Chitosan-Hyaluronan Nanoparticles for Vinblastine Sulfate Delivery: Characterization and Internalization Studies on K-562 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hydrolysis of Hyaluronic Acid
2.3. Purification of Chitosan
2.4. Preparation of Labelled Bacterial Lysate
2.5. Preparation of CS NPs Gelled with TPP and Coated with HY
2.6. Preparation of CS NPs by Polyelectrolyte Complexation with HY
2.7. Preparation of CS/HY NPs Loading VBL and CS/HY NPs Loading Labeled L. reuteri
2.8. Determination of Sizes and Surface Electrical Properties of NPs
2.9. Morphological Characterization of NPs
2.10. Molecular Characterization of Chemical and Physical Interactions in NPs
2.11. In Vitro VBL Release from NPs
2.12. In Vitro Biological Assays
2.12.1. Cell Cultures
2.12.2. Cell Internationalization Studies
- K-562 cells supplemented with unlabeled CS/HY NPs (negative control) or labeled CS/HY NPs;
- K-562 cells pretreated with an excess of HY (10 times higher than that used for the preparation of the NPs), left to incubate for 1 h, washed with PBS (pH, 7.4), and then supplemented of labeled CS/HY NPs;
- K-562 cells supplemented with unlabeled CS/TPP NPs (negative control) or labeled CS/TPP NPs;
- K-562 cells in RPMI 1640 culture medium (control);
- ZR-75-1 cells supplemented with unlabeled CS/HY NPs (negative control) or labeled CS/HY NPs;
- ZR-75-1 cells in RPMI 1640 culture medium (control).
2.12.3. Intracytoplasmic Staining of K562 Cells
2.12.4. Evaluation of Cytotoxic Activity
2.12.5. Statistical Analysis
3. Results and Discussion
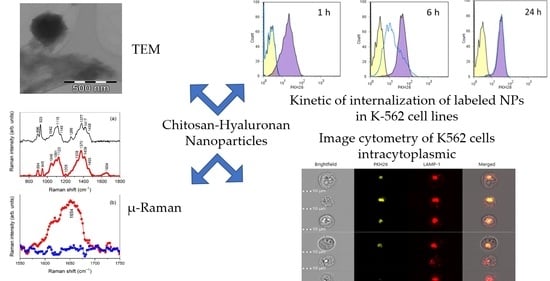
3.1. Dimensional and Morphological Characterizations of NPs
3.2. Molecular Characterization
3.3. Characterization of CS/HY NPs Loading VBL
3.4. In Vitro Cytotoxic Activity
3.5. Internalization Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keskin, D.; Tezcaner, A. Micelles As Delivery System for Cancer Treatment. Curr. Pharm. Des. 2017, 23, 5230–5241. [Google Scholar] [CrossRef] [PubMed]
- Olusanya, T.O.B.; Haj Ahmad, R.R.; Ibegbu, D.M.; Smith, J.R.; Elkordy, A.A. Liposomal Drug Delivery Systems and Anticancer Drugs. Molecules 2018, 23, 907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parveen, S.; Sahoo, S.K. Polymeric nanoparticles for cancer therapy. J. Drug Target. 2008, 16, 108–123. [Google Scholar] [CrossRef]
- Paus, C.; Van Der Voort, R.; Cambi, A. Nanomedicine in cancer therapy: Promises and hurdles of polymeric nanoparticles. Explor. Med. 2021, 2, 167–185. [Google Scholar] [CrossRef]
- Karlsson, J.; Vaughan, H.J.; Green, J.J. Biodegradable Polymeric Nanoparticles for Therapeutic Cancer Treatments. Annu. Rev. Chem. Biomol. Eng. 2018, 9, 105–127. [Google Scholar] [CrossRef]
- Sacco, P.; Pedroso-Santana, S.; Kumar, Y.; Joly, N.; Martin, P.; Bocchetta, P. Ionotropic Gelation of Chitosan Flat Structures and Potential Applications. Molecules 2021, 26, 660. [Google Scholar] [CrossRef]
- Pedroso-Santana, S.; Fleitas-Salazar, N. Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Polym. Int. 2020, 69, 443–447. [Google Scholar] [CrossRef]
- Rodrigues, S.; Dionísio, M.; López, C.R.; Grenha, A. Biocompatibility of chitosan carriers with application in drug delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef] [Green Version]
- Suh, J.K.F.; Matthew, H.W. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [CrossRef] [PubMed]
- Lončarević, A.; Ivanković, M.; Rogina, A. Lysozyme-induced degradation of chitosan: The characterisation of degraded chitosan scaffolds. J. Tissue Repair Regen. 2017, 1, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Desai, K.G. Chitosan Nanoparticles Prepared by Ionotropic Gelation: An Overview of Recent Advances. Crit. Rev. Ther. Drug Carrier Syst. 2016, 33, 107–158. [Google Scholar] [CrossRef] [PubMed]
- Trapani, A.; De Giglio, E.; Cafagna, D.; Denora, N.; Agrimi, G.; Cassano, T.; Gaetano, S.; Cuomo, V.; Trapani, G. Characterization and evaluation of chitosan nanoparticles for dopamine brain delivery. Int. J. Pharm. 2011, 419, 296–307. [Google Scholar] [CrossRef] [PubMed]
- De Gaetano, F.; Marino, A.; Marchetta, A.; Bongiorno, C.; Zagami, R.; Cristiano, M.C.; Paolino, D.; Pistarà, V.; Ventura, C.A. Development of Chitosan/Cyclodextrin Nanospheres for Levofloxacin Ocular Delivery. Pharmaceutics 2021, 13, 1293. [Google Scholar] [CrossRef] [PubMed]
- Zaki, N.M.; Nasti, A.; Tirelli, N. Nanocarriers for cytoplasmic delivery: Cellular uptake and intracellular fate of chitosan and hyaluronic acid-coated chitosan nanoparticles in a phagocytic cell model. Macromol. Biosci. 2011, 11, 1747–1760. [Google Scholar] [CrossRef]
- Furlani, F.; Donati, I.; Marsich, E.; Sacco, P. Characterization of Chitosan/Hyaluronan Complex Coacervates Assembled by Varying Polymers Weight Ratio and Chitosan Physical-Chemical Composition. Colloids Interfaces 2020, 4, 12. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, J.; Quispe, C.; Butnariu, M.; Rotariu, L.S.; Syta, O.; Sestito, S.; Rapposell, S.; Akram, M.; Iqbal, M.; Krishna, A.; et al. Chitosan nanoparticles as a promising tool in nanomedicine with particular emphasis on oncological treatment. Cancer Cell Int. 2021, 21, 318–339. [Google Scholar] [CrossRef]
- De La Fuente, M.; Seijo, B.; Alonso, M.J. Bioadhesive hyaluronan-chitosan nanoparticles can transport genes across the ocular mucosa and transfect ocular tissue. Gene. Ther. 2008, 15, 668–676. [Google Scholar] [CrossRef] [Green Version]
- Culty, M.; Nguyen, H.A.; Underhill, C.B. The hyaluronan receptor (CD44) participates in the uptake and degradation of hyaluronan. J. Cell Biol. 1992, 116, 1055–1062. [Google Scholar] [CrossRef] [Green Version]
- Platt, V.M.; Szoka, F.C., Jr. Anticancer therapeutics: Targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol. Pharm. 2008, 5, 474–486. [Google Scholar] [CrossRef] [Green Version]
- Akentieva, N.P.; Gizatullin, A.R.; Silvestre, O.; Savchuk, O.; Shkondina, N.I.; Prichodchenko, T.P.; Mitschenko, D.V.; Zhilenkov, A.V.; Troshin, P.A.; Sanina, N.A.; et al. Development of chitosan-hyaluronic acid nanoparticles and study of their physico-chemical properties for targeted delivery of anticancer drugs. IOP Conf. Ser. Mater. Sci. Eng. 2020, 848, 012002–012010. [Google Scholar] [CrossRef]
- Wickens, J.M.; Alsaab, H.O.; Kesharwani, P.; Bhise, K.; Amin, M.C.I.M.; Tekade, R.K.; Gupta, U.; Yier, A.R. Recent advances in hyaluronic acid-decorated nanocarriers for targeted cancer therapy. Drug Discov. Today 2017, 22, 665–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.Y.; Park, S.J.; Park, K.; Kim, K.S.; Lee, H.; Hahn, S.K. Target-specific gene silencing of layer-by-layer assembled gold-cysteamine/siRNA/PEI/HA nanocomplex. ACS Nano 2011, 5, 6138–6147. [Google Scholar] [CrossRef] [PubMed]
- Lallana, E.; Rios De La Rosa, J.M.; Tirella, A.; Pelliccia, M.; Gennari, A.; Stratford, I.J.; Puri, S.; Ashford, M.; Tirelli, N. Chitosan/Hyaluronic Acid Nanoparticles: Rational Design Revisited for RNA Delivery. Mol. Pharm. 2017, 14, 2422–2436. [Google Scholar] [CrossRef]
- Choi, K.Y.; Chung, H.; Min, K.H.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Jeong, S.Y. Self-assembled hyaluronic acid nanoparticles for active tumor targeting. Biomaterials 2010, 31, 106–114. [Google Scholar] [CrossRef]
- Martino, E.; Casamassima, G.; Castiglione, S.; Cellupica, E.; Pantalone, S.; Papagni, F.; Rui, M.; Siciliano, A.M.; Collina, S. Vinca alkaloids and analogues as anti-cancer agents: Looking back, peering ahead. Bioorg. Med. Chem. Lett. 2018, 28, 2816–2826. [Google Scholar] [CrossRef]
- Lucas, D.M.; Still, P.C.; Pérez, L.B.; Grever, M.R.; Kinghorn, A.D. Potential of plant-derived natural products in the treatment of leukemia and lymphoma. Curr. Drug Targets 2010, 11, 812–822. [Google Scholar] [CrossRef] [PubMed]
- Chand, V.K.; Link, B.K.; Ritchie, J.M.; Shannon, M.; Wooldridge, J.E. Neutropenia and febrile neutropenia in patients with Hodgkin’s lymphoma treated with doxorubicin (Adriamycin), bleomycin, vinblastine and dacarbazine (ABVD) chemotherapy. Leuk. Lymphoma 2006, 47, 657–663. [Google Scholar] [CrossRef]
- Makinson, A.; Martelli, N.; Peyrière, H.; Turriere, C.; Le Moing, V.; Reynes, J. Profound neutropenia resulting from interaction between antiretroviral therapy and vinblastine in a patient with HIV-associated Hodgkin’s disease. Eur. J. Haematol. 2007, 78, 358–360. [Google Scholar] [CrossRef]
- Zu, Y.; Zhang, Y.; Zhao, X.; Zhang, Q.; Liu, Y.; Jiang, R. Optimization of the preparation process of vinblastine sulfate (VBLS)-loaded folate-conjugated bovine serum albumin (BSA) nanoparticles for tumor-targeted drug delivery using response surface methodology (RSM). Int. J. Nanomed. 2009, 4, 321–333. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Liu, R.; Huang, H.; Zhu, Q. Vinblastine-Loaded Nanoparticles with Enhanced Tumor-Targeting Efficiency and Decreasing Toxicity: Developed by One-Step Molecular Imprinting Process. Mol. Pharm. 2019, 16, 2675–2689. [Google Scholar] [CrossRef]
- Zu, Y.; Zhao, Q.; Zhao, X.; Zu, S.; Meng, L. Process optimization for the preparation of oligomycin-loaded folate-conjugated chitosan nanoparticles as a tumor-targeted drug delivery system using a two-level factorial design method. Int. J. Nanomed. 2011, 6, 3429–3441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tømmeraas, K.; Melander, C. Kinetics of Hyaluronan Hydrolysis in Acidic Solution at Various pH Values. Biomacromolecules 2008, 9, 1535–1540. [Google Scholar] [CrossRef] [PubMed]
- Nasti, A.; Zaki, N.M.; De Leonardis, P.; Ungphaiboon, S.; Sansongsak, P.; Rimoli, M.G.; Tirelli, N. Chitosan/TPP and Chitosan/TPP-hyaluronic acid nanoparticles: Systematic optimisation of the preparative process and preliminary biological evaluation. Pharm. Res. 2009, 26, 1918–1930. [Google Scholar] [CrossRef] [PubMed]
- Culty, M.; Shizari, M.; Thompson, E.W.; Underhill, C.B. Binding and degradation of hyaluronan by human breast cancer cell lines expressing different forms of CD44: Correlation with invasive potential. J. Cell Physiol. 1994, 160, 275–286. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Gennari, A.; Rios De La Rosa, J.M.; Hohn, E.; Pelliccia, M.; Lallana, E.; Donno, R.; Tirella, A.; Tirelli, N. The different ways to chitosan/hyaluronic acid nanoparticles: Templated vs direct complexation. Influence of particle preparation on morphology, cell uptake and silencing efficiency. Beilstein J. Nanotechnol. 2019, 10, 2594–2608. [Google Scholar] [CrossRef] [Green Version]
- Cannavà, C.; Stancanelli, R.; Marabeti, M.R.; Venuti, V.; Cascio, C.; Guarneri, P.; Bongiorno, C.; Sortino, G.; Majolino, D.; Mazzaglia, A.; et al. Nanospheres based on PLGA/amphiphilic cyclodextrin assemblies as potential enhancers of Methylene Blue neuroprotective effect. RSC Adv. 2016, 6, 16720–16729. [Google Scholar] [CrossRef]
- Kotzianova, A.; Rebicek, J.; Zidek, O.; Pokorny, M.; Hrbac, J.; Velebny, V. Raman spectroscopy based method for the evaluation of compositional consistency of nanofibrous layers. Anal. Methods 2015, 7, 9900–9905. [Google Scholar] [CrossRef]
- Essendoubi, M.; Gobinet, C.; Reynaud, R.; Angiboust, J.F.; Manfait, M.; Piot, O. Human skin penetration of hyaluronic acid of different molecular weights as probed by Raman spectroscopy. Skin Res. Technol. 2016, 22, 55–62. [Google Scholar] [CrossRef]
- Gieroba, B.; Sroka-Bartnicka, A.; Kazimierczak, P.; Kalisz, G.; Lewalska-Graczyk, A.; Vivcharenko, V.; Nowakowski, R.; Pieta, I.S.; Przekora, A. Spectroscopic studies on the temperature-dependent molecular arrangements in hybrid chitosan/1,3-β-D-glucan polymeric matrices. Int. J. Biol. Macromol. 2020, 159, 911–921. [Google Scholar] [CrossRef]
- Ouasti, S.; Kingham, P.J.; Terenghi, G.; Tirelli, N. The CD44/integrins interplay and the significance of receptor binding and re-presentation in the uptake of RGD-functionalized hyaluronic acid. Biomaterials 2012, 33, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Heldin, P.; Hascall, V.C.; Karamanos, N.K.; Skandalis, S.S.; Markwald, R.R.; Ghatak, S. Hyaluronan-CD44 interactions as potential targets for cancer therapy. FEBS J. 2011, 278, 1429–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knudson, W.; Chow, G.; Knudson, C.B. CD44-mediated uptake and degradation of hyaluronan. Matrix Biol. 2002, 21, 15–23. [Google Scholar] [CrossRef]
- Tammi, R.; Rilla, K.; Pienimaki, J.P.; MacCallum, D.K.; Hogg, M.; Luukkonen, M.; Hascall, V.C.; Tammi, M. Hyaluronan enters keratinocytes by a novel endocytic route for catabolism. J. Biol. Chem. 2001, 276, 35111–35122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lokeshwar, V.B.; Fregien, N.; Bourguignon, L.Y. Ankyrin-binding domain of CD44(GP85) is required for the expression of hyaluronic acid-mediated adhesion function. J. Cell Biol. 1994, 126, 1099–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundell, B.I.; McCarthy, J.B.; Kovach, N.L.; Verfaillie, C.M. Activation of beta1 integrins on CML progenitors reveals cooperation between beta1 integrins and CD44 in the regulation of adhesion and proliferation. Leukemia 1997, 11, 822–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakizaki, I.; Ibori, N.; Kojima, K.; Yamaguchi, M.; Endo, M. Mechanism for the hydrolysis of hyaluronan oligosaccharides by bovine testicular hyaluronidase. FEBS J. 2010, 277, 1776–1786. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Li, Z.; Qiao, M.; Long, M.; Wang, M.; Zhang, X.; Tian, C.; Chen, D. Self-assembled pH-responsive hyaluronic acid-g-poly((L)-histidine) copolymer micelles for targeted intracellular delivery of doxorubicin. Acta Biomater. 2014, 10, 2024–2035. [Google Scholar] [CrossRef]











| Samples | CS (mg) | TPP (mg) |
|---|---|---|
| 1 | 4 | 1 |
| 2 | 4 | 2 |
| 3 | 4 | 4 |
| 4 | 3 | 1 |
| 5 | 2 | 1 |
| 6 | 1 | 1 |
| Samples | mg | RH (nm) ± S.D. | P.I. (%) | ζ (mV) ± S.D. | |
|---|---|---|---|---|---|
| 1 | CS | 4 | 180.54 ± 2.89 | 22 ± 2 | +26.54 ± 2.41 |
| TPP | 1 | ||||
| 2 | CS | 4 | 162.71 ± 5.41 | 79 ± 5 | −21.85 ± 2.31 |
| TPP | 1 | ||||
| HY | 0.5 | ||||
| 3 | CS | 2.82 | 110.23 ± 3.15 | 12 ± 3 | −13.51 ± 1.85 |
| HY | 0.56 | ||||
| Samples | Yield (%) | RH (nm) ± S.D. | P.I. (%) | E.E. (%) | D.C. (%) | ζ (mV) ± S.D. |
|---|---|---|---|---|---|---|
| Empty NPs | 75.02 ± 16.52 | 110.23 ± 3.15 | 12 ± 3 | -- | -- | −13.51 ± 1.85 |
| 5% VBL-CS/HY NPs | 69.74 ± 12.30 | 115.65 ± 9.87 | 16 ± 1 | 37.78 ± 2.89 | 2.41 ± 5.60 | −12.11 ± 0.87 |
| 15% VBL-CS/HY NPs | 71.00 ± 21.56 | 122.87 ± 10.22 | 15 ± 1 | 56.23 ± 3.54 | 7.45 ± 3.21 | −10.05 ± 0.56 |
| 20% VBL-CS/HY NPs | 55.78 ± 10.02 | 330.75 ± 25.89 | 15 ± 5 | 18.03 ± 5.27 | 5.36 ± 9.35 | −11.34 ± 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannavà, C.; De Gaetano, F.; Stancanelli, R.; Venuti, V.; Paladini, G.; Caridi, F.; Ghica, C.; Crupi, V.; Majolino, D.; Ferlazzo, G.; et al. Chitosan-Hyaluronan Nanoparticles for Vinblastine Sulfate Delivery: Characterization and Internalization Studies on K-562 Cells. Pharmaceutics 2022, 14, 942. https://doi.org/10.3390/pharmaceutics14050942
Cannavà C, De Gaetano F, Stancanelli R, Venuti V, Paladini G, Caridi F, Ghica C, Crupi V, Majolino D, Ferlazzo G, et al. Chitosan-Hyaluronan Nanoparticles for Vinblastine Sulfate Delivery: Characterization and Internalization Studies on K-562 Cells. Pharmaceutics. 2022; 14(5):942. https://doi.org/10.3390/pharmaceutics14050942
Chicago/Turabian StyleCannavà, Carmela, Federica De Gaetano, Rosanna Stancanelli, Valentina Venuti, Giuseppe Paladini, Francesco Caridi, Corneliu Ghica, Vincenza Crupi, Domenico Majolino, Guido Ferlazzo, and et al. 2022. "Chitosan-Hyaluronan Nanoparticles for Vinblastine Sulfate Delivery: Characterization and Internalization Studies on K-562 Cells" Pharmaceutics 14, no. 5: 942. https://doi.org/10.3390/pharmaceutics14050942