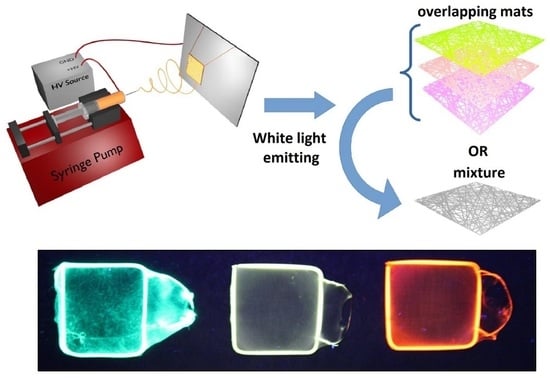
White-Light Emission of Dye-Doped Polymer Submicronic Fibers Produced by Electrospinning
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
3.1. Single-Dye-Doped Polymer Fibers
3.2. Dye-Doped Polymer Fibers Obtained from Mixed Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohammadzadehmoghadam, S.; Dong, Y.; Barbhuiya, S.; Guo, L.; Liu, D.; Umer, R.; Qi, X.; Tang, Y. Electrospinning: Current status and future trends. In Nano-Size Polymers: Preparation, Properties, Applications; Fakirov, S., Ed.; Springer: Cham, Switzerland, 2016; pp. 89–154. [Google Scholar]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Frenot, A.; Chronakis, I.S. Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Tan, S.; Huang, X.; Wu, B. Some fascinating phenomena in electrospinning processes and applications of electrospun nanofiber. Polym. Int. 2007, 56, 1330–1339. [Google Scholar] [CrossRef]
- Li, D.; Wang, Y.; Xia, Y. Electrospininning of Polymeric and Ceramic Nanofibers as Uniaxially Aligned Arrays. Nano Lett. 2003, 3, 1167–1171. [Google Scholar] [CrossRef]
- Stocco, T.D.; Rodrigues, B.V.M.; Marciano, F.R.; Lobo, A.O. Design of a novel electrospinning setup for the fabrication of biomimetic scaffolds for meniscus tissue engineering applications. Mater. Lett. 2017, 196, 221–224. [Google Scholar] [CrossRef]
- Li, D.; Babel, A.; Jenekhe, S.A.; Xia, Y. Nanofibers of Conjugated Polymers Prepared by Electrospinning with a Two-Capillary Spinneret. Adv. Mater. 2004, 76, 2062–2066. [Google Scholar] [CrossRef]
- Li, D.; McCann, J.T.; Xia, Y. Use of Electrospinning to Directly Fabricate Hollow Nanofibers with Functionalized Inner and Outer Surface. Small 2005, 1, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef] [PubMed]
- Persano, L.; Dagdeviren, C.; Su, Y.; Zhang, Y.; Girardo, S.; Pisignano, D.; Huang, Y.; Rogers, J.A. High performance piezoelectric devices based on aligned arrays of nanofibers of poly(vinylidenefluoride-co-trifluoroethylene). Nat. Commun. 2013, 4, 1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; Li, X.; Xia, Y. Putting Electrospun Nanofibers to Work for Biomedical Research. Macromol. Rapid Commun. 2008, 29, 1775–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, B.L.; Limaye, A.; Yarborough, J.; Freeman, J.W. Investigating processing techniques for bovine gelatin electrospun scaffolds for bone tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Liu, X.; Sui, B.; Liu, C.; Mo, X.; Sun, J. Development of fish collagen/bioactive glass/chitosan composite nanofibers as a GTR/GBR membrane for inducing periodontal tissue regeneration. Biomed. Mater. 2017, 12, 055004. [Google Scholar] [CrossRef] [PubMed]
- Moutsatsou, P.; Coopman, K.; Georgiadou, S. Biocompatibility Assessment of Conducting PANI/Chitosan Nanofibers for Wound Healing Applications. Polymers 2017, 9, 687. [Google Scholar] [CrossRef]
- Lin, W.-C.; Yeh, I.-T.; Niyama, E.; Huang, W.-R.; Ebara, M.; Wu, C.-S. Electrospun Poly(ε-caprolactone) Nanofibrous Mesh for Imiquimod Delivery in Melanoma Therapy. Polymers 2018, 10, 231. [Google Scholar] [CrossRef]
- Li, D.; Nie, W.; Chen, L.; Miao, Y.; Zhang, X.; Chen, F.; Yu, B.; Ao, R.; Yu, B.; He, C. Fabrication of curcumin-loaded mesoporous silica incorporated polyvinyl pyrrolidone nanofibers for rapid hemostasis and antibacterial treatment. RSC Adv. 2017, 7, 7973–7982. [Google Scholar] [CrossRef] [Green Version]
- Gao, X.; Gou, J.; Zhang, L.; Duan, S.; Li, C. A silk fibroin based green nano-filter for air filtration. RSC Adv. 2018, 8, 8181–8189. [Google Scholar] [CrossRef] [Green Version]
- Camerlo, A.; Vebert-Nardin, C.; Rossi, R.M.; Popa, A.M. Fragrance encapsulation in polymeric matrices by emulsion electrospinning. Eur. Polym. J. 2013, 49, 3806–3813. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, J.; Yin, X.; Yu, J.; Ding, B. Functional modification of breathable polyacrylonitrile/polyurethane/TiO2 nanofibrous membranes with robust ultraviolet resistant and waterproof performance. J. Colloid Interface Sci. 2017, 508, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.N.; Yan, X.; You, M.H.; Fu, J.; Nie, G.D.; Yu, M.; Ning, X.; Wan, Y.; Long, Y.Z. Reversible photochromic nanofibrous membranes with excellent water/windproof and breathable performance. J. Appl. Polym. Sci. 2018, 135, 46342. [Google Scholar] [CrossRef]
- Busuioc, C.; Evanghelidis, A.; Galatanu, A.; Enculescu, I. Direct and contactless electrical control of temperature of paper and textile foldable substrates using electrospun metallic-web transparent electrodes. Sci. Rep. 2016, 6, 34584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Yu, R.-H.; Shi, C.-M.; Tao, F.-R.; Li, T.-D.; Cui, Y.-Z. Electrospun nanofibrous membrane based on AIE-active compound for detecting picric acid in aqueous solution. Sens. Actuators B Chem. 2018, 262, 637–645. [Google Scholar] [CrossRef]
- Wolf, C.; Tscherner, M.; Köstler, S. Ultra-fast opto-chemical sensors by using electrospun nanofibers assensing layers. Sens. Actuators B Chem. 2015, 209, 1064–1069. [Google Scholar] [CrossRef]
- Resta, V.; Camposeo, A.; Montinaro, M.; Moffa, M.; Kazlauskas, K.; Jursenas, S.; Tomkeviciene, A.; Grazulevicius, J.V.; Pisignano, D. Nanoparticle-doped electrospun fiber random lasers with spatially extended light modes. Opt. Express 2017, 25, 24604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.-C.; Zhang, Z.-Y.; Shan, C.-X.; Feng, Z.-Q.; Li, J.-S.; Song, C.-L.; Bao, Y.-N.; Qi, X.-H.; Dong, B. A flexible and superhydrophobic upconversion-luminescence membrane as an ultrasensitive fluorescence sensor for single droplet detection. Light Sci. Appl. 2016, 5, e16136. [Google Scholar] [CrossRef]
- Tang, Y.; Liang, G.; Chen, J.; Yu, S.; Li, Z.; Rao, L.; Yu, B. Highly reflective nanofiber films based on electrospinning and their application on color uniformity and luminous efficacy improvement of white light-emitting diodes. Opt. Express 2017, 25, 20598. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Zhang, L.; Chen, Q.; Galipeau, D.; Fong, H.; Qiao, Q. Electrospun Carbon Nanofibers as Low-Cost Counter Electrode for Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2010, 2, 3572–3577. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Hu, Z.; Ma, Z.; Gecevičius, M.; Dong, G.; Zhou, S.; Qiu, J. Anisotropically Enhanced Nonlinear Optical Properties of Ensembles of Gold Nanorods Electrospun in Polymer Nanofiber Film. ACS Appl. Mater. Interfaces 2016, 8, 2048–2053. [Google Scholar] [CrossRef] [PubMed]
- Poduval, R.K.; Noimark, S.; Colchester, R.J.; Macdonald, T.J.; Parkin, I.P.; Desjardins, A.E.; Papakonstantinou, I. Optical fiber ultrasound transmitter with electrospun carbon nanotube-polymer composite. Appl. Phys. Lett. 2017, 110, 223701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Preda, N.; Evanghelidis, A.; Enculescu, M.; Florica, C.; Enculescu, I. Zinc oxide electroless deposition on electrospun PMMA fiber mats. Mater. Lett. 2015, 138, 238–242. [Google Scholar] [CrossRef]
- Beregoi, M.; Busuioc, C.; Evanghelidis, A.; Matei, E.; Iordache, F.; Radu, M.; Dinischiotu, A.; Enculescu, I. Electrochromic properties of polyaniline-coated fiber webs for tissue engineering applications. Int. J. Pharm. 2016, 510, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Beregoi, M.; Evanghelidis, A.; Diculescu, V.C.; Iovu, H.; Enculescu, I. Polypyrrole Actuator Based on Electrospun Microribbons. ACS Appl. Mater. Interfaces 2017, 9, 38068–38075. [Google Scholar] [CrossRef] [PubMed]
- Beregoi, M.; Evanghelidis, A.; Matei, E.; Enculescu, I. Polyaniline based microtubes as building-blocks for artificial muscle applications. Sens. Actuators B Chem. 2017, 253, 576–583. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Ton, L. Functionalized polymer nanofibers: A versatile platform for manipulating light at the nanoscale. Light Sci. Appl. 2013, 2, e102. [Google Scholar] [CrossRef]
- Camposeo, A.; Persano, L.; Pisignano, D. Light-Emitting Electrospun Nanofibers for Nanophotonics and Optoelectronics. Macromol. Mater. Eng. 2013, 298, 487–503. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. General Introduction to the Chemistry of Dyes. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2010; pp. 55–65. [Google Scholar]
- Kircher, M.F.; Gambhir, S.S.; Grimm, J. Noninvasive cell-tracking methods. Nat. Rev. Clin. Oncol. 2011, 8, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Dragulescu-Andrasi, A.; Yao, H. Fluorescence imaging in vivo: Recent advances. Curr. Opin. Biotechnol. 2007, 18, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Lievenbrück, M.; Ritter, H. Polymers and dyes: Developments and applications. Polymers 2015, 7, 717–746. [Google Scholar] [CrossRef]
- Bueno, L.; Amador, C.; Bakalis, S. Modeling the deposition of fluorescent whitening agents on cotton fabrics. AIChE J. 2018, 64, 1305–1316. [Google Scholar] [CrossRef]
- Morel, O.J.X.; Christie, R.M. Current Trends in the Chemistry of Permanent Hair Dyeing. Chem. Rev. 2011, 111, 2537–2561. [Google Scholar] [CrossRef] [PubMed]
- Seymour, S. Functional Aesthetics: Visions in Fashionable Technology, 1st ed.; Springer: Vienna, Austria, 2010. [Google Scholar]
- Robinson, J.R. Photodynamic insecticides: A review of studies on photosensitizing dyes as insect control agents, their practical application, hazards, and residues. In Residue Reviews, Residues of Pesticides and Other Contaminants in the Total Environment; Gunther, F.A., Ed.; Springer: New York, NY, USA, 2010; Volume 88, pp. 69–100. [Google Scholar]
- Enculescu, M.; Evanghelidis, A.; Enculescu, I. Influence of morphology on the emissive properties of dye-doped PVP nanofibers produced by electrospinning. J. Phys. Chem. Solids 2014, 75, 1365–1371. [Google Scholar] [CrossRef]
- Enculescu, M.; Evanghelidis, A.; Busuioc, C.; Florica, C.; Costas, A.; Oancea, M.; Preda, N.; Matei, E.; Enculescu, I. Dependence on the dye’s type and concentration of the emissive properties of electrospun dye-doped beaded nanofibers. Dig. J. Nanomater. Biostruct. 2014, 9, 809–816. [Google Scholar]
- Enculescu, M.; Matei, E. Influence of metallic and semiconducting nanostructures on the optical properties of dye-doped polymer thin films. Thin Solid Films 2016, 614, 31–35. [Google Scholar] [CrossRef]
- Görgülü, S.; Ekren, N. Energy saving in lighting system with fuzzy logic controller which uses light-pipe and dimmable ballast. Energy Build. 2013, 61, 172–176. [Google Scholar] [CrossRef]
- Krarti, M.; Erickson, P.M.; Hillman, T.C. A simplified method to estimate energy savings of artificial lighting use from daylighting. Build. Environ. 2005, 40, 747–754. [Google Scholar] [CrossRef]
- Pandharipande, A.; Caicedo, D. Daylight integrated illumination control of LED systems based on enhanced presence sensing. Energy Build. 2011, 43, 944–950. [Google Scholar] [CrossRef]
- Woelders, T.; Beersma, D.G.M.; Gordijn, M.C.M.; Hut, R.A.; Wams, E.J. Daily Light Exposure Patterns Reveal Phase and Period of the Human Circadian Clock. J. Biol. Rhythms 2017, 32, 274–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairchild, M.D. Seeing, adapting to, and reproducing the appearance of nature. Appl. Opt. 2015, 54, B107–B116. [Google Scholar] [CrossRef] [PubMed]
- Gueymard, C.A. Reference solar spectra: Their evolution, standardization issues, and comparison to recent measurements. Adv. Space Res. 2006, 37, 323–340. [Google Scholar] [CrossRef]
- Ma, S.; Wei, M.; Liang, J.; Wang, B.; Chen, Y.; Pointer, M.; Luo, M.R. Evaluation of whiteness metrics. Light. Res. Technol. 2018, 50, 429–445. [Google Scholar] [CrossRef]
- Persano, L.; Camposeo, A.; Pisignano, D. Active polymer nanofibers for photonics, electronics, energy generation and micromechanics. Prog. Polym. Sci. 2015, 43, 48–95. [Google Scholar] [CrossRef]
- Ner, Y.; Grote, J.G.; Stuart, J.A.; Sotzing, G.A. White Luminescence from Multiple-Dye-Doped Electrospun DNA Nanofibers by Fluorescence Resonance Energy Transfer. Angew. Chem. Int. Ed. 2009, 48, 5134–5138. [Google Scholar] [CrossRef] [PubMed]
- Camposeo, A.; Di Benedetto, F.; Cingolani, R.; Pisignano, D. Full color control and white emission from conjugated polymer nanofibers. Appl. Phys. Lett. 2009, 9, 043109. [Google Scholar] [CrossRef]
- Sui, X.M.; Shao, C.L.; Liu, Y.C. White-light emission of polyvinyl alcohol/ZnO hybrid nanofibers prepared by electrospinning. Appl. Phys. Lett. 2005, 87, 113115. [Google Scholar] [CrossRef]
- Liu, J.; Zhou, Q.; Cheng, Y.; Geng, Y.; Wang, L.; Ma, D.; Jing, X.; Wang, F. The First Single Polymer with Simultaneous Blue, Green, and Red Emission for White Electroluminescence. Adv. Mater. 2005, 17, 2974–2978. [Google Scholar] [CrossRef]
- Das, A.J.; Lafargue, C.; Lebental, M.; Zyss, J.; Narayan, K.S. Three-dimensional microlasers based on polymer fibers fabricated by electrospinning. Appl. Phys. Lett. 2011, 99, 263303. [Google Scholar] [CrossRef]
- Liu, Y.; He, J.-H.; Yu, J.-Y.; Zeng, H.-M. Controlling numbers and sizes of beads in electrospun nanofibers. Polym. Int. 2008, 57, 632–636. [Google Scholar] [CrossRef]
- Nezarati, R.M.; Eifert, M.B.; Cosgriff-Hernandez, E. Effects of Humidity and Solution Viscosity on Electrospun Fiber Morphology. Tissue Eng. Part C 2013, 19, 810–819. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, G.; Jackson, W.R.; Choi, C.Y.; Bergmark, W.R. Solvent Effects on Emission Yield and Lifetime for Coumarin Laser Dyes. Requirements for a Rotatory Decay Mechanism. J. Phys. Chem. 1985, 89, 294–300. [Google Scholar] [CrossRef]
- Cheng, J.-A.; Chang, C.-P.; Chen, C.-H.; Lin, M.-S. The Fluorescent Quantum Efficiency of Copolymers Containing Coumarin-6 at the Side-chain. J. Polym. Res. 2005, 12, 53–59. [Google Scholar] [CrossRef]
- Banerjee, R.; Mondal, S.; Purkayastha, P. Revival, enhancement and tuning of fluorescence from Coumarin 6: Combination of host–guest chemistry, viscosity and collisional quenching. RSC Adv. 2016, 6, 105347–105349. [Google Scholar] [CrossRef]
- Wagner, B.D. The Use of Coumarins as Environmentally-Sensitive Fluorescent Probes of Heterogeneous Inclusion Systems. Molecules 2009, 14, 210–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drexhage, K.H. Fluorescence Efficiency of Laser Dyes. J. Res. Natl. Bureau Stand. A. Phys. Chem. 1976, 80, 421–428. [Google Scholar] [CrossRef]
- Fischer, A.; Cremer, C.; Stelzer, E.H.K. Fluorescence of coumarins and xanthenes after two-photon absorption with a pulsed titanium–sapphire laser. Appl. Opt. 1995, 34, 1989–2003. [Google Scholar] [CrossRef] [PubMed]
- Seth, D.; Chakrabarty, D.; Chakraborty, A.; Sarkar, N. Study of energy transfer from 7-amino coumarin donors to rhodamine 6G acceptor in non-aqueous reverse micelles. Chem. Phys. Lett. 2005, 401, 546–552. [Google Scholar] [CrossRef]
- Speiser, S.; Chisena, F.L. Optical Bistability in Fluorescein Dyes. Appl. Phys. B 1988, 45, 137–144. [Google Scholar] [CrossRef]
- Sjoback, R.; Nygren, J.; Kubista, M. Absorption and fluorescence properties of fluorescein. Spectrochim. Acta Part A 1995, 51, L7–L21. [Google Scholar] [CrossRef]
- Jin, K.; Torkelson, J.M. Tg-confinement effects in strongly miscible blends of poly(2,6-dimethyl-1,4-phenylene oxide) and polystyrene: Roles of bulk fragility and chain segregation. Polymer 2017, 118, 85–96. [Google Scholar] [CrossRef]












| Dye | Chemical Formula | Molecular Structure | Absorption Wavelengths | Emission Wavelengths |
|---|---|---|---|---|
| Coumarin 7 | C20H19N3O2 |  | 437 and 455 nm | 485 and 505 nm |
| Coumarin 6 | C20H18N2O2S |  | 448 and 465 nm | 495 and 515 nm |
| Fluorescein | C20H12O5 |  | 430, 457, and 487 nm | 515, 550, and 575 nm |
| Rhodamine 6G | C28H31N2O3Cl |  | 534 nm | 560 nm |
| Sulforhodamine B | C27H29N2NaO7S2 |  | 550 nm | 580 nm |
| Sulforhodamine 101 | C31H29ClN2O6S2 |  | 575 nm | 590 nm |
| Solution | Percentage of Single-Dye Solution | |||||
|---|---|---|---|---|---|---|
| C7 | C6 | FL | Rh 6G | SRh B | SRh 101 | |
| Mix a | 20 | 20 | - | 20 | 20 | 20 |
| Mix b | 16.66 | 33.33 | - | 33.33 | 8.33 | 8.33 |
| Mix c | 12.5 | 25 | - | 25 | 12.5 | 25 |
| Mix 3 | 10 | 20 | 20 | 20 | 10 | 20 |
| Mix d | 12.5 | 22.5 | 22.5 | 22.5 | 10 | 10 |
| Mix e | 17 | 22.5 | 22.5 | 22.5 | 7.5 | 7.5 |
| Mix f | 5 | 25 | 25 | 25 | 5 | 5 |
| Mix 2 | 15 | 30 | 30 | 10 | 7.5 | 7.5 |
| Mix 1 | 12.5 | 25 | 40 | 7.5 | 7.5 | 7.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enculescu, M.; Evanghelidis, A.; Enculescu, I. White-Light Emission of Dye-Doped Polymer Submicronic Fibers Produced by Electrospinning. Polymers 2018, 10, 737. https://doi.org/10.3390/polym10070737
Enculescu M, Evanghelidis A, Enculescu I. White-Light Emission of Dye-Doped Polymer Submicronic Fibers Produced by Electrospinning. Polymers. 2018; 10(7):737. https://doi.org/10.3390/polym10070737
Chicago/Turabian StyleEnculescu, Monica, Alexandru Evanghelidis, and Ionut Enculescu. 2018. "White-Light Emission of Dye-Doped Polymer Submicronic Fibers Produced by Electrospinning" Polymers 10, no. 7: 737. https://doi.org/10.3390/polym10070737