Enhanced Cytotoxic Effect of TAT–PLGA-Embedded DOXO Carried by Biomimetic Magnetic Nanoparticles upon Combination with Magnetic Hyperthermia and Photothermia
Abstract
:1. Introduction
2. Materials and Methods
2.1. BMNP Production
2.2. BMNP Functionalization
2.3. PLGA Encapsulation of BMNPs and DOXO-BMNPs, and TAT Peptide Functionalization
2.4. TAT–PLGA(DOXO-BMNPs) Nanoassembly Characterization
2.5. Magnetic Hyperthermia
2.6. Photothermia
2.7. Stability and Drug Release
2.8. Cell Culture
2.9. Internalization of TAT–PLGA(DOXO-BMNPs)
2.10. In Vitro Hyperthermia Cytotoxicity Assay
2.11. In Vitro Photothermal Cytotoxicity Assay
2.12. Statistical Analysis
3. Results and Discussion
3.1. TAT–PLGA(DOXO-BMNPs) Characterization
3.2. TAT–PLGA(DOXO-BMNPs) Nanoassemblies as Hyperthermic Agents
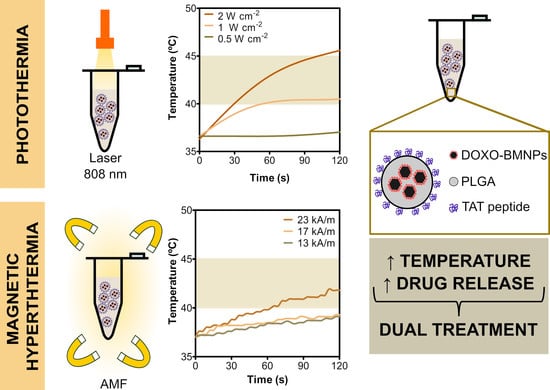
3.2.1. Magnetic Hyperthermia
3.2.2. Photothermia
3.3. TAT–PLGA(DOXO-BMNPs) Nanoassemblies as Nanocarriers
3.4. Cell Uptake
3.5. TAT–PLGA(DOXO-BMNPs) Cytotoxicity Combined with Magnetic Hyperthermia or with Photothermia
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valverde-Tercedor, C.; Montalbán-López, M.; Perez-Gonzalez, T.; Sanchez-Quesada, M.S.; Prozorov, T.; Pineda-Molina, E.; Fernandez-Vivas, M.A.; Rodriguez-Navarro, A.B.; Trubitsyn, D.; Bazylinski, D.A.; et al. Size control of in vitro synthesized magnetite crystals by the MamC protein of Magnetococcus marinus strain MC-1. Appl. Microbiol. Biotechnol. 2015, 99, 5109–5121. [Google Scholar] [CrossRef] [PubMed]
- Nudelman, H.; Valverde-tercedor, C.; Kolusheva, S.; Perez, T.; Widdrat, M.; Grimberg, N.; Levi, H.; Nelkenbaum, O.; Davidov, G.; Faivre, D.; et al. Structure—Function studies of the magnetite-biomineralizing magnetosome-associated protein MamC. J. Struct. Biol. 2016, 194, 244–252. [Google Scholar] [CrossRef] [PubMed]
- García Rubia, G.; Peigneux, A.; Jabalera, Y.; Puerma, J.; Oltolina, F.; Elert, K.; Colangelo, D.; Gómez Morales, J.; Prat, M.; Jimenez-Lopez, C. pH-Dependent adsorption release of doxorubicin on MamC-Biomimetic Magnetite Nanoparticles. Langmuir 2018, 34, 13713–13724. [Google Scholar] [CrossRef]
- Glasgow, M.D.K.; Chougule, M.B. Recent developments in active tumor targeted multifunctional nanoparticles for combination chemotherapy in cancer treatment and imaging. J. Biomed. Nanotechnol. 2015, 11, 1859–1898. [Google Scholar] [CrossRef]
- Alphandéry, E.; Faure, S.; Raison, L.; Duguet, E.; Howse, P.A.; Bazylinski, D.A. Heat production by bacterial magnetosomes exposed to an oscillating magnetic field. J. Phys. Chem. C 2011, 115, 18–22. [Google Scholar] [CrossRef] [Green Version]
- El-Boubbou, K. Magnetic iron oxide nanoparticles as drug carriers: Preparation, conjugation and delivery. Nanomedicine 2018, 13, 929–952. [Google Scholar] [CrossRef]
- Dobson, J. Magnetic micro- and nano-particle-based targeting for drug and gene delivery. Nanomedicine 2006, 1, 31–37. [Google Scholar] [CrossRef]
- Prozorov, T.; Bazylinski, D.A.; Mallapragada, S.K.; Prozorov, R. Novel magnetic nanomaterials inspired by magnetotactic bacteria: Topical review. Mater. Sci. Eng. R 2013, 74, 133–172. [Google Scholar] [CrossRef]
- Iglesias, G.R.; Jabalera, Y.; Peigneux, A.; Fernández, B.L.C.; Delgado, Á.V.; Jimenez-Lopez, C. Enhancement of magnetic hyperthermia by mixing synthetic inorganic and biomimetic magnetic nanoparticles. Pharmaceutics 2019, 11, 273. [Google Scholar] [CrossRef] [Green Version]
- Jabalera, Y.; Garcia-Pinel, B.; Ortiz, R.; Iglesias, G.; Cabeza, L.; Prados, J.; Jimenez-Lopez, C.; Melguizo, C. Oxaliplatin–biomimetic magnetic nanoparticle assemblies for colon cancer-targeted chemotherapy: An in vitro study. Pharmaceutics 2019, 11, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jabalera, Y.; Oltolina, F.; Peigneux, A.; Sola-Leyva, A.; Carrasco-Jiménez, M.P.; Prat, M.; Jimenez-Lopez, C.; Iglesias, G.R. Nanoformulation design including MamC-mediated biomimetic nanoparticles allows the simultaneous application of targeted drug delivery and magnetic hyperthermia. Polymers 2020, 12, 1832. [Google Scholar] [CrossRef]
- Peigneux, A.; Oltolina, F.; Colangelo, D.; Iglesias, G.R.; Delgado, A.V.; Prat, M.; Jimenez-Lopez, C. Functionalized Biomimetic Magnetic Nanoparticles as effective nanocarriers for targeted chemotherapy. Part. Part. Syst. Charact. 2019, 36, 1900057. [Google Scholar] [CrossRef]
- Plan Sangnier, A.; Preveral, S.; Curcio, A.; Silva, A.K.A.; Lefèvre, C.T.; Pignol, D.; Lalatonne, Y.; Wilhelm, C. Targeted thermal therapy with genetically engineered magnetite magnetosomes@RGD: Photothermia is far more efficient than magnetic hyperthermia. J. Control. Release 2018, 279, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Jaque, D.; Martínez Maestro, L.; Del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martín Rodríguez, E.; García Solé, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Estelrich, J.; Antònia Busquets, M. Iron oxide nanoparticles in photothermal therapy. Molecules 2018, 23, 1567. [Google Scholar] [CrossRef] [Green Version]
- Jabalera, Y.; Sola-Leyva, A.; Carrasco-Jiménez, M.P.; Iglesias, G.R.; Jimenez-Lopez, C. Synergistic Photothermal-Chemotherapy Based on the Use of Biomimetic Magnetic Nanoparticles. Pharmaceutics 2021, 13, 625. [Google Scholar] [CrossRef]
- Espinosa, A.; Di Corato, R.; Kolosnjaj-Tabi, J.; Flaud, P.; Pellegrino, T.; Wilhelm, C. Duality of Iron Oxide Nanoparticles in Cancer Therapy: Amplification of Heating Efficiency by Magnetic Hyperthermia and Photothermal Bimodal Treatment. ACS Nano 2016, 10, 2436–2446. [Google Scholar] [CrossRef]
- Espinosa, A.; Kolosnjaj-Tabi, J.; Abou-Hassan, A.; Plan Sangnier, A.; Curcio, A.; Silva, A.K.A.; Di Corato, R.; Neveu, S.; Pellegrino, T.; Liz-Marzán, L.M.; et al. Magnetic (Hyper)Thermia or Photothermia? Progressive Comparison of Iron Oxide and Gold Nanoparticles Heating in Water, in Cells, and In Vivo. Adv. Funct. Mater. 2018, 28, 1803660. [Google Scholar] [CrossRef]
- Di Corato, R.; Espinosa, A.; Lartigue, L.; Tharaud, M.; Chat, S.; Pellegrino, T.; Ménager, C.; Gazeau, F.; Wilhelm, C. Magnetic hyperthermia efficiency in the cellular environment fordifferent nanoparticle designs. Biomaterials 2014, 35, 6400–6411. [Google Scholar] [CrossRef]
- Oltolina, F.; Peigneux, A.; Colangelo, D.; Clemente, N.; D’urso, A.; Valente, G.; Iglesias, G.R.; Jiménez-Lopez, C.; Prat, M. Biomimetic magnetite nanoparticles as targeted drug nanocarriers and mediators of hyperthermia in an experimental cancer model. Cancers 2020, 12, 2564. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pinel, B.; Jabalera, Y.; Ortiz, R.; Cabeza, L.; Jimenez-Lopez, C.; Melguizo, C.; Prados, J. Biomimetic Magnetoliposomes as Oxaliplatin Nanocarriers: In Vitro Study for Potential Application in Colon Cancer. Pharmaceutics 2020, 12, 589. [Google Scholar] [CrossRef]
- Vurro, F.; Jabalera, Y.; Mannucci, S.; Glorani, G.; Sola-Leyva, A.; Gerosa, M.; Romeo, A.; Romanelli, M.G.; Malatesta, M.; Calderan, L.; et al. Improving the Cellular Uptake of Biomimetic Magnetic Nanoparticles. Nanomaterials 2021, 11, 766. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Lü, J.M.; Wang, X.; Marin-Muller, C.; Wang, H.; Lin, P.H.; Yao, Q.; Chen, C. Current advances in research and clinical applications of PLGA-based nanotechnology. Expert Rev. Mol. Diagn. 2009, 9, 325–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paswan, S.K.; Saini, T.R.; Jahan, S.; Ganesh, N. Designing and Formulation Optimization of Hyaluronic Acid Conjugated PLGA Nanoparticles of Tamoxifen for Tumor Targeting. Pharm. Nanotechnol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Bastaki, S.; Aravindhan, S.; Ahmadpour Saheb, N.; Afsari Kashani, M.; Dorofeev, A.E.; Karoon Kiani, F.; Jahandideh, H.; Beigi Dargani, F.; Aksoun, M.; Nikkhoo, A.; et al. Codelivery of STAT3 and PD-L1 siRNA by hyaluronate-TAT trimethyl/thiolated chitosan nanoparticles suppresses cancer progression in tumor-bearing mice. Life Sci. 2021, 266. [Google Scholar] [CrossRef] [PubMed]
- Joshi, N.; Hajizadeh, F.; Ansari Dezfouli, E.; Zekiy, A.O.; Nabi Afjadi, M.; Mousavi, S.M.; Hojjat-Farsangi, M.; Karpisheh, V.; Mahmoodpoor, A.; Hassannia, H.; et al. Silencing STAT3 enhances sensitivity of cancer cells to doxorubicin and inhibits tumor progression. Life Sci. 2021, 275. [Google Scholar] [CrossRef]
- Takke, A.; Shende, P. Magnetic-core-based silibinin nanopolymeric carriers for the treatment of renal cell cancer. Life Sci. 2021, 275. [Google Scholar] [CrossRef] [PubMed]
- Perez-Gonzalez, T.; Rodriguez-Navarro, A.; Jimenez-Lopez, C. Inorganic magnetite precipitation at 25 °C: A low-cost inorganic coprecipitation method. J. Supercond. Nov. Magn. 2011, 24, 549–557. [Google Scholar] [CrossRef]
- Gaglio, S.C.; De Rosa, C.; Piccinelli, F.; Romeo, A.; Perduca, M. Complexes of rare earth ions embedded in poly(lactic-co-glycolic acid)(PLGA)nanoparticles: Characterization and spectroscopic study. Opt. Mater. 2019, 94, 249–256. [Google Scholar] [CrossRef]
- Obaidat, I.; Issa, B.; Haik, Y. Magnetic Properties of Magnetic Nanoparticles for Efficient Hyperthermia. Nanomaterials 2015, 5, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A. On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J. Phys. D Appl. Phys. 2014, 47, 495003. [Google Scholar] [CrossRef]
- Jabalera, Y.; Sola-Leyva, A.; Peigneux, A.; Vurro, F.; Iglesias, G.R.; Vilchez-Garcia, J.; Pérez-Prieto, I.; Aguilar-Troyano, F.J.; López-Cara, L.C.; Carrasco-Jiménez, M.P.; et al. Biomimetic magnetic nanocarriers drive choline kinase alpha inhibitor inside cancer cells for combined chemo-hyperthermia therapy. Pharmaceutics 2019, 11, 408. [Google Scholar] [CrossRef] [Green Version]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia—biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Dutz, S.; Hergt, R. Magnetic nanoparticle heating and heat transfer on a microscale: Basic principles, realities and physical limitations of hyperthermia for tumour therapy. Int. J. Hyperth. 2013, 29, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Geisow, M.J.; Evans, W.H. pH in the endosome. Measurements during pinocytosis and receptor-mediated endocytosis. Exp. Cell Res. 1984, 150, 36–46. [Google Scholar] [CrossRef]
- Mosquera, V.; Villar-Alvarez, E.; Cambón, A.; Pardo, A.; Mosquera, V.X.; Bouzas-Mosquera, A.; Topete, A.; Barbosa, S.; Taboada, P. Gold Nanorod-Based Nanohybrids for Combinatorial Therapeutics. ACS Omega 2018, 3, 12633–12647. [Google Scholar] [CrossRef]
- Santra, S.; Yang, H.; Stanley, J.T.; Holloway, P.H.; Moudgil, B.M.; Walter, G.; Mericle, R.A. Rapid and effective labeling of brain tissue using TAT-conjugated CdS:Mn/ZnS quantum dots. Chem. Commun. 2005, 3144–3146. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Venkatraman, S.S.; Yang, Y.Y.; Guo, K.; Lu, J.; He, B.; Moochhala, S.; Kan, L. Polymeric micelles anchored with TAT for delivery of antibiotics across the blood-brain barrier. In Proceedings of the Biopolymers—Peptide Science Section. Biopolymers 2008, 90, 617–623. [Google Scholar] [CrossRef]
- Wang, H.; Xu, K.; Liu, L.; Tan, J.P.K.; Chen, Y.; Li, Y.; Fan, W.; Wei, Z.; Sheng, J.; Yang, Y.Y.; et al. The efficacy of self-assembled cationic antimicrobial peptide nanoparticles against Cryptococcus neoformans for the treatment of meningitis. Biomaterials 2010, 31, 2874–2881. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Martin, A.; Han, X.; Shirihai, O.S.; Grinstaff, M.W. Biodegradable PLGA Nanoparticles Restore Lysosomal Acidity and Protect Neural PC-12 Cells against Mitochondrial Toxicity. Ind. Eng. Chem. Res. 2019, 58, 13910–13917. [Google Scholar] [CrossRef]
- Elmowafy, E.M.; Tiboni, M.; Soliman, M.E. Biocompatibility, biodegradation and biomedical applications of poly(lactic acid)/poly(lactic-co-glycolic acid) micro and nanoparticles. J. Pharm. Investig. 2019, 49, 347–380. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabalera, Y.; Sola-Leyva, A.; Gaglio, S.C.; Carrasco-Jiménez, M.P.; Iglesias, G.R.; Perduca, M.; Jimenez-Lopez, C. Enhanced Cytotoxic Effect of TAT–PLGA-Embedded DOXO Carried by Biomimetic Magnetic Nanoparticles upon Combination with Magnetic Hyperthermia and Photothermia. Pharmaceutics 2021, 13, 1168. https://doi.org/10.3390/pharmaceutics13081168
Jabalera Y, Sola-Leyva A, Gaglio SC, Carrasco-Jiménez MP, Iglesias GR, Perduca M, Jimenez-Lopez C. Enhanced Cytotoxic Effect of TAT–PLGA-Embedded DOXO Carried by Biomimetic Magnetic Nanoparticles upon Combination with Magnetic Hyperthermia and Photothermia. Pharmaceutics. 2021; 13(8):1168. https://doi.org/10.3390/pharmaceutics13081168
Chicago/Turabian StyleJabalera, Ylenia, Alberto Sola-Leyva, Salvatore Calogero Gaglio, María P. Carrasco-Jiménez, Guillermo R. Iglesias, Massimiliano Perduca, and Concepcion Jimenez-Lopez. 2021. "Enhanced Cytotoxic Effect of TAT–PLGA-Embedded DOXO Carried by Biomimetic Magnetic Nanoparticles upon Combination with Magnetic Hyperthermia and Photothermia" Pharmaceutics 13, no. 8: 1168. https://doi.org/10.3390/pharmaceutics13081168