Biological Activity of NHC-Gold-Alkynyl Complexes Derived from 3-Hydroxyflavones
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Synthesis of the [Au(L)(NHC)] Complexes


2.3. Distribution Coefficient (LogP)
2.4. Stability
2.5. Disk Diffusion Test
2.6. Bacterial Growth and MIC Calculation E. coli
2.7. Dihydrofolate Reductase and TrxR Activities Activity Assay in E. coli
2.8. Preparation of E. coli for Scanning Electron Microscopy (SEM)
2.9. Caco-2 Cell Culture and Treatment
2.10. Antiproliferative Activity and IC50 Calculation
2.11. Measurement of Intracellular ROS Levels
2.12. Measurement of TrxR Activity
3. Results and Discussion
3.1. Synthesis
3.2. Solution Stability and Lipophilicity
3.3. Biological Activity
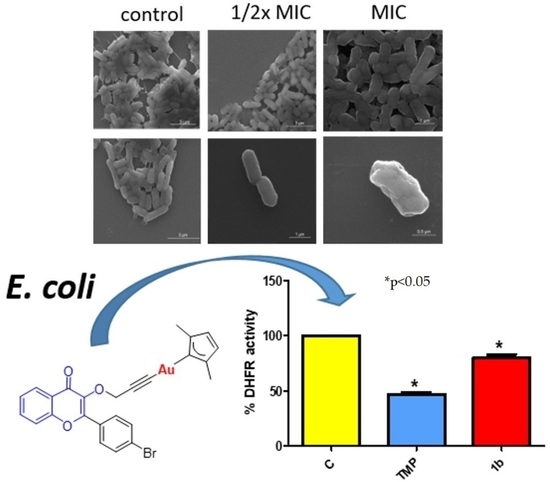
3.3.1. Antibacterial Effect
3.3.2. Antiproliferative Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.D.; Zhang, B.; Guo, S. Transition Metal Complexes Supported by N-Heterocyclic Carbene-Based Pincer Platforms: Synthesis, Reactivity and Applications. Eur. J. Inorg. Chem. 2021, 2021, 188–204. [Google Scholar] [CrossRef]
- Reshi, N.U.D.; Bera, J.K. Recent advances in annellated NHCs and their metal complexes. Coord. Chem. Rev. 2020, 422, 213334–213413. [Google Scholar] [CrossRef]
- Romain, C.; Bellemin-Laponnaz, S.; Dagorne, S. Recent progress on NHC-stabilized early transition metal (group 3–7) complexes: Synthesis and applications. Coord. Chem. Rev. 2020, 422, 213411–213442. [Google Scholar] [CrossRef]
- Scattolin, T.; Nolan, S.P. Synthetic Routes to Late Transition Metal-NHC Complexes. Trends Chem. 2020, 2, 721–736. [Google Scholar] [CrossRef]
- Hahn, F.E.; Jahnke, M.C. Heterocyclic carbenes: Synthesis and coordination chemistry. Angew. Chem. Int. Ed. 2008, 47, 3122–3172. [Google Scholar] [CrossRef]
- Doddi, A.; Peters, M.; Tamm, M. N-Heterocyclic Carbene Adducts of Main Group Elements and Their Use as Ligands in Transition Metal Chemistry. Chem. Rev. 2019, 119, 6994–7112. [Google Scholar] [CrossRef]
- Nolan, S.P. The Development and Catalytic Uses of N-Heterocyclic Carbene Gold Complexes. Acc. Chem. Res. 2011, 44, 91–100. [Google Scholar] [CrossRef]
- Marion, N.; Nolan, S.P. N-heterocyclic carbenes in gold catalysis. Chem. Soc. Rev. 2008, 37, 1776–1782. [Google Scholar] [CrossRef]
- Hopkinson, M.N.; Richter, C.; Schedler, M.; Glorius, F. An overview of N-heterocyclic carbenes. Nature 2014, 510, 485–496. [Google Scholar] [CrossRef]
- Nolan, S.P. N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis; Wiley-VCH Verlag GmbH&Co.: Weinheim, Germany, 2014. [Google Scholar]
- Liu, J.; Xing, X.N.; Huang, J.H.; Lu, L.Q.; Xiao, W.J. Light opens a new window for N-heterocyclic carbene catalysis. Chem. Sci. 2020, 11, 10605–10613. [Google Scholar] [CrossRef]
- Liu, W.K.; Gust, R. Update on metal N-heterocyclic carbene complexes as potential anti-tumor Metallodrugs. Coord. Chem. Rev. 2016, 329, 191–213. [Google Scholar] [CrossRef]
- Liu, W.K.; Gust, R. Metal N-heterocyclic carbene complexes as potential antitumor metallodrugs. Chem. Soc. Rev. 2013, 42, 755–773. [Google Scholar] [CrossRef] [PubMed]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent advances in gold-NHC complexes with biological properties. Chem. Soc. Rev. 2019, 48, 447–462. [Google Scholar] [CrossRef] [PubMed]
- Porchia, M.; Pellei, M.; Marinelli, M.; Tisato, F.; Del Bello, F.; Santini, C. New insights in Au-NHCs complexes as anticancer agents. Eur. J. Med. Chem. 2018, 146, 709–746. [Google Scholar] [CrossRef]
- Marinelli, M.; Santini, C.; Pellei, M. Recent Advances in Medicinal Applications of Coinage-Metal (Cu and Ag) N-Heterocyclic Carbene Complexes. Curr. Top. Med. Chem. 2016, 16, 2995–3017. [Google Scholar] [CrossRef]
- Gautier, A.; Cisnetti, F. Advances in metal-carbene complexes as potent anti-cancer agents. Metallomics 2012, 4, 23–32. [Google Scholar] [CrossRef]
- Tong, K.C.; Hu, D.; Wan, P.K.; Lok, C.N.; Che, C.M. Anti-cancer gold, platinum and iridium compounds with porphyrin and/or N-heterocyclic carbene ligand(s). In Advances in Inorganic Chemistry; Sadler, P.J., VanEldik, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 75, pp. 87–119. [Google Scholar]
- Patil, S.A.; Hoagland, A.P.; Patil, S.A.; Bugarin, A. N-heterocyclic carbene-metal complexes as bio-organometallic antimicrobial and anticancer drugs, an update (2015–2020). Future Med. Chem. 2020, 12, 2239–2275. [Google Scholar] [CrossRef]
- Hindi, K.M.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. The Medicinal Applications of Imidazolium Carbene-Metal Complexes. Chem. Rev. 2009, 109, 3859–3884. [Google Scholar] [CrossRef]
- Fernandez, G.A.; Gurovic, M.S.V.; Olivera, N.L.; Chopa, A.B.; Silbestri, G.F. Antibacterial properties of water-soluble gold(I) N-heterocyclic carbene complexes. J. Inorg. Biochem. 2014, 135, 54–57. [Google Scholar] [CrossRef]
- Samanta, T.; Roymahapatra, G.; Porto, W.F.; Seth, S.; Ghorai, S.; Saha, S.; Sengupta, J.; Franco, O.L.; Dinda, J.; Mandal, S.M. N,N′-Olefin Functionalized Bis-Imidazolium Gold(I) Salt Is an Efficient Candidate to Control Keratitis-Associated Eye Infection. PLoS ONE 2013, 8, e58346. [Google Scholar] [CrossRef]
- Glisic, B.D.; Djuran, M.I. Gold complexes as antimicrobial agents: An overview of different biological activities in relation to the oxidation state of the gold ion and the ligand structure. Dalton T 2014, 43, 5950–5969. [Google Scholar] [CrossRef]
- Owings, J.P.; McNair, N.N.; Mui, Y.F.; Gustafsson, T.N.; Holmgren, A.; Contel, M.; Goldberg, J.B.; Mead, J.R. Auranofin and N-heterocyclic carbene gold-analogs are potent inhibitors of the bacteria Helicobacter pylori. FEMS Microbiol. Lett. 2016, 363, fnw148. [Google Scholar] [CrossRef] [PubMed]
- Ott, I. Metal N-heterocyclic carbene complexes in medicinal chemistry. In Advances in Inorganic Chemistry; Sadler, P.J., VanEldik, R., Eds.; Academic Press: Cambridge, MA, USA, 2020; Volume 75, pp. 121–148. [Google Scholar]
- Zhao, W.H.; Ferro, V.; Baker, M.V. Carbohydrate-N-heterocyclic carbene metal complexes: Synthesis, catalysis and biological studies. Coord. Chem. Rev. 2017, 339, 1–16. [Google Scholar] [CrossRef]
- Marmol, I.; Quero, J.; Rodriguez-Yoldi, M.J.; Cerrada, E. Gold as a Possible Alternative to Platinum-Based Chemotherapy for Colon Cancer Treatment. Cancers 2019, 11, 780. [Google Scholar] [CrossRef] [PubMed]
- Oberkofler, J.; Aikman, B.; Bonsignore, R.; Pothig, A.; Platts, J.; Casini, A.; Kuhn, F.E. Exploring the Reactivity and Biological Effects of Heteroleptic N-Heterocyclic Carbene Gold(I)-Alkynyl Complexes. Eur. J. Inorg. Chem. 2020, 2020, 1040–1051. [Google Scholar] [CrossRef]
- Zou, T.; Lum, C.T.; Lok, C.-N.; To, W.-P.; Low, K.-H.; Che, C.-M. A Binuclear Gold(I) Complex with Mixed Bridging Diphosphine and Bis(N-Heterocyclic Carbene) Ligands Shows Favorable Thiol Reactivity and Inhibits Tumor Growth and Angiogenesis In Vivo. Angew. Chem. Int. Ed. 2014, 53, 5810–5814. [Google Scholar] [CrossRef]
- Dominelli, B.; Jakob, C.H.G.; Oberkofler, J.; Fischer, P.J.; Esslinger, E.-M.; Reich, R.M.; Marques, F.; Pinheiro, T.; Correia, J.D.G.; Kühn, F.E. Mechanisms underlying the cytotoxic activity of syn/anti-isomers of dinuclear Au(I) NHC complexes. Eur. J. Med. Chem. 2020, 203, 112576. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G.; Metzler-Nolte, N. Small organometallic compounds as antibacterial agents. Dalton T 2012, 41, 6350–6358. [Google Scholar] [CrossRef]
- Hussaini, S.Y.; Haque, R.A.; Razali, M.R. Recent progress in silver(I)-, gold(I)/(III)- and palladium(II)-N-heterocyclic carbene complexes: A review towards biological perspectives. J. Organomet. Chem. 2019, 882, 96–111. [Google Scholar] [CrossRef]
- Ratia, C.; Soengas, R.G.; Soto, S.M. Gold-Derived Molecules as New Antimicrobial Agents. Front. Microbiol. 2022, 13, 772. [Google Scholar] [CrossRef]
- Roder, C.; Thomson, M.J. Auranofin: Repurposing an Old Drug fro a Golden New Age. Drugs R&D 2015, 15, 13–20. [Google Scholar]
- Gamberi, T.; Chiappetta, G.; Fiaschi, T.; Modesti, A.; Sorbi, F.; Magherini, F. Upgrade of an old drug: Auranofin in innovative cancer therapies to overcome drug resistance and to increase drug effectiveness. Med. Res. Rev. 2022, 42, 1111–1146. [Google Scholar] [CrossRef] [PubMed]
- Abdalbari, F.H.; Telleria, C.M. The gold complex auranofin: New perspectives for cancer therapy. Discov. Oncol. 2021, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- AbdelKhalek, A.; Abutaleb, N.S.; Mohammad, H.; Seleem, M.N. Antibacterial and antivirulence activities of auranofin against Clostridium difficile. Int. J. Antimicrob. Ag. 2019, 53, 54–62. [Google Scholar] [CrossRef]
- Aguinagalde, L.; Diez-Martinez, R.; Yuste, J.; Royo, I.; Gil, C.; Lasa, I.; Martin-Fontecha, M.; Marin-Ramos, N.I.; Ardanuy, C.; Linares, J.; et al. Auranofin efficacy against MDR Streptococcus pneumoniae and Staphylococcus aureus infections. J. Antimicrobiol. Chemother. 2015, 70, 2608–2617. [Google Scholar] [CrossRef]
- Marzo, T.; Cirri, D.; Pollini, S.; Prato, M.; Fallani, S.; Cassetta, M.I.; Novelli, A.; Rossolini, G.M.; Messori, L. Auranofin and its Analogues Show Potent Antimicrobial Activity against Multidrug-Resistant Pathogens: Structure-Activity Relationships. ChemMedChem 2018, 13, 2448–2454. [Google Scholar] [CrossRef]
- Abutaleb, N.S.; Seleem, M.N. Antivirulence activity of auranofin against vancomycin-resistant enterococci: In vitro and in vivo studies. Int. J. Antimicrob. Ag. 2020, 55, 105828. [Google Scholar] [CrossRef]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef]
- Harbut, M.B.; Vilcheze, C.; Luo, X.Z.; Hensler, M.E.; Guo, H.; Yang, B.Y.; Chatterjee, A.K.; Nizet, V.; Jacobs, W.R.; Schultz, P.G.; et al. Auranofin exerts broad-spectrum bactericidal activities by targeting thiol-redox homeostasis. Proc. Natl. Acad. Sci. USA 2015, 112, 4453–4458. [Google Scholar] [CrossRef]
- Dias, M.V.B.; Santos, J.C.; Libreros-Zúñiga, G.A.; Ribeiro, J.A.; Chavez-Pacheco, S.M. Folate biosynthesis pathway: Mechanisms and insights into drug design for infectious diseases. Future Med. Chem. 2018, 10, 935–959. [Google Scholar] [CrossRef]
- Thangamani, S.; Mohammad, H.; Abushahba, M.F.N.; Sobreira, T.J.P.; Hedrick, V.E.; Paul, L.N.; Seleem, M.N. Antibacterial activity and mechanism of action of auranofin against multi-drug resistant bacterial pathogens. Sci. Rep. 2016, 6, 22571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, B.; Yang, X.J.; Yan, M.D. Synthesis and Structure-Activity Relationship Study of Antimicrobial Auranofin against ESKAPE Pathogens. J. Med. Chem. 2019, 62, 7751–7768. [Google Scholar] [CrossRef] [PubMed]
- Marmol, I.; Castellnou, P.; Alvarez, R.; Gimeno, M.C.; Rodriguez-Yoldi, M.J.; Cerrada, E. Alkynyl Gold(I) complexes derived from 3-hydroxyflavones as multi-targeted drugs against colon cancer. Eur. J. Med. Chem. 2019, 183, 111661–111675. [Google Scholar] [CrossRef] [PubMed]
- Bantreil, X.; Nolan, S.P. Synthesis of N-heterocyclic carbene ligands and derived ruthenium olefin metathesis catalysts. Nat. Protocol. 2011, 6, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Visbal, R.; Laguna, A.; Gimeno, M.C. Simple and efficient synthesis of [MCI(NHC)] (M = Au, Ag) complexes. Chem. Commun. 2013, 49, 5642–5644. [Google Scholar] [CrossRef] [PubMed]
- Atrian-Blasco, E.; Gascon, S.; Rodriguez-Yoldi, M.J.; Laguna, M.; Cerrada, E. Novel Gold(I) Thiolate Derivatives Synergistic with 5-Fluorouracil as Potential Selective Anticancer Agents in Colon Cancer. Inorg. Chem. 2017, 56, 8562–8579. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Disk Susceptibility Tests; Approved Standard; CLSI: Wayne, PA, USA, 2012. [Google Scholar]
- Marmol, I.; Virumbrales-Munoz, M.; Quero, J.; Sanchez-De-Diego, C.; Fernandez, L.; Ochoa, I.; Cerrada, E.; Yoldi, M.J.R. Alkynyl gold(I) complex triggers necroptosis via ROS generation in colorectal carcinoma cells. J. Inorg. Biochem. 2017, 176, 123–133. [Google Scholar] [CrossRef]
- Marmol, I.; Sanchez-De-Diego, C.; Dieste, A.P.; Cerrada, E.; Yoldi, M.J.R. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef]
- Quero, J.; Cabello, S.; Fuertes, T.; Marmol, I.; Laplaza, R.; Polo, V.; Gimeno, M.C.; Rodriguez-Yoldi, M.J.; Cerrada, E. Proteasome versus Thioredoxin Reductase Competition as Possible Biological Targets in Antitumor Mixed Thiolate-Dithiocarbamate Gold(III) Complexes. Inorg. Chem. 2018, 57, 10832–10845. [Google Scholar] [CrossRef]
- De Fremont, P.; Scott, N.M.; Stevens, E.D.; Nolan, S.P. Synthesis and structural characterization of N-heterocyclic carbene gold(I) complexes. Organometallics 2005, 24, 2411–2418. [Google Scholar] [CrossRef]
- Cassetta, M.I.; Marzo, T.; Fallani, S.; Novelli, A.; Messori, L. Drug repositioning: Auranofin as a prospective antimicrobial agent for the treatment of severe staphylococcal infections. Biometals 2014, 27, 787–791. [Google Scholar] [CrossRef] [PubMed]
- Sannella, A.R.; Casini, A.; Gabbiani, C.; Messori, L.; Bilia, A.R.; Vincieri, F.F.; Majori, G.; Severini, C. New uses for old drugs. Auranofin, a clinically established antiarthritic metallodrug, exhibits potent antimalarial effects in vitro: Mechanistic and pharmacological implications. FEBS Lett. 2008, 582, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Bussing, R.; Karge, B.; Lippmann, P.; Jones, P.G.; Bronstrup, M.; Ott, I. Gold(I) and Gold(III) N-Heterocyclic Carbene Complexes as Antibacterial Agents and Inhibitors of Bacterial Thioredoxin Reductase. ChemMedChem 2021, 16, 3402–3409. [Google Scholar] [CrossRef]
- Schmidt, C.; Karge, B.; Misgeld, R.; Prokop, A.; Franke, R.; Brönstrup, M.; Ott, I. Gold(I) NHC Complexes: Antiproliferative Activity, Cellular Uptake, Inhibition of Mammalian and Bacterial Thioredoxin Reductases, and Gram-Positive Directed Antibacterial Effects. Chem. Eur. J. 2017, 23, 1869–1880. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, H.; Dietze, P.; Preu, L.; Bandow, J.E.; Ott, I. Evaluation of Ruthenium(II) N-Heterocyclic Carbene Complexes as Antibacterial Agents and Inhibitors of Bacterial Thioredoxin Reductase. Molecules 2021, 26, 4282. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Qiao, W.; An, Q.; Yang, T.; Luo, Y. Dihydrofolate reductase inhibitors for use as antimicrobial agents. Eur. J. Med. Chem. 2020, 195, 112268. [Google Scholar] [CrossRef] [PubMed]
- Gonen, N.; Assaraf, Y.G. Antifolates in cancer therapy: Structure, activity and mechanisms of drug resistance. Drug Resist. Updates 2012, 15, 183–210. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Chauhan, P.M. Dihydrofolate reductase as a therapeutic target for infectious diseases: Opportunities and challenges. Future Med. Chem. 2012, 4, 1335–1365. [Google Scholar] [CrossRef]
- Srinivasan, B.; Tonddast-Navaei, S.; Roy, A.; Zhou, H.Y.; Skolnick, J. Chemical space of Escherichia coli dihydrofolate reductase inhibitors: New approaches for discovering novel drugs for old bugs. Med. Res. Rev. 2019, 39, 684–705. [Google Scholar] [CrossRef]
- Raimondi, M.V.; Randazzo, O.; La Franca, M.; Barone, G.; Vignoni, E.; Rossi, D.; Collina, S. DHFR Inhibitors: Reading the Past for Discovering Novel Anticancer Agents. Molecules 2019, 24, 1140. [Google Scholar] [CrossRef]
- Galassi, R.; Oumarou, C.S.; Burini, A.; Dolmella, A.; Micozzi, D.; Vincenzetti, S.; Pucciarelli, S. A study on the inhibition of dihydrofolate reductase (DHFR) from Escherichia coli by gold(i) phosphane compounds. X-ray crystal structures of (4,5-dichloro-1H-imidazolate-1-yl)-triphenylphosphane-gold(i) and (4,5-dicyano-1H-imidazolate-1-yl)-triphenylphosphane-gold(i). Dalton T 2015, 44, 3043–3056. [Google Scholar] [CrossRef]
- Galassi, R.; Luciani, L.; Gambini, V.; Vincenzetti, S.; Lupidi, G.; Amici, A.; Marchini, C.; Wang, J.; Pucciarelli, S. Multi-Targeted Anticancer Activity of Imidazolate Phosphane Gold(I) Compounds by Inhibition of DHFR and TrxR in Breast Cancer Cells. Front. Chem. 2021, 8, 602845. [Google Scholar] [CrossRef] [PubMed]
- Naik, K.K.; Thangavel, S.; Alam, A.; Kumar, S. Flavone Analogues as Antimicrobial Agents. Recent Pat. Inflamm. Allergy Drug Discov. 2017, 11, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Montenegro, C.D.; Goncalves, G.F.; de Oliveira, A.A.; Lira, A.B.; Cassiano, T.T.M.; de Lima, N.T.R.; Barbosa, J.M.; Diniz, M.; Pessoa, H.L.F. In Silico Study and Bioprospection of the Antibacterial and Antioxidant Effects of Flavone and Its Hydroxylated Derivatives. Molecules 2017, 22, 869. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Fu, Y.; Liu, M.; Zhang, J.; Wang, W.; Li, J.; Zeng, Q.; Wang, T.; Li, Y. Mechanisms of Action of Luteolin Against Single- and Dual-Species of Escherichia coli and Enterobacter cloacae and Its Antibiofilm Activities. Appl. Biochem. Biotech. 2021, 193, 1397–1414. [Google Scholar] [CrossRef]
- Cheung, H.-Y.; Wong, M.M.-K.; Cheung, S.-H.; Liang, L.Y.; Lam, Y.-W.; Chiu, S.-K. Differential Actions of Chlorhexidine on the Cell Wall of Bacillus subtilis and Escherichia coli. PLoS ONE 2012, 7, e36659. [Google Scholar] [CrossRef]
- Schnaider, L.; Brahmachari, S.; Schmidt, N.W.; Mensa, B.; Shaham-Niv, S.; Bychenko, D.; Adler-Abramovich, L.; Shimon, L.J.W.; Kolusheva, S.; DeGrado, W.F.; et al. Self-assembling dipeptide antibacterial nanostructures with membrane disrupting activity. Nat. Commun. 2017, 8, 1365. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef]






| Bacterial Strain | Inhibition Zone (mm) a | ||||
|---|---|---|---|---|---|
| 1b | 1d | 2b | 2c | Phenol | |
| E. coli 25922 | 9.04 ± 2.17 | - | - | - | 23.39 ± 1.26 |
| E. coli 8739 | 11.10 ± 0.08 | - | 8.97 ± 0.49 | - | 22.71 ± 1.37 |
| E. coli 13216 | 10.95 ± 0.64 | - | 8.10 ± 0.60 | - | 20.70 ± 2.97 |
| P. aeruginosa | - | - | - | - | 30.48 ± 1.45 |
| S. enterica 25928 | 8.90 ± 1.56 | - | - | - | 19.20 ± 0.42 |
| S. enterica 14028 | 9.39 ± 1.26 | - | - | - | 27.79 ± 1.11 |
| E. faecalis | 12.50 ± 1.41 | 9.47 ± 1.67 | 12.1 ± 0.99 | 10.25 ± 1.41 | 13.5 ± 2.12 |
| L. monocytogenes | 18.50 ± 1.41 | 14.25 ± 1.06 | 17.17 ± 0.24 | 15.33 ± 0.47 | 16.58 ± 0.12 |
| S. epidermidis | 25.05 ± 1.48 | 17.23 ± 0.87 | 20.95 ± 0.91 | 18.06 ± 2.04 | 22.67 ± 4.49 |
| S. aureus 2593 | 13.76 ± 0.54 | 9.70 ± 0.02 | 12.79 ± 1.11 | 10.49 ± 1.13 | 12.05 ± 0.73 |
| S. aureus 13565 | 16.58 ± 1.63 | 11.86 ± 0.20 | 14.03 ± 0.17 | 12.19 ± 0.88 | 17.25 ± 3.38 |
| Complex | IC50 (μM) | LogP7.4 |
|---|---|---|
| [AuCl(IMe)] | >100 | - |
| [Au(L2a)(IMe)] (1a) | 43.34 ± 5.06 | 0.34 |
| [Au(L2b)(IMe)] (1b) | 23.01 ± 2.42 | 1.32 |
| [Au(L2c)(IMe)] (1c) | 15.05 ± 3.04 | 1.96 |
| [Au(L2d)(IMe)] (1d) | 16.33 ± 1.04 | 0.22 |
| [AuCl(IPr)] | 9.09 ± 3.22 | - |
| [Au(L2a)(IPr)] (2a) | 16.34 ± 2.04 | 0.42 |
| [Au(L2b)(IPr)] (2b) | 53.85 ± 22.72 | 1.47 |
| [Au(L2c)(IPr)] (2c) | 46.15 ± 22.54 | 1.11 |
| [Au(L2d)(IPr)] (2d) | 48.22 ± 14.56 | 3.40 |
| Auranofin | 1.80 ± 0.1 | −2.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mármol, I.; Quero, J.; Azcárate, P.; Atrián-Blasco, E.; Ramos, C.; Santos, J.; Gimeno, M.C.; Rodríguez-Yoldi, M.J.; Cerrada, E. Biological Activity of NHC-Gold-Alkynyl Complexes Derived from 3-Hydroxyflavones. Pharmaceutics 2022, 14, 2064. https://doi.org/10.3390/pharmaceutics14102064
Mármol I, Quero J, Azcárate P, Atrián-Blasco E, Ramos C, Santos J, Gimeno MC, Rodríguez-Yoldi MJ, Cerrada E. Biological Activity of NHC-Gold-Alkynyl Complexes Derived from 3-Hydroxyflavones. Pharmaceutics. 2022; 14(10):2064. https://doi.org/10.3390/pharmaceutics14102064
Chicago/Turabian StyleMármol, Inés, Javier Quero, Paula Azcárate, Elena Atrián-Blasco, Carla Ramos, Joana Santos, María Concepción Gimeno, María Jesús Rodríguez-Yoldi, and Elena Cerrada. 2022. "Biological Activity of NHC-Gold-Alkynyl Complexes Derived from 3-Hydroxyflavones" Pharmaceutics 14, no. 10: 2064. https://doi.org/10.3390/pharmaceutics14102064