.; Guo, X.X.; Wang, J.Y.; Li, H.J.; Li, P.Y.; Chen, S.Q.; He, W.D. Hybrid cryogels composed of P(NIPAM-co-AMPS) and metal nanoparticles for rapid reduction of p-nitrophenol. Polymer 2020, 193, 122352. [Google Scholar] [CrossRef]
- Tan, B.; Baycan, F. A new donor-acceptor conjugated polymer-gold nanoparticles biocomposite materials for enzymatic determination of glucose. Polymer 2020, 210, 123066. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F.; Astruc, D.; Lin, W.; Gu, H. Highly-branched amphiphilic organometallic dendronized diblock copolymer: ROMP synthesis, self-assembly and long-term Au and Ag nanoparticle stabilizer for high-efficiency catalysis. Polymer 2019, 173, 1–10. [Google Scholar] [CrossRef]
- Hernández Montoto, A.; Llopis-Lorente, A.; Gorbe, M.; Terrés, J.M.; Cao-Milán, R.; Díaz de Greñu, B.; Alfonso, M.; Ibañez, J.; Marcos, M.D.; Orzáez, M.; et al. Janus Gold Nanostars–Mesoporous Silica Nanoparticles for NIR-Light-Triggered Drug Delivery. Chem. A Eur. J. 2019, 25, 8471–8478. [Google Scholar] [CrossRef]
- Crini, G. Review: A history of cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Szejtli, J. Past, present, and future of cyclodextrin research. Pure Appl. Chem. 2004, 76, 1825–1845. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.E.; Brewster, M.E. Cyclodextrin-based pharmaceutics: Past, present and future. Nat. Rev. Drug Discov. 2004, 3, 1023–1035. [Google Scholar] [CrossRef]
- Silva, N.; Riveros, A.; Yutronic, N.; Lang, E.; Chornik, B.; Guerrero, S.; Samitier, J.; Jara, P.; Kogan, M. Photothermally Controlled Methotrexate Release System Using β-Cyclodextrin and Gold Nanoparticles. Nanomaterials 2018, 8, 985. [Google Scholar] [CrossRef] [Green Version]
- Sierpe, R.; Noyong, M.; Simon, U.; Aguayo, D.; Huerta, J.; Kogan, M.J.; Yutronic, N. Construction of 6-thioguanine and 6-mercaptopurine carriers based on βcyclodextrins and gold nanoparticles. Carbohydr. Polym. 2017, 177, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Asela, I.; Noyong, M.; Simon, U.; Andrades-Lagos, J.; Campanini-Salinas, J.; Vásquez-Velásquez, D.; Kogan, M.; Yutronic, N.; Sierpe, R. Gold nanoparticles stabilized with βcyclodextrin-2-amino-4-(4-chlorophenyl) thiazole complex: A novel system for drug transport. PLoS ONE 2017, 12, e0185652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sierpe, R.; Lang, E.; Jara, P.; Guerrero, A.R.; Chornik, B.; Kogan, M.J.; Yutronic, N. Gold Nanoparticles Interacting with β-Cyclodextrin-Phenylethylamine Inclusion Complex: A Ternary System for Photothermal Drug Release. ACS Appl. Mater. Interfaces 2015, 7, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Osman, S.K.; Brandl, F.P.; Zayed, G.M.; Teßmar, J.K.; Göpferich, A.M. Cyclodextrin based hydrogels: Inclusion complex formation and micellization of adamantane and cholesterol grafted polymers. Polymer 2011, 52, 4806–4812. [Google Scholar] [CrossRef]
- Kilsdonk, E.P.C.; Yancey, P.G.; Stoudt, G.W.; Bangerter, F.W.; Johnson, W.J.; Phillips, M.C.; Rothblat, G.H. Cellular cholesterol efflux mediated by cyclodextrins. J. Biol. Chem. 1995, 270, 17250–17256. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xiao, H.; Li, J.; Zhong, Y. Drug carrier systems based on water-soluble cationic β-cyclodextrin polymers. Int. J. Pharm. 2004, 278, 329–342. [Google Scholar] [CrossRef]
- Herrera, B.A.; Bruna, T.C.; Sierpe, R.A.; Lang, E.P.; Urzúa, M.; Flores, M.I.; Jara, P.S.; Yutronic, N.I. A surface functionalized with per-(6-amino-6-deoxy)-β-cyclodextrin for potential organic pollutant removal from water. Carbohydr. Polym. 2020, 233, 115865. [Google Scholar] [CrossRef]
- Yi, W.J.; Li, L.J.; He, H.; Hao, Z.; Liu, B.; Shen, Y.; Chao, Z.S. Poly(L-lactide)/cyclodextrin/citrate networks modified hydroxyapatite and its role as filler in the promotion to the properties of poly(L-lactide) biomaterials. Polymer 2018, 145, 1–10. [Google Scholar] [CrossRef]
- Shao, Y.; Jia, Y.G.; Shi, C.; Luo, J.; Zhu, X.X. Block and random copolymers bearing cholic acid and oligo(ethylene glycol) pendant groups: Aggregation, thermosensitivity, and drug loading. Biomacromolecules 2014, 15, 1837–1844. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Liu, H.; He, H.; Ribbe, A.E.; Thayumanavan, S. Blended Assemblies of Amphiphilic Random and Block Copolymers for Tunable Encapsulation and Release of Hydrophobic Guest Molecules. Macromolecules 2020, 53, 2713–2723. [Google Scholar] [CrossRef]
- Guo, Q.; Zhang, T.; An, J.; Wu, Z.; Zhao, Y.; Dai, X.; Zhang, X.; Li, C. Block versus Random Amphiphilic Glycopolymer Nanopaticles as Glucose-Responsive Vehicles. Biomacromolecules 2015, 16, 3345–3356. [Google Scholar] [CrossRef]
- Malanga, M.; Szemán, J.; Fenyvesi, É.; Puskás, I.; Csabai, K.; Gyémánt, G.; Fenyvesi, F.; Szente, L. “Back to the Future”: A New Look at Hydroxypropyl Beta-Cyclodextrins. J. Pharm. Sci. 2016, 105, 2921–2931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiss, T.; Fenyvesi, F.; Bácskay, I.; Váradi, J.; Fenyvesi, É.; Iványi, R.; Szente, L.; Tósaki, Á.; Vecsernyés, M. Evaluation of the cytotoxicity of β-cyclodextrin derivatives: Evidence for the role of cholesterol extraction. Eur. J. Pharm. Sci. 2010, 40, 376–380. [Google Scholar] [CrossRef]
- Furuya, T.; Koga, T. Theoretical study of inclusion complex formation of cyclodextrin and single polymer chain. Polymer 2017, 131, 193–201. [Google Scholar] [CrossRef]
- Zhong, N.; Ohvo-Rekilä, H.; Ramstedt, B.; Slotte, J.P.; Bittman, R. Selective removal of palmitic acid from Langmuir monolayers by complexation with new quaternary ammonium β-cyclodextrin derivatives. Langmuir 2001, 17, 5319–5323. [Google Scholar] [CrossRef]
- Chen, H.; Kou, X.; Yang, Z.; Ni, W.; Wang, J. Shape- and size-dependent refractive index sensitivity of gold nanoparticles. Langmuir 2008, 24, 5233–5237. [Google Scholar] [CrossRef]
- Isaacs, S.R.; Cutler, E.C.; Park, J.S.; Lee, T.R.; Shon, Y.S. Synthesis of tetraoctylammonium-protected gold nanoparticles with improved stability. Langmuir 2005, 21, 5689–5692. [Google Scholar] [CrossRef]
- George Thomas, K.; Zajicek, J.; Kamat, P.V. Surface binding properties of tetraoctylammonium bromide-capped gold nanoparticles. Langmuir 2002, 18, 3722–3727. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Y.; Wu, G. Stabilized and size-tunable gold nanoparticles formed in a quaternary ammonium-based room-temperature ionic liquid under γ-irradiation. Nanotechnology 2005, 16, 2360–2364. [Google Scholar] [CrossRef] [Green Version]
- Astruc, D.; Lu, F.; Aranzaes, J.R. Nanoparticles as recyclable catalysts: The frontier between homogeneous and heterogeneous catalysis. Angew. Chemie Int. Ed. 2005, 7852–7872. [Google Scholar] [CrossRef]
- Vivek, J.P.; Burgess, I.J. Quaternary ammonium bromide surfactant adsorption on low-index surfaces of gold. 2. Au(100) and the role of crystallographic-dependent adsorption in the formation of anisotropic nanoparticles. Langmuir 2012, 28, 5040–5047. [Google Scholar] [CrossRef] [PubMed]
- Farren-Dai, M.; Awoonor-Williams, E.; Macneil, C.S.; Mahimwalla, Z.; Ghandi, K. A novel gold nanoparticle stabilization and its muon chemistry. Chem. Phys. Lett. 2014, 610–611, 331–334. [Google Scholar] [CrossRef]
- Vivek, J.P.; Burgess, I.J. Quaternary ammonium bromide surfactant adsorption on low-index surfaces of gold. 1. Au(111). Langmuir 2012, 28, 5031–5039. [Google Scholar] [CrossRef]
- Wankar, J.; Kotla, N.G.; Gera, S.; Rasala, S.; Pandit, A.; Rochev, Y.A. Recent Advances in Host–Guest Self-Assembled Cyclodextrin Carriers: Implications for Responsive Drug Delivery and Biomedical Engineering. Adv. Funct. Mater. 2020, 30, 1909049. [Google Scholar] [CrossRef]
- Peng, L.; Liu, S.; Feng, A.; Yuan, J. Polymeric Nanocarriers Based on Cyclodextrins for Drug Delivery: Host-Guest Interaction as Stimuli Responsive Linker. Mol. Pharm. 2017, 14, 2475–2486. [Google Scholar] [CrossRef]
- Irsfeld, M.; Spadafore, M.; Prüß, B.M. β-phenylethylamine, a small molecule with a large impact. Webmedcentral 2013, 4, 4409. [Google Scholar]
- Szabo, A.; Billett, E.; Turner, J. Phenylethylamine, a possible link to the antidepressant effects of exercise? Br. J. Sports Med. 2001, 35, 342–343. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.A.; Hong, S.S.; Han, X.H.; Hwang, J.S.; Oh, G.J.; Lee, K.S.; Lee, M.K.; Hwang, B.Y.; Ro, J.S. Piperine from the Fruits of Piper longum with Inhibitory Effect on Monoamine Oxidase and Antidepressant-Like Activity. Chem. Pharm. Bull. 2005, 53, 823–835. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, S.K.; Bhutani, M.K.; Bishnoi, M. Antidepressant activity of curcumin: Involvement of serotonin and dopamine system. Psychopharmacology 2008, 201, 435–442. [Google Scholar] [CrossRef]
- Zarai, Z.; Boujelbene, E.; Ben Salem, N.; Gargouri, Y.; Sayari, A. Antioxidant and antimicrobial activities of various solvent extracts, piperine and piperic acid from Piper nigrum. LWT Food Sci. Technol. 2013, 50, 634–641. [Google Scholar] [CrossRef]
- Kumar, V.; Patil, V.; Apte, A.; Harale, N.; Patil, P.; Kulkarni, S. Ultrasensitive Gold Nanostar-Polyaniline Composite for Ammonia Gas Sensing. Langmuir 2015, 31, 13247–13256. [Google Scholar] [CrossRef]
- Otto, L.; Budde, K.; Kastenmüller, G.; Kaul, A.; Völker, U.; Völzke, H.; Adamski, J.; Kühn, J.P.; Krumsiek, J.; Artati, A.; et al. Associations between adipose tissue volume and small molecules in plasma and urine among asymptomatic subjects from the general population. Sci. Rep. 2020, 10, 1487. [Google Scholar] [CrossRef] [Green Version]
- Minati, L.; Benetti, F.; Chiappini, A.; Speranza, G. One-step synthesis of star-shaped gold nanoparticles. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 441, 623–628. [Google Scholar] [CrossRef]
- Ezawa, T.; Inoue, Y.; Tunvichien, S.; Suzuki, R.; Kanamoto, I. Changes in the Physicochemical Properties of Piperine/ β -Cyclodextrin due to the Formation of Inclusion Complexes. Int. J. Med. Chem. 2016, 2016, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian Inc.: Wallingford, UK, 2013. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Balan, C.; Pop, L.C.; Baia, M. IR, Raman and SERS analysis of amikacin combined with DFT-based calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Barone, V. Anharmonic vibrational properties by a fully automated second-order perturbative approach. J. Chem. Phys. 2005, 122, 014108. [Google Scholar] [CrossRef] [PubMed]
- Kooij, E.S.; Ahmed, W.; Hellenthal, C.; Zandvliet, H.J.W.; Poelsema, B. From nanorods to nanostars: Tuning the optical properties of gold nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2012, 413, 231–238. [Google Scholar] [CrossRef]
- De Puig, H.; Tam, J.O.; Yen, C.W.; Gehrke, L.; Hamad-Schifferli, K. Extinction Coefficient of Gold Nanostars. J. Phys. Chem. C 2015, 119, 17408–17415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Sun, Y.; Ma, P.; Zhang, D.; Li, S.; Wang, X.; Song, D. Gold nanostar-enhanced surface plasmon resonance biosensor based on carboxyl-functionalized graphene oxide. Anal. Chim. Acta 2016, 913, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Babij, N.R.; McCusker, E.O.; Whiteker, G.T.; Canturk, B.; Choy, N.; Creemer, L.C.; Amicis, C.V.D.; Hewlett, N.M.; Johnson, P.L.; Knobelsdorf, J.A.; et al. NMR Chemical Shifts of Trace Impurities: Industrially Preferred Solvents Used in Process and Green Chemistry. Org. Process Res. Dev. 2016, 20, 661–667. [Google Scholar] [CrossRef]
- Ouellette, R.J.; Rawn, J.D. Organic Chemistry-Structure, Mechanism, and Synthesis. In Organic Chemistry; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-800780-8. [Google Scholar]
- Ezawa, T.; Inoue, Y.; Murata, I.; Takao, K.; Sugita, Y.; Kanamoto, I. Characterization of the Dissolution Behavior of Piperine/Cyclodextrins Inclusion Complexes. AAPS PharmSciTech 2018, 19, 923–933. [Google Scholar] [CrossRef]
- Ternes, W.; Krause, E.L. Characterization and determination of piperine and piperine isomers in eggs. Anal. Bioanal. Chem. 2002, 374, 155–160. [Google Scholar] [CrossRef]
- Abou-Okeil, A.; Rehan, M.; El-Sawy, S.M.; El-bisi, M.K.; Ahmed-Farid, O.A.; Abdel-Mohdy, F.A. Lidocaine/β-cyclodextrin inclusion complex as drug delivery system. Eur. Polym. J. 2018, 108, 304–310. [Google Scholar] [CrossRef]
- Abarca, R.L.; Rodríguez, F.J.; Guarda, A.; Galotto, M.J.; Bruna, J.E. Characterization of beta-cyclodextrin inclusion complexes containing an essential oil component. Food Chem. 2016, 196, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Aytac, Z.; Kusku, S.I.; Durgun, E.; Uyar, T. Encapsulation of gallic acid/cyclodextrin inclusion complex in electrospun polylactic acid nanofibers: Release behavior and antioxidant activity of gallic acid. Mater. Sci. Eng. C 2016, 63, 231–239. [Google Scholar] [CrossRef]
- Zhao, X.; Xiao, D.; Alonso, J.P.; Wang, D.Y. Inclusion complex between beta-cyclodextrin and phenylphosphonicdiamide as novel bio-based flame retardant to epoxy: Inclusion behavior, characterization and flammability. Mater. Des. 2017, 114, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Chhour, P.; Naha, P.C.; Cheheltani, R.; Benardo, B.; Mian, S.; Cormode, D.P. Gold nanoparticles for biomedical applications: Synthesis and in vitro evaluation. In Methods in Pharmacology and Toxicology; Humana Press: New York, NY, USA, 2016; pp. 87–111. [Google Scholar]
- Moskovits, M. Surface-enhanced spectroscopy. Rev. Mod. Phys. 1985, 57, 783–826. [Google Scholar] [CrossRef]
- Aroca, R. Surface-Enhanced Vibrational Spectroscopy; Wiley: Chichester, UK, 2007; ISBN 9780471607311. [Google Scholar]
- He, S.; Kang, M.W.C.; Khan, F.J.; Tan, E.K.M.; Reyes, M.A.; Kah, J.C.Y. Optimizing gold nanostars as a colloid-based surface-enhanced Raman scattering (SERS) substrate. J. Opt. 2015, 17, 114013. [Google Scholar] [CrossRef]
- Nalbant Esenturk, E.; Hight Walker, A.R. Surface-enhanced Raman scattering spectroscopy via gold nanostars. J. Raman Spectrosc. 2009, 40, 89–91. [Google Scholar] [CrossRef]
- Abalde-Cela, S.; Aldeanueva-Potel, P.; Mateo-Mateo, C.; Rodríguez-Lorenzo, L.; Alvarez-Puebla, R.A.; Liz-Marzán, L.M. Surface-enhanced Raman scattering biomedical applications of plasmonic colloidal particles. J. R. Soc. Interface 2010, 7, S435–S450. [Google Scholar] [CrossRef] [Green Version]










| H of PhEA | δPhEA (ppm) | δCCD/P-PhEA-PIP (ppm) | Δδ (ppm) | H of PIP | δPIP (ppm) | δCCD/P-PhEA-PIP (ppm) | Δδ (ppm) |
|---|---|---|---|---|---|---|---|
| Hb | 2.658 | 2.683 | 0.025 | Ho | 1.475 | 1.475 | 0 |
| Ha | 2.795 | 2.819 | 0.024 | Hp | 1.594 | 1.594 | 0 |
| He | 7.176 | 7.171 | −0.005 | Hn | 3.521 | - | - |
| Hc | 7.205 | 7.198 | −0.007 | Hm | 6.043 | 6.040 | −0.003 |
| Hd | 7.285 | 7.278 | −0.007 | Hi | 6.671 | 6.666 | −0.005 |
| Hj | 6.894 | 6.893 | −0.001 | ||||
| Hl | 6.922 | 6.922 | 0 | ||||
| Hk | 6.961 | 6.960 | −0.001 | ||||
| Hf | 6.990/6.850 | 6.990/6.850 | 0 | ||||
| Hh/Hg | 7.171/7.219 | 7.165/7.215 | −0.006/−0.004 |
| System | Wavelength of Absmax (nm) | Plasmon Bandwidth (nm) | Hydrodynamic Diameter (nm) | PDI | Surface Charge (mV) | TEM Diameter (nm) |
|---|---|---|---|---|---|---|
| AuNSs | 639 | 275 | 121 ± 18 | 0.220 | −48.9 ± 3.1 | 83 ± 30 |
| AuNS-CCD/P | 610 | 245 | 167 ± 36 | 0.418 | −15.9 ± 0.6 | 87 ± 27 |
| AuNS-CCD/P-PhEA-PIP | 618 | 246 | 178 ± 39 | 0.551 | −15.5 ± 0.2 | 78 ± 23 |
| Phenylethylamine | Piperine | ||||||
|---|---|---|---|---|---|---|---|
| Raman Signal (cm−1) | Theoretical Raman (cm−1) | SERS Signal (cm−1) | Assigned Molecular Vibration | Raman Signal (cm−1) | Theoretical Raman (cm−1) | SERS Signal (cm−1) | Assigned Molecular Vibration |
| 622 | 629 | 624 | -Symmetrical aryl ring deformation | 1111 | 1085 | 1106 | -Aryl ring H scissoring |
| 829 | 810 | 827 | -Amine H wagging | 1122 | 1116 | 1124 | - H of piperidine aliphatic chain twisting and H of methylene dioxy group twisting |
| 1009 | 999 | 1008 | -C=C aryl ring symmetrical bending | 1141 | 1146 | 1146 | - H of piperidine aliphatic chain twisting, C-C diene chain stretching and aryl ring H scissoring |
| 1040 | 1036 | 1036 | -Aryl ring H rocking and aryl deformation | 1160 | 1150 | 1163 | -C. B.: (i) Aryl ring out of plane torsion and diene chain out of plane torsion; (ii) aryl ring bending deformation |
| 1164 | 1167 | 1163 | -Aryl ring H scissoring | 1214 | 1226 | 1210 | - H of piperidine aliphatic chain twisting and H of diene chain rocking |
| 1214 | 1207 | 1210 | -C. B.: (i) Aryl ring out of plane torsion and deformation; (ii) aryl ring H wagging | 1237 | 1277 | 1242 | -Aryl ring H rocking |
| 1597 | 1583 | 1602 | -C=C aryl ring symmetrical stretching | 1302 | 1312 | 1299 | -Diene chain H scissoring |
| 1617 | 1602 | 1614 | -C=C aryl ring symmetrical stretching | 1376 | 1360 | 1369 | -Diene Chain H rocking |
| 1457 | --- | 1459 | ------ | ||||
| 1595 | 1599 | 1590 | -C. B.: (i) C-C-C piperidine aliphatic chain bending, C-C-N piperidine aliphatic chain-amide group bending and diene chain bending; (ii) piperidine aliphatic chain H twisting and diene chain H rocking | ||||
| 1610 | 1606 | 1614 | -C. B.: (i) Piperidine aliphatic chain H rocking, diene chain bending and aryl ring bending deformation; (ii) piperidine aliphatic chain H twisting, C-C diene chain stretching and aryl ring H scissoring | ||||
| 1636 | 1635 | 1637 | -C. B.: (i) C-C-C piperidine aliphatic chain bending; (ii) diene chain H rocking, aryl ring hydrogen scissoring and methylene dioxy group H twisting | ||||
| 1645 | 1646 | - | -C. B.: (i) Piperidine aliphatic chain H rocking and N-C(=O)-C amide group bending; (ii) piperidine aliphatic chain H twisting, C-C diene chain stretching and aryl ring H scissoring | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donoso-González, O.; Lodeiro, L.; Aliaga, Á.E.; Laguna-Bercero, M.A.; Bollo, S.; Kogan, M.J.; Yutronic, N.; Sierpe, R. Functionalization of Gold Nanostars with Cationic β-Cyclodextrin-Based Polymer for Drug Co-Loading and SERS Monitoring. Pharmaceutics 2021, 13, 261. https://doi.org/10.3390/pharmaceutics13020261
Donoso-González O, Lodeiro L, Aliaga ÁE, Laguna-Bercero MA, Bollo S, Kogan MJ, Yutronic N, Sierpe R. Functionalization of Gold Nanostars with Cationic β-Cyclodextrin-Based Polymer for Drug Co-Loading and SERS Monitoring. Pharmaceutics. 2021; 13(2):261. https://doi.org/10.3390/pharmaceutics13020261
Chicago/Turabian StyleDonoso-González, Orlando, Lucas Lodeiro, Álvaro E. Aliaga, Miguel A. Laguna-Bercero, Soledad Bollo, Marcelo J. Kogan, Nicolás Yutronic, and Rodrigo Sierpe. 2021. "Functionalization of Gold Nanostars with Cationic β-Cyclodextrin-Based Polymer for Drug Co-Loading and SERS Monitoring" Pharmaceutics 13, no. 2: 261. https://doi.org/10.3390/pharmaceutics13020261