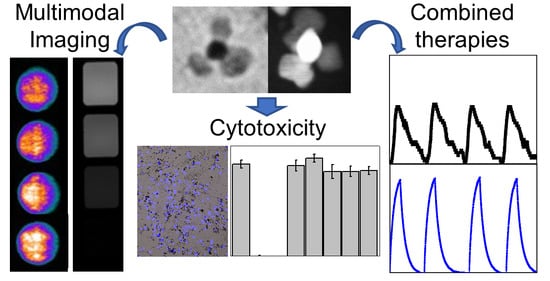
Iron–Gold Nanoflowers: A Promising Tool for Multimodal Imaging and Hyperthermia Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Iron–Gold Nanoflowers
2.2.1. Synthesis of Iron Oleate
2.2.2. Synthesis of Au Seeds
2.2.3. Synthesis of Au@Fe NPs
2.3. Functionalization of Au@Fe NPs
2.4. Characterization
2.5. Cytotoxicity Assays
2.5.1. Cell Culture
2.5.2. Cytotoxicity Assays
2.6. Statistical Analysis
3. Results and Discussion
3.1. Synthesis and Characterization of Au@FeNPs
3.2. In Vitro Cytotoxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fass, L. Imaging and cancer: A review. Mol. Oncol. 2008, 2, 115–152. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Martin, A.; Stanway, S.; Johnston, S.R.; Constantinidou, A. Imaging in oncology—Over a century of advances. Nat. Rev. Clin. Oncol. 2012, 9, 728–737. [Google Scholar] [CrossRef] [PubMed]
- Siddique, S.; Chow, J.C.L. Application of Nanomaterials in Biomedical Imaging and Cancer Therapy. Nanomaterials 2020, 10, 1700. [Google Scholar] [CrossRef]
- Caro, C.; Garcia-Martin, M.L.; Pernia Leal, M. Manganese-Based Nanogels as pH Switches for Magnetic Resonance Imaging. Biomacromolecules 2017, 18, 1617–1623. [Google Scholar] [CrossRef]
- Rogosnitzky, M.; Branch, S. Gadolinium-based contrast agent toxicity: A review of known and proposed mechanisms. Biometals 2016, 29, 365–376. [Google Scholar] [CrossRef] [Green Version]
- Pasternak, J.J.; Williamson, E.E. Clinical pharmacology, uses, and adverse reactions of iodinated contrast agents: A primer for the non-radiologist. Mayo Clin. Proc. 2012, 87, 390–402. [Google Scholar] [CrossRef] [Green Version]
- Caro, C.; Dalmases, M.; Figuerola, A.; Garcia-Martin, M.L.; Leal, M.P. Highly water-stable rare ternary Ag-Au-Se nanocomposites as long blood circulation time X-ray computed tomography contrast agents. Nanoscale 2017, 9, 7242–7251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Nurgali, K.; Jagoe, R.T.; Abalo, R. Editorial: Adverse Effects of Cancer Chemotherapy: Anything New to Improve Tolerance and Reduce Sequelae? Front. Pharmacol. 2018, 9, 245. [Google Scholar] [CrossRef]
- van Rhoon, G.C.; Franckena, M.; Ten Hagen, T.L.M. A moderate thermal dose is sufficient for effective free and TSL based thermochemotherapy. Adv. Drug. Deliv. Rev. 2020, 163–164, 145–156. [Google Scholar] [CrossRef]
- Szasz, A.M.; Minnaar, C.A.; Szentmartoni, G.; Szigeti, G.P.; Dank, M. Review of the Clinical Evidences of Modulated Electro-Hyperthermia (mEHT) Method: An Update for the Practicing Oncologist. Front. Oncol. 2019, 9, 1012. [Google Scholar] [CrossRef] [Green Version]
- Payne, M.; Bossmann, S.H.; Basel, M.T. Direct treatment versus indirect: Thermo-ablative and mild hyperthermia effects. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1638. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Fiorentini, G.; Szasz, A.M.; Szigeti, G.; Szasz, A.; Minnaar, C.A. Quo Vadis Oncological Hyperthermia (2020)? Front. Oncol. 2020, 10, 1690. [Google Scholar] [CrossRef] [PubMed]
- Bettaieb, A.; Wrzal, P.; Averill-Bates, D. Hyperthermia: Cancer Treatment and Beyond. In Cancer Treatment: Conventional and Innovative Approaches; Rangel, L., Ed.; IntechOpen: London, UK, 2013. [Google Scholar]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [Green Version]
- Iyer, A.K.; Khaled, G.; Fang, J.; Maeda, H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov. Today 2006, 11, 812–818. [Google Scholar] [CrossRef]
- Fornaguera, C.; Garcia-Celma, M.J. Personalized Nanomedicine: A Revolution at the Nanoscale. J. Pers. Med. 2017, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pernia Leal, M.; Caro, C.; Garcia-Martin, M.L. Shedding light on zwitterionic magnetic nanoparticles: Limitations for in vivo applications. Nanoscale 2017, 9, 8176–8184. [Google Scholar] [CrossRef] [PubMed]
- Caro, C.; Egea-Benavente, D.; Polvillo, R.; Royo, J.L.; Pernia Leal, M.; Garcia-Martin, M.L. Comprehensive Toxicity Assessment of PEGylated Magnetic Nanoparticles for in vivo applications. Colloids Surf. B Biointerfaces 2019, 177, 253–259. [Google Scholar] [CrossRef]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; Garcia-Martin, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 40. [Google Scholar] [CrossRef]
- Besenhard, M.O.; Panariello, L.; Kiefer, C.; LaGrow, A.P.; Storozhuk, L.; Perton, F.; Begin, S.; Mertz, D.; Thanh, N.T.K.; Gavriilidis, A. Small iron oxide nanoparticles as MRI T1 contrast agent: Scalable inexpensive water-based synthesis using a flow reactor. Nanoscale 2021, 13, 8795–8805. [Google Scholar] [CrossRef]
- Guardia, P.; Di Corato, R.; Lartigue, L.; Wilhelm, C.; Espinosa, A.; Garcia-Hernandez, M.; Gazeau, F.; Manna, L.; Pellegrino, T. Water-soluble iron oxide nanocubes with high values of specific absorption rate for cancer cell hyperthermia treatment. ACS Nano 2012, 6, 3080–3091. [Google Scholar] [CrossRef] [PubMed]
- Nica, V.; Caro, C.; Paez-Munoz, J.M.; Leal, M.P.; Garcia-Martin, M.L. Bi-Magnetic Core-Shell CoFe2O4@MnFe2O4 Nanoparticles for In Vivo Theranostics. Nanomaterials 2020, 10, 907. [Google Scholar] [CrossRef]
- Hakami, T.M.; Davarpanah, A.M.; Rahdar, A.; Barrett, S.D. Structural and magnetic study and cytotoxicity evaluation of tetra-metallic nanoparticles of Co0.5Ni0.5CrxFe2−xO4 prepared by co-precipitation. J. Mol. Struct. 2018, 1165, 344–348. [Google Scholar] [CrossRef]
- Storozhuk, L.; Besenhard, M.O.; Mourdikoudis, S.; LaGrow, A.P.; Lees, M.R.; Tung, L.D.; Gavriilidis, A.; Thanh, N.T.K. Stable Iron Oxide Nanoflowers with Exceptional Magnetic Heating Efficiency: Simple and Fast Polyol Synthesis. ACS Appl. Mater. Interfaces 2021, 13, 45870–45880. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neurooncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Nardecchia, S.; Sánchez-Moreno, P.; de Vicente, J.; Marchal, J.A.; Boulaiz, H. Clinical Trials of Thermosensitive Nanomaterials: An Overview. Nanomaterials 2019, 9, 191. [Google Scholar] [CrossRef] [Green Version]
- Hassanisaadi, M.; Bonjar, G.H.S.; Rahdar, A.; Pandey, S.; Hosseinipour, A.; Abdolshahi, R. Environmentally Safe Biosynthesis of Gold Nanoparticles Using Plant Water Extracts. Nanomaterials 2021, 11, 2033. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Wang, X.; Burda, C.; Basilion, J.P. Recent Development of Gold Nanoparticles as Contrast Agents for Cancer Diagnosis. Cancers 2021, 13, 1825. [Google Scholar] [CrossRef]
- Ferber, S.; Baabur-Cohen, H.; Blau, R.; Epshtein, Y.; Kisin-Finfer, E.; Redy, O.; Shabat, D.; Satchi-Fainaro, R. Polymeric nanotheranostics for real-time non-invasive optical imaging of breast cancer progression and drug release. Cancer Lett. 2014, 352, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Suo, Y.; Shi, H.; Liu, R.; Wu, F.; Wang, T.; Ma, L.; Liu, H.; Cheng, Z. Deep-Tissue Photothermal Therapy Using Laser Illumination at NIR-IIa Window. Nanomicro Lett. 2020, 12, 38. [Google Scholar] [CrossRef]
- Vines, J.B.; Yoon, J.H.; Ryu, N.E.; Lim, D.J.; Park, H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7, 167. [Google Scholar] [CrossRef] [Green Version]
- Efremova, M.V.; Naumenko, V.A.; Spasova, M.; Garanina, A.S.; Abakumov, M.A.; Blokhina, A.D.; Melnikov, P.A.; Prelovskaya, A.O.; Heidelmann, M.; Li, Z.A.; et al. Magnetite-Gold nanohybrids as ideal all-in-one platforms for theranostics. Sci. Rep. 2018, 8, 11295. [Google Scholar] [CrossRef] [Green Version]
- Caro, C.; Gamez, F.; Quaresma, P.; Paez-Munoz, J.M.; Dominguez, A.; Pearson, J.R.; Pernia Leal, M.; Beltran, A.M.; Fernandez-Afonso, Y.; De la Fuente, J.M.; et al. Fe3O4-Au Core-Shell Nanoparticles as a Multimodal Platform for In Vivo Imaging and Focused Photothermal Therapy. Pharmaceutics 2021, 13, 416. [Google Scholar] [CrossRef]
- Efremova, M.V.; Nalench, Y.A.; Myrovali, E.; Garanina, A.S.; Grebennikov, I.S.; Gifer, P.K.; Abakumov, M.A.; Spasova, M.; Angelakeris, M.; Savchenko, A.G.; et al. Size-selected Fe3O4-Au hybrid nanoparticles for improved magnetism-based theranostics. Beilstein J. Nanotechnol. 2018, 9, 2684–2699. [Google Scholar] [CrossRef] [Green Version]
- Mirrahimi, M.; Hosseini, V.; Kamrava, S.K.; Attaran, N.; Beik, J.; Kooranifar, S.; Ghaznavi, H.; Shakeri-Zadeh, A. Selective heat generation in cancer cells using a combination of 808 nm laser irradiation and the folate-conjugated Fe2O3@Au nanocomplex. Artif. Cells Nanomed. Biotechnol. 2018, 46, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Hadi, F.; Tavakkol, S.; Laurent, S.; Pirhajati, V.; Mahdavi, S.R.; Neshastehriz, A.; Shakeri-Zadeh, A. Combinatorial effects of radiofrequency hyperthermia and radiotherapy in the presence of magneto-plasmonic nanoparticles on MCF-7 breast cancer cells. J. Cell Physiol. 2019, 234, 20028–20035. [Google Scholar] [CrossRef] [PubMed]
- Yankeelov, T.E.; Abramson, R.G.; Quarles, C.C. Quantitative multimodality imaging in cancer research and therapy. Nat. Rev. Clin. Oncol. 2014, 11, 670–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zhang, X.N.; Li, X.D.; Chang, J. Multimodality imaging in nanomedicine and nanotheranostics. Cancer Biol. Med. 2016, 13, 339–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef] [Green Version]
- Hu, C.M.; Aryal, S.; Zhang, L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther. Deliv. 2010, 1, 323–334. [Google Scholar] [CrossRef]
- Mukha, I.; Chepurna, O.; Vityuk, N.; Khodko, A.; Storozhuk, L.; Dzhagan, V.; Zahn, D.R.T.; Ntziachristos, V.; Chmyrov, A.; Ohulchanskyy, T.Y. Multifunctional Magneto-Plasmonic Fe3O4/Au Nanocomposites: Approaching Magnetophoretically-Enhanced Photothermal Therapy. Nanomaterials 2021, 11, 1113. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Cheng, K.; Qi, S.; Liu, H.; Jiang, Y.; Jiang, H.; Li, J.; Chen, K.; Zhang, H.; Cheng, Z. Affibody modified and radiolabeled gold-iron oxide hetero-nanostructures for tumor PET, optical and MR imaging. Biomaterials 2013, 34, 2796–2806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; An, K.; Hwang, Y.; Park, J.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Tancredi, P.; da Costa, L.S.; Calderon, S.; Moscoso-Londoño, O.; Socolovsky, L.M.; Ferreira, P.J.; Muraca, D.; Zanchet, D.; Knobel, M. Exploring the synthesis conditions to control the morphology of gold-iron oxide heterostructures. Nano Res. 2019, 12, 1781–1788. [Google Scholar] [CrossRef]
- Pozo-Torres, E.; Caro, C.; Avasthi, A.; Paez-Munoz, J.M.; Garcia-Martin, M.L.; Fernandez, I.; Pernia Leal, M. Clickable iron oxide NPs based on catechol derived ligands: Synthesis and characterization. Soft Matter 2020, 16, 3257–3266. [Google Scholar] [CrossRef]
- Thanh, N.T.; Maclean, N.; Mahiddine, S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Baek, K.H. Novel green synthesis of gold nanoparticles using Citrullus lanatus rind and investigation of proteasome inhibitory activity, antibacterial, and antioxidant potential. Int. J. Nanomed. 2015, 10, 7253–7264. [Google Scholar] [CrossRef] [Green Version]
- Kurien, U.; Hu, Z.; Lee, H.; Dastoor, A.P.; Ariya, P.A. Radiation enhanced uptake of Hg0(g) on iron (oxyhydr)oxide nanoparticles. RSC Adv. 2017, 7, 45010–45021. [Google Scholar] [CrossRef] [Green Version]
- Amendola, V.; Pilot, R.; Frasconi, M.; Marago, O.M.; Iati, M.A. Surface plasmon resonance in gold nanoparticles: A review. J. Phys. Condens. Matter 2017, 29, 203002. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Campbell, J.; Burkitt, S.; Dong, N.; Zavaleta, C. Chapter 9—Nanoparticle characterization techniques. In Nanoparticles for Biomedical Applications; Chung, E.J., Leon, L., Rinaldi, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 129–144. [Google Scholar] [CrossRef]
- Umut, E.; Pineider, F.; Arosio, P.; Sangregorio, C.; Corti, M.; Tabak, F.; Lascialfari, A.; Ghigna, P. Magnetic, optical and relaxometric properties of organically coated gold–magnetite (Au–Fe3O4) hybrid nanoparticles for potential use in biomedical applications. J. Magn. Magn. Mater. 2012, 324, 2373–2379. [Google Scholar] [CrossRef]
- Zhang, X.; Blasiak, B.; Marenco, A.J.; Trudel, S.; Tomanek, B.; van Veggel, F.C.J.M. Design and Regulation of NaHoF4 and NaDyF4 Nanoparticles for High-Field Magnetic Resonance Imaging. Chem. Mater. 2016, 28, 3060–3072. [Google Scholar] [CrossRef]
- Fernandez-Barahona, I.; Gutierrez, L.; Veintemillas-Verdaguer, S.; Pellico, J.; Morales, M.D.P.; Catala, M.; Del Pozo, M.A.; Ruiz-Cabello, J.; Herranz, F. Cu-Doped Extremely Small Iron Oxide Nanoparticles with Large Longitudinal Relaxivity: One-Pot Synthesis and in Vivo Targeted Molecular Imaging. ACS Omega 2019, 4, 2719–2727. [Google Scholar] [CrossRef] [Green Version]
- Nyborg, W.L. Solutions of the bio-heat transfer equation. Phys. Med. Biol. 1988, 33, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale Heat Transfer Transduced by Surface Plasmon Resonant Gold Nanoparticles. J. Phys. Chem. C Nanomater. Interfaces 2007, 111, 3636–3641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leon Felix, L.; Sanz, B.; Sebastian, V.; Torres, T.E.; Sousa, M.H.; Coaquira, J.A.H.; Ibarra, M.R.; Goya, G.F. Gold-decorated magnetic nanoparticles design for hyperthermia applications and as a potential platform for their surface-functionalization. Sci. Rep. 2019, 9, 4185. [Google Scholar] [CrossRef] [Green Version]
- Castellanos-Rubio, I.; Arriortua, O.; Iglesias-Rojas, D.; Baron, A.; Rodrigo, I.; Marcano, L.; Garitaonandia, J.S.; Orue, I.; Fdez-Gubieda, M.L.; Insausti, M. A Milestone in the Chemical Synthesis of Fe3O4 Nanoparticles: Unreported Bulklike Properties Lead to a Remarkable Magnetic Hyperthermia. Chem. Mater. 2021, 33, 8693–8704. [Google Scholar] [CrossRef]
- Lee, J.H.; Jang, J.T.; Choi, J.S.; Moon, S.H.; Noh, S.H.; Kim, J.W.; Kim, J.G.; Kim, I.S.; Park, K.I.; Cheon, J. Exchange-coupled magnetic nanoparticles for efficient heat induction. Nat. Nanotechnol. 2011, 6, 418–422. [Google Scholar] [CrossRef]
- Reyes-Ortega, F.; Checa Fernandez, B.L.; Delgado, A.V.; Iglesias, G.R. Hyperthermia-Triggered Doxorubicin Release from Polymer-Coated Magnetic Nanorods. Pharmaceutics 2019, 11, 517. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, S.; Fernandez, T.; Metze, S.; Balakrishnan, P.B.; Mai, B.T.; Conteh, J.; De Mei, C.; Turdo, A.; Di Franco, S.; Stassi, G.; et al. Magnetic Nanoparticle-Based Hyperthermia Mediates Drug Delivery and Impairs the Tumorigenic Capacity of Quiescent Colorectal Cancer Stem Cells. ACS Appl. Mater. Interfaces 2021, 13, 15959–15972. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christou, E.; Pearson, J.R.; Beltrán, A.M.; Fernández-Afonso, Y.; Gutiérrez, L.; de la Fuente, J.M.; Gámez, F.; García-Martín, M.L.; Caro, C. Iron–Gold Nanoflowers: A Promising Tool for Multimodal Imaging and Hyperthermia Therapy. Pharmaceutics 2022, 14, 636. https://doi.org/10.3390/pharmaceutics14030636
Christou E, Pearson JR, Beltrán AM, Fernández-Afonso Y, Gutiérrez L, de la Fuente JM, Gámez F, García-Martín ML, Caro C. Iron–Gold Nanoflowers: A Promising Tool for Multimodal Imaging and Hyperthermia Therapy. Pharmaceutics. 2022; 14(3):636. https://doi.org/10.3390/pharmaceutics14030636
Chicago/Turabian StyleChristou, Evangelia, John R. Pearson, Ana M. Beltrán, Yilian Fernández-Afonso, Lucía Gutiérrez, Jesús M. de la Fuente, Francisco Gámez, María L. García-Martín, and Carlos Caro. 2022. "Iron–Gold Nanoflowers: A Promising Tool for Multimodal Imaging and Hyperthermia Therapy" Pharmaceutics 14, no. 3: 636. https://doi.org/10.3390/pharmaceutics14030636