The Establishment of an Efficient Callus Induction System for Lotus (Nelumbo nucifera)
Abstract
:1. Introduction
2. Results
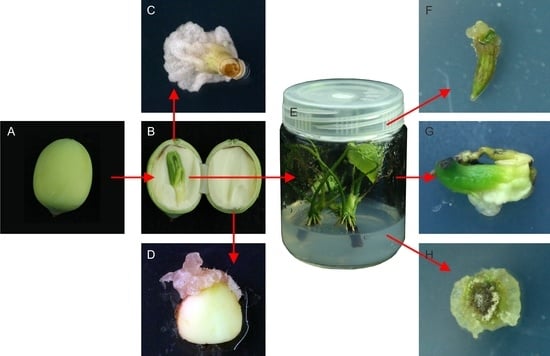
2.1. Callus Induction in Different Explants
2.2. Callus Induction of Different Genotypes
2.3. Effects of Plant Growth Regulators on Callus Induction
2.4. Optimizing Conditions for Lotus Callus Induction
2.5. Aseptic Seedlings as Explants for Callus Induction
2.6. Accumulation of Benzylisoquilonine Alkaloids (BIA) in Lotus Callus
3. Discussion
4. Material and Methods
4.1. Plant Materials
4.2. Preparation of Lotus Explants
4.3. Conditions for Callus Induction
4.4. Quantification of Lotus Alkaloids and Quantitative RT-PCR
4.5. Data Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guo, H.B. Cultivation of lotus (Nelumbo nucifera Gaertn. ssp. nucifera) and its utilization in China. Genet. Resour. Crop. Evol. 2009, 56, 323–330. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, L.; Pan, C.; Xu, L.; Liu, Y.; Ke, W.; Yang, P. Transcriptomic analysis of the regulation of rhizome formation in temperate and tropical lotus (Nelumbo nucifera). Sci. Rep. UK 2015, 5, 13059. [Google Scholar] [CrossRef] [PubMed]
- Grienke, U.; Mair, C.E.; Saxena, P.; Baburin, I.; Scheel, O.; Ganzera, M.; Schuster, D.; Hering, S.; Rollinger, J.M. Human ether-à-go-go related gene (hERG) channel blocking aporphine alkaloids from lotus leaves and their quantitative analysis in dietary weight loss aupplements. J. Agric. Food Chem. 2015, 63, 5634–5639. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Li, G.; He, Y.; Xu, B.; Mi, X.; Wang, H.; Wang, Z. Pronuciferine and nuciferine inhibit lipogenesis in 3T3-L1 adipocytes by activating the AMPK signaling pathway. Life Sci. 2015, 136, 120–125. [Google Scholar] [CrossRef]
- Liu, W.; Y, D.; Guo, J.; Xiang, Z.; Deng, L.; He, L. Nuciferine, extracted from Nelumbo nucifera Gaertn, inhibits tumor-promoting effect of nicotine involving Wnt/β-catenin signaling in non-small cell lung cancer. J. Ethnopharmacol. 2015, 165, 83–93. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wu, T.; Li, B.; Liu, T.; Wang, R.; Liu, Q.; Liu, Z.; Gong, Y.; Shao, C. Isoliensinine induces apoptosis in triple-negative human breast cancer cells through ROS generation and p38 MAPK/JNK activation. Sci. Rep. UK 2015, 5, 12579. [Google Scholar] [CrossRef]
- Nguyen, K.H.; Ta, T.N.; Pham, T.H.M.; Nguyen, Q.T.; Pham, H.D.; Mishra, S.; Nyomba, B.L.G. Nuciferine stimulates insulin secretion from beta cells-an in vitro comparison with glibenclamide. J. Ethnopharmacol. 2012, 142, 488–495. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Aoshima, A.; Ikeshiro, Y.; Chen, Y.; Furukawa, H.; Itoigawa, M.; Fujioka, T.; Mihashi, K.; Cosentino, L.M.; Morris-Natschke, S.L.; et al. Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure activity correlations with related alkaloids. Bioorg. Med. Chem. 2005, 13, 443–448. [Google Scholar] [CrossRef]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. Vitr. Cell Dev. Plant 2019, 1, 1–15. [Google Scholar] [CrossRef]
- Mostafa, H.H.A.; Wang, H.; Song, J.; Li, X. Effect of genotypes and explants on garlic callus production and endogenous hormones. Sci. Rep. UK 2020, 10, 4867. [Google Scholar] [CrossRef] [Green Version]
- Molina, D.M.; Aponte, M.E.; Cortina, H.; Moreno, G. The effect of genotye and explant age on somatic embryogenesis of coffee. Plant. Cell Tissue Org. 2002, 71, 117–123. [Google Scholar] [CrossRef]
- Yang, M.; Han, Y.; Van Buren, R.; Ming, R.; Xu, L.; Han, Y.; Liu, Y. Genetic linkage maps for Asian and American lotus constructed using novel SSR markers derived from the genome of sequenced cultivar. BMC Genom. 2012, 13, 653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.; Chen, S.; Yin, X.; Wang, K.; Liu, Y.; Li, S.; Yang, P. Systematic qualitative and quantitative assessment of anthocyanins, flavones and flavonols in the petals of 108 lotus (Nelumbo nucifera) cultivars. Food Chem. 2013, 139, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Fu, Z.; Chen, S.; Damaris, R.N.; Wang, K.; Li, T.; Yang, P. Proteomic and epigenetic analyses of lotus (Nelumbo nucifera) petals between red and white cultivars. Plant. Cell Physiol. 2015, 56, 1546–1555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Fu, J.; Li, M.; Fragner, L.; Weckwerth, W.; Yang, P. Metabolomic and proteomic profiles reveal the dynamics of primary metabolism during seed development of lotus (Nelumbo nucifera). Front. Plant. Sci. 2016, 7, 750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, X.; Zhu, L.; Fang, T.; Vimolmangkang, S.; Yang, D.; Ogutu, C.; Liu, Y.; Han, Y. Analysis of isoquinoline alkaloid composition and wound-induced variation in Nelumbo using HPLC-MS/MS. J. Agric. Food Chem. 2016, 64, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhao, L.; Fang, T.; Xiong, Y.; Ogutu, C.; Yang, D.; Vimolmangkang, S.; Liu, Y.; Han, Y. Investigation of benzylisoquinoline alkaloid biosynthetic pathway and its transcriptional regulation in lotus. Hortic. Res. 2018, 5, 29. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.; Wang, K.; Yang, P. The evolution of plant microRNAs: Insights from a basal eudicot sacred lotus. Plant. J. 2017, 89, 442–457. [Google Scholar] [CrossRef]
- Huang, L.; Yang, M.; Li, L.; Li, H.; Yang, D.; Shi, T.; Yang, P. Whole genome re-sequencing reveals evolutionary patterns of sacred lotus (Nelumbo nucifera). J. Integr. Plant. Biol. 2018, 60, 2–15. [Google Scholar] [CrossRef]
- Ming, R.; Van Buren, R.; Liu, Y.; Yang, M.; Han, Y.; Li, L.T.; Zhang, Q.; Kim, M.J.; Schatz, M.C.; Campbell, M.; et al. Genome of the long-living sacred lotus (Nelumbo nucifera Gaertn.). Genome Biol. 2013, 14, R41. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fan, G.; Liu, Y.; Sun, F.; Shi, C.; Liu, X.; Peng, J.; Chen, W.; Huang, X.; Cheng, S.; et al. The sacred lotus genome provides insights into the evolution of flowering plants. Plant. J. 2013, 76, 557–567. [Google Scholar] [CrossRef]
- Gui, S.; Peng, J.; Wang, X.; Wu, Z.; Cao, R.; Salse, J.; Zhang, H.; Zhu, Z.; Xia, Q.; Quan, Z.; et al. Improving Nelumbo nucifera genome assemblies using high-resolution genetic maps and BioNano genome mapping reveals ancient chromosome rearrangements. Plant. J. 2018, 94, 721–734. [Google Scholar] [CrossRef] [Green Version]
- Shi, T.; Rahmani, R.S.; Gugger, P.F.; Wang, M.; Li, H.; Zhang, Y.; Li, Z.; Wang, Q.; de Peer, Y.V.; Marchal, K.; et al. Distinct expression and methylation patterns for genes with different fates following a single whole-genome duplication in flowering plants. Mol. Biol. Evol. 2020, 37, 2394–2413. [Google Scholar] [CrossRef]
- Lin, Z.; Zhang, C.; Cao, D.; Damaris, R.N.; Yang, P. The latest studies on lotus (Nelumbo nucifera)-an emerging horticultural model plant. Int. J. Mol. Sci. 2019, 20, 3680. [Google Scholar] [CrossRef] [Green Version]
- Weigel, D.; Glazebrook, J. Arabidopsis: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2002; pp. 1–37. [Google Scholar]
- Abe, T.; Futsuhara, Y. Genotypic variability for callus formation and plant regeneration in rice (Oryza satzva L.). Theor. Appl. Genet. 1986, 72, 3–10. [Google Scholar] [CrossRef]
- Gupta, G.R.; Guha, S.; Maheshwari, S.C. Differentiation of buds from leaves of Nicotiana tabacum L. in sterile cultures. Phytomorphology 1966, 16, 175–782. [Google Scholar]
- McCabe, D.E.; Swain, W.F.; Martinell, B.J.; Christou, P. Stable transformation of soybean (Glycine max) by particle acceleration. Nat. BioTechnol. 1988, 6, 923–926. [Google Scholar] [CrossRef]
- McCormick, S.; Niedermeyer, J.; Fry, J.; Barnason, A.; Horsch, R.; Fraley, R. Leaf disc transformation of cultivated tomato (L. esculentum) using Agrobacterium tumefaciens. Plant. Cell Rep. 1986, 5, 81–84. [Google Scholar] [CrossRef]
- Shou, S.; Miao, L.; Zai, W.; Huang, X.; Guo, D. Factors influencing shoot multiplication of lotus (Nelumbo nucifera). Biol. Plantarum. 2008, 52, 529–532. [Google Scholar] [CrossRef]
- Mahmad, N.; Taha, R.M.; Othman, R.; Saleh, A.; Hasbullah, N.A.; Elias, H. Effects of NAA and BAP, double-layered media, and light distance on in vitro regeneration of Nelumbo nucifera Gaertn. (Lotus), and aquatic edible plant. Sci. World 2014, 12, 74518. [Google Scholar]
- Yu, X.; Sheng, J.; Zhao, L.; Diao, Y.; Zheng, X.; Xie, K.; Zhou, M.; Hu, Z. In Vitro plant regeneration of lotus (Nelumbo nucifera). Open Life Sci. 2015, 10, 142–146. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Zhang, D.; Liu, F.; Qin, M.; Tian, D. Micropropagation of Nelumbo nucifera ‘Weishan Hong’ through germfree mature embryos. Vitr. Cell Dev. Biol. Anim. 2019, 55, 305–312. [Google Scholar] [CrossRef]
- He, Z.; Liu, S. A study on callus introduction and regeneration of plantlet from embryo culture of lotus. Acta Hydrobiol. Sin. 1987, 11, 278–280. (In Chinese) [Google Scholar]
- Arunyanart, S.; Chaitrayagun, M. Induction of somatic embryogenesis in lotus (Nelumbo nucifera Geartn.). Sci. Hortic. 2005, 105, 411–420. [Google Scholar] [CrossRef]
- Buathong, R.; Saetiew, K.; Phansiri, S.; Parinthawong, N.; Arunyanart, S. Tissue culture and transformation of the antisense DFR gene into lotus (Nelumbo nucifera Gaertn.) through particle bombardment. Sci. Hortic. 2013, 161, 216–222. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plantarum. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Dias, M.I.; Sousa, M.J.; Alves, R.C.; Ferreira, I.C.F.R. Exploring plant tissue culture to improve the production of phenolic compounds: A review. Ind. Crop. Prod. 2016, 9–22. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Xiong, Y.; Li, Y.; Ye, S.; Yin, Q.; Gao, S.; Yang, D.; Yang, M.; Palva, T.E.; Deng, X. Comprehensive analysis and functional studies of WRKY transcription factors in Nelumbo nucifera. Int. J. Mol. Sci. 2019, 20, 5006. [Google Scholar] [CrossRef] [Green Version]
- Lai, K.L.; Liu, L. Induction and plant regeneration of callus from immature embryos of rice plants (Oryza satlva L.). Jpn. J. Crop. Sci. 1982, 51, 70–74. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.E.; Lineberger, R.D.; Miller, A.R. Effects of tomato cultivar, leaf age, and bacterial strain on transformation by Agrobacterium tumefaciens. Plant. Cell Tissue Organ. Cult. 1991, 24, 115–121. [Google Scholar] [CrossRef]
- Hall, R.D. An introduction to plant-cell culture. In Methods in Molecular Biology, Plant Cell Culture Protocols; Hall, R.D., Ed.; Humana Press: Totowa, NJ, USA, 1999; Volume 111, Chapter 1; pp. 1–18. [Google Scholar]
- Skoog, F.; Miller, C.O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Soc. Exp. Biol. Symp. 1957, 11, 118–131. [Google Scholar]
- Duncan, D.R. Cotton Transformation/Cotton, Biotechnology in Agriculture and Forestry; Zehr, U.B., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 65, pp. 65–77. [Google Scholar]
- Bailey, M.A.; Boerma, H.R.; Parrott, W.A. Genotype effects on proliferative embryogenesis and plant regeneration of soybean. Vitr. Cell Dev. Biol. 1993, 29, 102–108. [Google Scholar] [CrossRef]
- Gordon-Kamm, W.J.; Spencer, T.M.; Mangano, M.L.; Adams, T.R.; Daines, R.J.; Start, W.G.; O’Brien, J.V.; Chambers, S.A.; Adams, W.R., Jr.; Willetts, N.G.; et al. Transformation of maize cells and regeneration of fertile transgenic plants. Plant. Cell 1990, 2, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Song, Y. Insight into the mode of action of 2,4-dichlorophenoxyacetic acid (2,4-D) as an herbicide. J. Intigr. Plant. Biol. 2014, 56, 106–113. [Google Scholar] [CrossRef]
- Silvertand, B.; van Rooyen, A.; Lavrijsen, P.; van Harten, A.M.; Jacobsen, E. Plant regeneration via organogenesis and somatic embryogenesis in callus cultures derived from mature zygotic embryos of leek (Allium ampeloprasum L.). Euphytica 1996, 91, 261–270. [Google Scholar] [CrossRef]
- Farhadi, N.; Panahandeh, J.; Aza, A.M.; Salte, S.A. Effects of explant type, growth regulators and light intensity on callus induction and plant regeneration in four ecotypes of Persian shallot (Allium hirtifolium). Sci. Hortic. 2017, 218, 80–86. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, J.; Hou, T.; Pan, I. Effects of light intensity and plant growth regulators on callus proliferation and shoot regeneration in the ornamental succulent Haworthia. Bot. Stud. 2019, 60, 10. [Google Scholar] [CrossRef]
- Halliday, K.J.; Martínez-García, J.F.; Josse, E.M. Integration of light and auxin signaling. Cold Spring Harb. Perspect. Biol. 2009, 1, a001586. [Google Scholar] [CrossRef] [Green Version]





| Plant Growth Regulators (mg/L) | Class I Callus (%) | Class II Callus (%) | Total Callus (%) | |||
|---|---|---|---|---|---|---|
| NAA | 6-BA | 2,4-D | ZT | |||
| 1 | 0.5 | – | – | 2.08 ± 1.80 e | 0.00 ± 1.10 f | 2.08 ± 1.80 f |
| 3 | 0.5 | – | – | 9.41 ± 1.10 b | 4.65 ± 1.33 e | 14.06 ± 1.65 d,e |
| 5 | 0.5 | – | – | 4.49 ± 0.28 d | 8.98 ± 0.56 d | 13.47 ± 0.84 e |
| 7 | 0.5 | – | – | 6.89 ± 0.47 c | 9.19 ± 0.63 c,d | 16.08 ± 1.10 d |
| – | 0.5 | 7 | – | 9.20 ± 0.44 b | 10.73 ± 0.52 b,c | 19.93 ± 0.96 c |
| – | 0.5 | 3 | – | 14.37 ± 0.15 a | 15.56 ± 0.88 a | 29.94 ± 0.73 a |
| – | – | 3 | 1 | 9.28 ± 0.81 b | 12 ± 1.18 b | 21.28 ± 1.46 c |
| – | – | 7 | 1 | 10.09 ± 0.15 b | 14.27 ± 1.26 a | 24.36 ± 1.11 b |
| – | – | 0.5 | 0.1 | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g |
| – | – | 1 | 0.1 | 0.00 ± 0.00 g | 0.00 ± 0.00 g | 0.00 ± 0.00 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Xiong, Y.; Li, J.; Yang, D.; Liu, J.; Sun, H.; Song, H.; Wang, Y.; Ma, J.; Liu, Y.; et al. The Establishment of an Efficient Callus Induction System for Lotus (Nelumbo nucifera). Plants 2020, 9, 1436. https://doi.org/10.3390/plants9111436
Deng X, Xiong Y, Li J, Yang D, Liu J, Sun H, Song H, Wang Y, Ma J, Liu Y, et al. The Establishment of an Efficient Callus Induction System for Lotus (Nelumbo nucifera). Plants. 2020; 9(11):1436. https://doi.org/10.3390/plants9111436
Chicago/Turabian StyleDeng, Xianbao, Yaqian Xiong, Jing Li, Dong Yang, Juan Liu, Heng Sun, Heyun Song, Yunmeng Wang, Junyu Ma, Yanling Liu, and et al. 2020. "The Establishment of an Efficient Callus Induction System for Lotus (Nelumbo nucifera)" Plants 9, no. 11: 1436. https://doi.org/10.3390/plants9111436