Excipient-Free Inhalable Microparticles of Azithromycin Produced by Electrospray: A Novel Approach to Direct Pulmonary Delivery of Antibiotics
Abstract
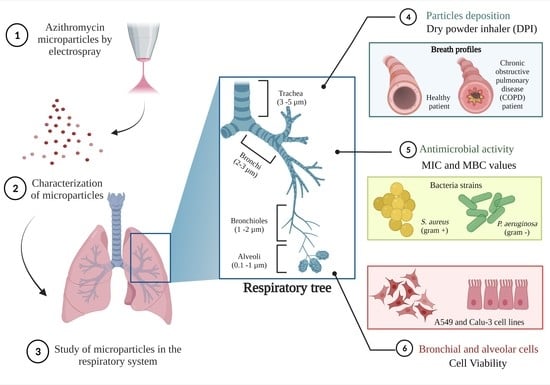
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrospray
2.3. Scanning Electron Microscopy (SEM)
2.4. X-ray Diffraction (XRD)
2.5. Differential Scanning Calorimetric (DSC)
2.6. Solid State Fourier Transform Infrared Spectroscopy (FTIR)
2.7. Drug Quantification
2.8. Equilibrium Solubility
2.9. Aerosol Properties
2.9.1. Aerodynamic Particle Size
2.9.2. Physical Particle Size
2.10. Antibacterial Activity
2.10.1. Optical Density (OD600) Measurements
2.10.2. Agar Dilution Method
2.11. In Vitro Cytotoxicity Studies
2.11.1. Cell Culture Conditions
2.11.2. Alamar Blue Assay
3. Results and Discussion
3.1. Production of Dry Microparticles
3.2. Characterization of Microparticles
3.3. In Vitro Aerosol Performance
3.4. Antimicrobial Activity
3.5. Cytotoxicity Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mangal, S.; Huang, J.; Shetty, N.; Park, H.; Lin, Y.W.; Yu, H.H.; Zemlyanov, D.; Velkov, T.; Li, J.; Zhou, Q. Effects of the antibiotic component on in-vitro bacterial killing, physico-chemical properties, aerosolization and dissolution of a ternary-combinational inhalation powder formulation of antibiotics for pan-drug resistant Gram-negative lung infections. Int. J. Pharm. 2019, 561, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Munner, S.; Wang, T.; Rintoul, L.; Ayoko, G.A.; Nazrul, I.; Izake, E.L. Development and characterization of meropenem dry powder inhaler formulation for pulmonary drug delivery. Int. J. Pharm. 2020, 587, 119684. [Google Scholar] [CrossRef] [PubMed]
- Maselli, D.J.; Keyt, H.; Restrepo, M.I. Inhaled antibiotic therapy in chronic respiratory diseases. Int. J. Mol. Sci. 2017, 18, 1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Vogt, F.G.; Hayes, D.; Mansour, H.M. Physicochemical characterization and aerosol dispersion performance of organic solution advanced spray-dried microparticulate/nanoparticulate antibiotic dry powders of tobramycin and azithromycin for pulmonary inhalation aerosol delivery. Eur. J. Pharm. Sci. 2014, 52, 191–205. [Google Scholar] [CrossRef]
- Uzun, I.N.; Sipahigil, O.; Dincer, S. Coprecipitation of Cefuroxime Axetil-PVP composite microparticles by batch supercritical antisolvent process. J. Supercrit. Fluids 2011, 55, 1059–1069. [Google Scholar] [CrossRef]
- Kalogiannis, C.G.; Michailof, C.M.; Panayiotou, C.G. Microencapsulation of amoxicillin in poly(L-lactic acid) by supercritical antisolvent precipitation. Ind. Eng. Chem. Res. 2006, 45, 8738–8743. [Google Scholar] [CrossRef]
- Adi, H.; Young, P.M.; Chan, H.K.; Agus, H.; Traini, D. Co-spray-dried mannitol-ciprofloxacin dry powder inhaler formulation for cystic fibrosis and chronic obstructive pulmonary disease. Eur. J. Pharm. Sci. 2010, 40, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Ye, T.; Yu, J.; Luo, Q.; Wang, S.; Chan, H.K. Inhalable clarithromycin liposomal dry powders using ultrasonic spray freeze drying. Powder Technol. 2017, 305, 63–70. [Google Scholar] [CrossRef]
- World Health Organization (WHO). World health organization model list of essential medicines. Ment. Holist. Heal. Some Int. Perspect. 2019, 21, 119–134. [Google Scholar]
- Echeverría-esnal, D.; Martin-ontiyuelo, C.; Navarrete-Rouco, M.E.; Cuscó, M.D.; Ferrández, O.; Horcajada, J.P. Azithromycin in the treatment of COVID-19: A review. Expert Rev. Anti. Infect. Ther. 2021, 19, 147–163. [Google Scholar] [CrossRef]
- Parnham, M.J.; Haber, V.E.; Giamarellos-Bourboulis, E.J.; Perletti, G.; Verleden, G.M.; Vos, R. Azithromycin: Mechanisms of action and their relevance for clinical applications. Pharmacol. Ther. 2014, 143, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Leroy, A.; Caillon, J.; Caroff, N.; Broquet, A.; Leroy, A. Could Azithromycin Be Part of Pseudomonas aeruginosa Acute Pneumonia Treatment? Front. Microbiol. 2021, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- Adeli, E. The Journal of Supercritical Fluids A comparative evaluation between utilizing SAS supercritical fluid technique and solvent evaporation method in preparation of Azithromycin solid dispersions for dissolution rate enhancement. J. Supercrit. Fluids 2014, 87, 9–21. [Google Scholar] [CrossRef]
- Firth, A.; Prathapan, P. Azithromycin: The First Broad-spectrum Therapeutic. Eur. J. Med. Chem. 2020, 207, 112739. [Google Scholar] [CrossRef] [PubMed]
- Sader, H.S.; Duncan, L.R.; Mendes, R.E. Antimicrobial activity of dalbavancin and comparators against Staphylococcus aureus causing pneumonia in patients with and without cystic fibrosis. Int. J. Infect. Dis. 2021, 107, 69–71. [Google Scholar] [CrossRef]
- Mangal, S.; Nie, H.; Xu, R.; Guo, R.; Cavallaro, A.; Zemlyanov, D.; Zhou, Q. Physico-Chemical Properties, Aerosolization and Dissolution of Co-Spray Dried Azithromycin Particles with L-Leucine for Inhalation. Pharm. Res. 2018, 35, 28. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Mi, G.; Hickey, D.; Li, Y.; Tu, J.; Webster, T.J.; Shen, Y. Azithromycin-loaded respirable microparticles for targeted pulmonary delivery for the treatment of pneumonia. Biomaterials 2018, 160, 107–123. [Google Scholar] [CrossRef]
- Meenach, S.A.; Anderson, K.W.; Hilt, J.Z.; McGarry, R.C.; Mansour, H.M. High-Performing Dry Powder Inhalers of Paclitaxel DPPC/DPPG Lung Surfactant-Mimic Multifunctional Particles in Lung Cancer: Physicochemical Characterization, In Vitro Aerosol Dispersion, and Cellular Studies. Am. Assoc. Pharm. Sci. Tech. 2014, 15, 1574–1587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yıldız-Peköz, A.; Ehrhardt, C. Advances in pulmonary drug delivery. Pharmaceutics 2020, 12, 911. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Gladki, E. Dry powder inhalers (DPIs)-A review of device reliability and innovation. Int. J. Pharm. 2008, 360, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Lin, X.; Liu, X.; Tian, B.; Tang, X. High azithromycin loading powders for inhalation and their in vivo evaluation in rats. Int. J. Pharm. 2010, 395, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Healy, A.M.; Amaro, M.I.; Paluch, K.J.; Tajber, L. Dry powders for oral inhalation free of lactose carrier particles. Adv. Drug Deliv. Rev. 2014, 75, 32–52. [Google Scholar] [CrossRef] [PubMed]
- Steipel, R.T.; Gallovic, M.D.; Batty, C.J.; Bachelder, E.M.; Ainslie, K.M. Electrospray for generation of drug delivery and vaccine particles applied in vitro and in vivo. Mater. Sci. Eng. C 2019, 105, 110070. [Google Scholar] [CrossRef] [PubMed]
- Yaqoubi, S.; Adibkia, K.; Nokhodchi, A.; Emami, S.; Alizadeh, A.A.; Hamishehkar, H.; Barzegar-Jalali, M. Co-electrospraying technology as a novel approach for dry powder inhalation formulation of montelukast and budesonide for pulmonary co-delivery. Int. J. Pharm. 2020, 591, 119970. [Google Scholar] [CrossRef]
- Wang, B.; Liu, F.; Xiang, J.; He, Y.; Zhang, Z.; Cheng, Z.; Liu, W.; Tan, S. A critical review of spray-dried amorphous pharmaceuticals: Synthesis, analysis and application. Int. J. Pharm. 2021, 594, 120165. [Google Scholar] [CrossRef]
- National Formulary, United States Pharmacopeia. Validation of Compendial Methods. Twenty-Sixth Revision, 21st ed.; The United States Pharmacopeial Convention Inc.: Rockville, MD, USA, 2003. [Google Scholar]
- Magnusson, B.; Örnemark, U. (Eds.) Eurachem Guide: The Fitness for Purpose of Analytical Methods–A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; 2014; ISBN 978-91-87461-59-0; Available online: https://www.eurachem.org.xn--ivg (accessed on 10 November 2014).
- Pharmacopeial U.S. Physical tests and determinations: Aerosols. In Nasal Sprays, Metered-Dose Inhalers, and Dry Powder Inhalers; Pharmacopeial Forum: Rockville, MD, USA, 2012; pp. 615–628. [Google Scholar]
- Delvadia, R.R.; Wei, X.; Longest, P.W.; Venitz, J.; Byron, P.R. In Vitro Tests for Aerosol Deposition. IV: Simulating Variations in Human Breath Profiles for Realistic DPI Testing. J. Aerosol Med. Pulm. Drug Deliv. 2016, 29, 196–206. [Google Scholar] [CrossRef] [Green Version]
- Champan, K.R.; Fogarty, C.M.; Lassen, C.; Jadayel, D.; Dederichs, J.; Dalvi, M.; Kramer, B. Delivery characteristics and patients’ handling of two single-dose dry-powder inhalers used in COPD. Int. J. COPD 2011, 6, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Byron, P.R.; Wei, X.; Delvadia, R.R.; Longest, P.W.; Venitz, J. Breath Profiles for Testing New DPI Devices in Development. Respir. Drug Deliv. 2014, 295–302. [Google Scholar]
- Jabłczyńska, K.; Janczewska, M.; Kulikowska, A.; Sosnowski, T.R. Preparation and characterization of biocompatible polymer particles as potential nanocarriers for inhalation therapy. Int. J. Polym. Sci. 2015, 2015, 763020. [Google Scholar] [CrossRef]
- Liu, Y.; Moore, J.H.; Kolling, G.L.; McGrath, J.S.; Papin, J.A.; Swami, N.S. Minimum bactericidal concentration of ciprofloxacin to Pseudomonas aeruginosa determined rapidly based on pyocyanin secretion. Sens. Actuators B Chem. 2020, 312, 127936. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.B.; Cockerill, F.R.; Bradford, P.A.; Eliopoulus, G.M.; Hindler, J.A.; Jenkins, S.G.; Lewis, J.S.; Limbago, B.; Miller, L.A.; Nicolau, D.P.; et al. M07—A10: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Tenth Edition; CLSI (Clinical Lab. Standars Institute): Wayne, PA, USA, 2015. [Google Scholar] [CrossRef]
- Kurakula, M.; Naveen, N.R. Electrospraying: A facile technology unfolding the chitosan based drug delivery and biomedical applications. Eur. Polym. J. 2021, 147, 110326. [Google Scholar] [CrossRef]
- Boda, S.K.; Li, X.; Xie, J. Electrospraying an enabling technology for pharmaceutical and biomedical applications: A review. J. Aerosol Sci. 2018, 125, 164–181. [Google Scholar] [CrossRef] [PubMed]
- Adeli, E. The use of spray freeze drying for dissolution and oral bioavailability improvement of Azithromycin. Powder Technol. 2017, 319, 323–331. [Google Scholar] [CrossRef]
- Aboulfotouh, K.; Zhang, Y.; Maniruzzaman, M.; Williams, R.O. Amorphous solid dispersion dry powder for pulmonary drug delivery: Advantages and challenges. Int. J. Pharm. 2020, 587, 119711. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Okuda, T.; Lu, X.Y.; Chan, H.K. Amorphous powders for inhalation drug delivery. Adv. Drug Deliv. Rev. 2016, 100, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Biswadev, B. Killing of Staphylococcus aureus in murine macrophages by chloroquine used alone and in combination with ciprofloxacin or azithromycin. J. Inflamm. Res. 2015, 8, 29–47. [Google Scholar] [PubMed] [Green Version]
- ICRP. Human Respiratory Tract Model for Radiological Protection; ICRP 24; ICRP: Ottawa, ON, Canada, 1994. [Google Scholar]
- Fuentes, F.; Izquierdo, J.; Martín, M.M.; Gomez-Lus, M.L.; Prieto, J. Postantibiotic and sub-MIC effects of azithromycin and isepamicin against Staphylococcus aureus and Escherichia coli. Antimicrob. Agents Chemother. 1998, 42, 414–418. [Google Scholar] [CrossRef] [Green Version]
- Imperi, F.; Leoni, L.; Visca, P. Antivirulence activity of azithromycin in Pseudomonas aeruginosa. Front. Microbiol. 2014, 5, 178. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Zhou, Y.; Qu, Q.; Cui, W.; God’spower, B.O.; Liu, Y.; Chen, X.; Chen, M.; Yang, Y.; Li, Y. Azithromycin inhibits biofilm formation by Staphylococcus xylosus and affects histidine biosynthesis pathway. Front. Pharmacol. 2018, 9, 740. [Google Scholar] [CrossRef]
- Imamura, Y.; Higashiyama, Y.; Tomono, K.; Izumikawa, K.; Yanagihara, K.; Ohno, H.; Miyazaki, Y.; Hirakata, Y.; Mizuta, Y.; Kadota, J.; et al. Azithromycin Exhibits Bactericidal Effects on Pseudomonas aeruginosa through Interaction with the Outer Membrane Azithromycin Exhibits Bactericidal Effects on Pseudomonas aeruginosa through Interaction with the Outer Membrane. Antimicrob. Agents Chemother. 2005, 49, 1377–1380. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Constant Flow | Breath Profile | ||
|---|---|---|---|
| 100 L/min | Healthy | Chronic Obstructive Pulmonary Disease (COPD) | |
| Emitted Dose (ED) (%) | 90.20 ± 0.40 | 86.65 ± 0.75 | 84.85 ± 0.80 |
| Fine Particle Dose (FPD) (mg) | 17.45 ± 1.00 | 12.55 ± 2.70 | 12.95 ± 3.35 |
| Fine Particle Fraction (FPF) (%) | 19.35 ± 1.05 | 14.50 ± 3.05 | 15.30 ± 4.05 |
| Respirable Fraction (RF) | 31.00 ± 4.15 | 29.10 ± 3.75 | 28.30 ± 4.10 |
| Median Mass Aerodynamic Diameter (MMAD) (µm) | 1.55 ± 0.08 | 1.56 ± 0.05 | 1.80 ± 0.10 |
| Dv10 (µm) | Dv50 (µm) | Dv90 (µm) | Span |
|---|---|---|---|
| 2.09 ± 0.06 | 9.96 ± 0.50 | 144.83 ± 16.95 | 14.330 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arauzo, B.; Lopez-Mendez, T.B.; Lobera, M.P.; Calzada-Funes, J.; Pedraz, J.L.; Santamaria, J. Excipient-Free Inhalable Microparticles of Azithromycin Produced by Electrospray: A Novel Approach to Direct Pulmonary Delivery of Antibiotics. Pharmaceutics 2021, 13, 1988. https://doi.org/10.3390/pharmaceutics13121988
Arauzo B, Lopez-Mendez TB, Lobera MP, Calzada-Funes J, Pedraz JL, Santamaria J. Excipient-Free Inhalable Microparticles of Azithromycin Produced by Electrospray: A Novel Approach to Direct Pulmonary Delivery of Antibiotics. Pharmaceutics. 2021; 13(12):1988. https://doi.org/10.3390/pharmaceutics13121988
Chicago/Turabian StyleArauzo, Beatriz, Tania B. Lopez-Mendez, Maria Pilar Lobera, Javier Calzada-Funes, Jose Luis Pedraz, and Jesus Santamaria. 2021. "Excipient-Free Inhalable Microparticles of Azithromycin Produced by Electrospray: A Novel Approach to Direct Pulmonary Delivery of Antibiotics" Pharmaceutics 13, no. 12: 1988. https://doi.org/10.3390/pharmaceutics13121988