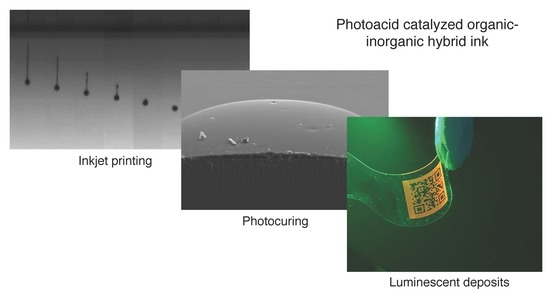
Digital Luminescence Patterning via Inkjet Printing of a Photoacid Catalysed Organic-Inorganic Hybrid Formulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
2.2.1. Ink Preparation
2.2.2. Substrates and Cleaning Procedure
2.2.3. Substrate Treatments
2.2.4. Inkjet Printing
2.2.5. UV-Curing Fixation
2.3. Characterization
2.3.1. Ink Properties Characterization
2.3.2. Flying Droplet Characterization
2.3.3. Deposited Ink and Film Characterization
3. Results and Discussion
3.1. Ink Formulation
3.2. Inkjet Printing
3.3. Photocuring process
3.4. Luminescent Properties of the Printed Films
3.5. Digital Patterning on Different Substrates
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kobayashi, S.; Mikoshiba, S.; Lim, S. Flat Panel Display Manufacturing. In LCD Backlights; John Wiley & Sons, Ltd.: West Sussex, UK, 2009. [Google Scholar]
- Konstantatos, G.; Sargent, E.H. Colloidal Quantum Dot Optoelectronics and Photovoltaics; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Debije, M.G.; Verbunt, P.P.C. Thirty Years of Luminescent Solar Concentrator Research: Solar Energy for the Built Environment. Adv. Energy Mater. 2012, 2, 12–35. [Google Scholar] [CrossRef]
- Liang, R.-Q.; Li, W.; Li, Y.; Tan, C.-Y.; Li, J.-X.; Jin, Y.-X.; Ruan, K.-C. An oligonucleotide microarray for microRNA expression analysis based on labeling RNA with quantum dot and nanogold probe. Nucleic Acids Res. 2005, 33, e17. [Google Scholar] [CrossRef] [PubMed]
- Brites, C.D.S.; Lima, P.P.; Silva, N.J.O.; Millan, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. A luminescent molecular thermometer for long-term absolute temperature measurements at the nanoscale. Adv. Mater. 2010, 22, 4499–4504. [Google Scholar] [CrossRef] [PubMed]
- Baride, A.; Meruga, J.M.; Douma, C.; Langerman, D.; Crawford, G.; Kellar, J.J.; Cross, W.M.; May, P.S. A NIR-to-NIR upconversion luminescence system for security printing applications. RSC Adv. 2015, 5, 101338–101346. [Google Scholar] [CrossRef]
- You, M.; Lin, M.; Wang, S.; Wang, X.; Zhang, G.; Hong, Y.; Dong, Y.; Jin, G.; Xu, F. Three-dimensional quick response code based on inkjet printing of upconversion fluorescent nanoparticles for drug anti-counterfeiting. Nanoscale 2016, 8, 10096–10104. [Google Scholar] [CrossRef] [PubMed]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Li, M.; Li, Y.; Jiang, J.; Gu, Z.; Zhang, X.; Jiang, L.; Song, Y. Patterning fluorescent quantum dot nanocomposites by reactive inkjet printing. Small 2015, 11, 1649–1654. [Google Scholar] [CrossRef] [PubMed]
- Shirasaki1, Y.; Supran, G.J.; Bawendi, M.G.; Bulovic, V. Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photon. 2013, 7, 13–23. [Google Scholar] [CrossRef]
- Guillou, O.; Daiguebonne, C.; Calvez, G.; Bernot, K. A long journey in lanthanide chemistry: From fundamental crystallogenesis studies to commercial anticounterfeiting taggants. Acc. Chem. Res. 2016, 49, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Frath, D.; Massue, J.; Ulrich, G.; Ziessel, R. Luminescent materials: Locking p-conjugated and heterocyclic ligands with boron(III). Angew. Chem. Int. Ed. 2014, 53, 2290–2310. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xie, Z.; Zhang, B.; Liu, Y.; Yang, W.; Liu, C.Y. Down- and up-conversion luminescent carbon dot fluid: Inkjet printing and gel glass fabrication. Nanoscale 2014, 6, 3818–3823. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.; Yang, Z.; Zheng, Z.; Liu, N.; Ling, J. Photoluminescent nanoparticles in water with tunable emission for coating and ink-jet printing. J. Mater. Chem. C 2015, 3, 3666–3675. [Google Scholar] [CrossRef]
- Lee, W.-H.; Park, Y.D. Inkjet Etching of Polymers and Its Applications in Organic Electronic Devices. Polymers 2017, 9, 441. [Google Scholar] [CrossRef]
- Homola, T.; Shekargoftar, M.; Dzik, P.; Krumpolec, R.; Durasova, Z.; Vesely, M.; Cernak, M. Low-temperature (70 °C) ambient air plasma-fabrication of inkjet-printed mesoporous TiO2 flexible photoanodes. Flex. Print. Electron. 2017, 2, 035010. [Google Scholar] [CrossRef]
- Ma, S.; Ribeiro, F.; Powell, K.; Lutian, J.; Møller, C.; Large, T.; Holbery, J. Fabrication of Novel Transparent Touch Sensing Device via Drop-on-Demand Inkjet Printing Technique. ACS Appl. Mater. Interfaces 2015, 7, 21628–21633. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Z.; Guo, Y.Z.; Cui, B.; Chu, F.Q.; Li, H.Z.; Li, Y.; He, M.; Ding, D.; Liu, R.P.; Li, L.H.; et al. Inkjet printing bendable circuits based on an oil-water interface reaction. Appl. Surf. Sci. 2018, 445, 391–397. [Google Scholar] [CrossRef]
- Sun, J.Z.; Yun, C.; Cui, B.; Li, P.; Liu, G.; Wang, X.; Chu, F. A Facile Approach for Fabricating Microstructured Surface Based on Etched Template by Inkjet Printing Technology. Polymers 2018, 10, 1209. [Google Scholar] [CrossRef]
- Sun, J.Z.; Cui, B.; Chu, F.Q.; Yun, C.H.; He, M.; Li, L.H.; Song, Y.L. Printable nanomaterials for the fabrication of high-performance supercapacitors. Nanomaterials 2018, 8, 528. [Google Scholar] [CrossRef] [PubMed]
- Bollgruen, P.; Gleissner, U.; Wolfer, T.; Megnin, C.; Mager, D.; Overmeyer, L.; Korvink, J.G.; Hanemann, T. Ink-jet printed fluorescent materials as light sources for planar optical waveguides on polymer foils. Opt. Eng. 2016, 55, 107107. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.-B.; Liu, M.; Lei, X.; Wen, T.; Zhang, J. Digital controlled luminescent emission via patterned deposition of lanthanide coordination compounds. ACS Appl. Mater. Interfaces 2014, 6, 12594–12599. [Google Scholar] [CrossRef] [PubMed]
- daLuz, L.L.; Milani, R.; Felix, J.F.; Ribeiro, I.R.B.; Talhavini, M.; Neto, B.A.D.; Chojnacki, J.; Rodrigues, M.O.; Júnior, S.A. Inkjet printing of lanthanide−organic frameworks for anti-counterfeiting applications. ACS Appl. Mater. Interfaces 2015, 7, 27115–27123. [Google Scholar] [CrossRef] [PubMed]
- Robin, M.; Kuai, W.; Amela-Cortes, M.; Cordier, S.; Molard, Y.; Mohammed-Brahim, T.; Jacques, E.; Harnois, M. Epoxy based ink as versatile material for inkjet-printed devices. ACS Appl. Mater. Interfaces 2015, 7, 21975–21984. [Google Scholar] [CrossRef] [PubMed]
- Haverinen, H.M.; Myllyla, R.A.; Jabbour, G.E. Inkjet printing of light emitting quantum dots. Appl. Phys. Lett. 2009, 94, 073108. [Google Scholar] [CrossRef]
- Andres, J.; Hersch, R.D.; Moser, J.-E.; Chauvin, A.-S. A new anti-counterfeiting feature relying on invisible luminescent full color images printed with lanthanide-based inks. Adv. Funct. Mater. 2014, 24, 5029–5036. [Google Scholar] [CrossRef]
- You, M.; Zhong, J.; Hong, Y.; Duan, Z.; Lin, M.; Xu, F. Inkjet printing of upconversion nanoparticles for anti-counterfeit applications. Nanoscale 2015, 7, 4423. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-M.; Tian, X.-T.; Zhang, H.; Yang, Z.-R.; Yin, X.-B. Anticounterfeiting quick response code with emission color of invisible metal—Organic frameworks as encoding information. ACS Appl. Mater. Interfaces 2018, 10, 22445–22452. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Haverinen, H.M.; Dhagat, P.; Jabbour, G.E. Inkjet printing-process and its applications. Adv. Mater. 2010, 22, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Derby, B. Inkjet printing of functional and structural materials: Fluid property requirements, feature, stability, and resolution. Annu. Rev. Mater. Res. 2010, 40, 395–414. [Google Scholar] [CrossRef]
- Alaman, J.; Alicante, R.; Pena, J.; Sanchez-Somolinos, C. Inkjet printing of functional materials for optical and photonic applications. Materials 2016, 9, 910. [Google Scholar] [CrossRef] [PubMed]
- Coenen, M.J.J.; Slaats, T.M.W.L.; Eggenhuisen, T.M.; Groen, P. Inkjet printing the three organic functional layers of two-colored organic light emitting diodes. Thin Solid Films 2015, 583, 194–200. [Google Scholar] [CrossRef]
- Samusjew, A.; Kratzer, M.; Moser, A.; Teichert, C.; Krawczyk, K.K.; Griesser, T. Inkjet Printing of Soft, Stretchable Optical Waveguides through the Photopolymerization of High-Profile Linear Patterns. ACS Appl. Mater. Interfaces 2017, 9, 4941–4947. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Martin-Olmos, C.; Baek, N.S.; Brugger, J. Simple and easily controllable parabolic-shaped microlenses printed on polymeric mesas. J. Mater. Chem. C 2013, 1, 2152–2157. [Google Scholar] [CrossRef]
- Descombes, L.J.; Cadarso, V.J.; Schleunitz, A.; Grutzner, S.; Klein, J.J.; Brugger, J.; Schift, H.; Grutzner, G. Organic-inorganic-hybrid-polymer microlens arrays with tailored optical characteristics and multi-focal properties. Opt. Express 2015, 23, 25365–25376. [Google Scholar] [CrossRef] [PubMed]
- Eggenhuisen, T.M.; Galagan, Y.; Biezemans, A.F.K.V.; Slaats, T.M.W.L.; Voorthuijzen, W.P.; Kommeren, S.; Shanmugam, S.; Teunissen, J.P.; Hadipour, A.; Verhees, W.J.H.; et al. High efficiency, fully inkjet printed organic solar cells with freedom of design. J. Mater. Chem. A 2015, 3, 7255–7262. [Google Scholar] [CrossRef] [Green Version]
- Tekin, E.; Smith, P.J.; Hoeppener, S.; van den Berg, A.M.J.; Susha, A.S.; Rogach, A.L.; Feldmann, J.; Schubert, U.S. Inkjet printing of luminescent CdTe nanocrystal–polymer composites. Adv. Funct. Mater. 2007, 17, 23–28. [Google Scholar] [CrossRef]
- Tekin, E.; de Gans, B.-J.; Schubert, U.S. Ink-jet printing of polymers from single dots to thin film libraries. J. Mater. Chem. 2004, 14, 2627–2632. [Google Scholar] [CrossRef]
- Jacot-Descombes, L.; Gullo, M.R.; Cadarso, V.J.; Brugger, J. Fabrication of epoxy spherical microstructures by controlled drop-on-demand inkjet printing. J. Micromech. Microeng. 2012, 22, 074012. [Google Scholar] [CrossRef] [Green Version]
- Bollgruen, P.; Gleissner, U.; Megnin, C.; Mager, D.; Korvink, J.; Hanemann, T. Ink-jet printing of host-guest systems based on acrylates with fluorescent dopants. SPIE Proc. 2016. [Google Scholar] [CrossRef]
- Serra, A.; Ramis, X.; Fernández-Francos, X. Epoxy sol-gel hybrid thermosets. Coatings 2016, 6, 8. [Google Scholar] [CrossRef]
- Sanchez, C.; Lebeau, B.; Chaput, F.; Boilot, J.-P. Optical properties of functional hybrid organic-inorganic nanocomposites. Adv. Mater. 2003, 15, 1969–1994. [Google Scholar] [CrossRef]
- Houbertz, R.; Frohlich, L.; Popall, M.; Streppel, U.; Dannberg, P.; Bräuer, A.; Serbin, J.; Chichkov, B.N. Inorganic-Organic Hybrid Polymers for Information Technology: From Planar Technology to 3D Nanostructures. Adv. Eng. Mater. 2003, 5, 551–555. [Google Scholar] [CrossRef]
- Sanchez, C.; Belleville, P.; Popall, M.; Nicole, L. Applications of advanced hybrid organic–inorganic nanomaterials: From laboratory to market. Chem. Soc. Rev. 2011, 40, 696–753. [Google Scholar] [CrossRef] [PubMed]
- Parola, S.; Julian-Lopez, B.; Carlos, L.D.; Sanchez, C. Optical properties of hybrid organic-inorganic materials and their applications. Adv. Funct. Mater. 2016, 26, 6506–6544. [Google Scholar] [CrossRef]
- Sriramulu, D.; Turaga, S.P.; Yi, A.X.; Bettiol, A.A.; Valiyaveettil, S. Synthesis, characterization and application of luminescent silica nanomaterials. J. Mater. Chem. C 2016, 4, 11190–11197. [Google Scholar] [CrossRef]
- Hong, Y.; Chen, Z.; Trofimov, A.A.; Lei, J.; Chen, J.; Yuan, L.; Zhu, W.; Xiao, H.; Xu, D.; Jacobsohn, L.G.; et al. Direct inkjet printing of miniaturized luminescent YAG:Er3+ from sol-gel precursor. Opt. Mater. 2017, 68, 11–18. [Google Scholar] [CrossRef]
- Alamán, J.; López-Valdeolivas, M.; Alicante, R.; Medel, F.J.; Silva-Treviño, J.; Peña, J.I.; Sánchez-Somolinos, C. Photoacid catalyzed organic–inorganic hybrid inks for the manufacturing of inkjet-printed photonic devices. J. Mater. Chem. C 2018, 6, 3882–3894. [Google Scholar] [CrossRef]
- Chemtob, A.; Versace, D.-L.; Belon, C.; Croutxe-Barghorn, C.; Rigolet, S. Concomitant organic-inorganic UV-curing catalyzed by photoacids. Macromolecules 2008, 41, 7390–7398. [Google Scholar] [CrossRef]
- Chemtob, A.; Peter, M.; Belon, C.; Dietlin, C.; Croutxé- Barghorn, C.; Vidal, L.; Rigolet, S. Macroporous organosilica films via a template-free photoinduced sol–gel process. J. Mater. Chem. 2010, 20, 9104–9112. [Google Scholar] [CrossRef]
- Croutxé-Barghon, C.; Belon, C.; Chemtob, A. Polymerization of hybrid sol-gel materials catalyzed by photoacids generation. J. Photopolym. Sci. Technol. 2010, 23, 129–134. [Google Scholar] [CrossRef]
- Danzebrink, R.; Aegerter, M.A. Deposition of micropatterned coating using an ink-jet technique. Thin Solid Films 1999, 351, 115–118. [Google Scholar] [CrossRef]
- Danzebrink, R.; Aegerter, M.A. Deposition of optical microlens arrays by ink-jet processes. Thin Solid Films 2001, 392, 223–225. [Google Scholar] [CrossRef]
- Fujii, T.; Isbii, A.; Anpo, M. Absorption and fluorescence spectra of rhodamine B molecules encapsulated in silica gel networks and their thermal stability. J. Photochem. Photobiol. A 1990, 54, 231–237. [Google Scholar] [CrossRef]
- Severin-Vantilt, M.M.E.; Oomen, E.W.J.L. The incorporation of Rhodamine B in silica sol-gel layers. J. Non-Cryst. Solids 1993, 159, 38–48. [Google Scholar] [CrossRef]
- Zareba-Grodz, I.; Pazik, R.; Hermanowicz, K.; Strek, W.; Maruszewski, K. Preparation and optical properties of hybrid coatings based on epoxy-modified silane and rhodamine B. J. Lumin. 2006, 119–120, 148–152. [Google Scholar] [CrossRef]
- del Monte, F.; Levy, D. Formation of fluorescent Rhodamine B J-dimers in sol-gel glasses induced by the adsorption geometry on the silica surface. J. Phys. Chem. B 1998, 102, 8036–8041. [Google Scholar] [CrossRef]
- Schulz-Ekloff, G.; Wöhrle, D.; van Duffel, B.; Schoonheydt, R.A. Chromophores in porous silicas and minerals: Preparation and optical properties. Microporous Mesoporous Mater. 2002, 51, 91–138. [Google Scholar] [CrossRef]
- Nedelcev, T.; Racko, D.; Krupa, I. Preparation and characterization of a new derivative of rhodamine B with an alkoxysilane moiety. Dyes Pigm. 2008, 76, 550–556. [Google Scholar] [CrossRef]
- Negishi, N.; Fujino, M.; Yamashita, H.; Fox, M.A.; Anpo, M. Photophysical properties and photochemical stability of Rhodamine B encapsulated in S1O2 and Si-Ti binary oxide matrices by the sol-gel method. Langmuir 1994, 10, 1772–1776. [Google Scholar] [CrossRef]
- Fink, C.K.; Nakamura, K.; Ichimura, S.; Jenkins, S.J. Silicon oxidation by ozone. J. Phys. Condens. Matter 2009, 21, 183001–183020. [Google Scholar] [CrossRef] [PubMed]
- Schuhmacher, B.; Muschenborn, W.; Stratmann, M.; Schultrich, B.; Klages, C.-P.; Kretschmer, M.; Seyfert, U.; Forster, F.; Tiller, H.-J. Novel coating systems and surface technologies for continuous processing of steel sheet. Adv. Eng. Mater. 2001, 3, 681–689. [Google Scholar] [CrossRef]
- Standard ASTM D3359-02. Standard Test Methods for Measuring Adhesion by Tape Test; ASTM International: West Conshocken, PA, USA, 2002. [Google Scholar]
- Hoath, S.D.; Hutchings, I.M.; Martin, G.D.; Tuladhar, T.R.; Mackley, M.R.; Vadillo, D. Links between ink rheology, drop-on-demand jet formation, and printability. J. Imaging Sci. Technol. 2009, 53, 412081–412088. [Google Scholar] [CrossRef]
- Hoath, S.D.; Vadillo, D.C.; Harlen, O.G.; McIlroy, C.; Morrison, N.F.; Hsiao, W.-K.; Tuladhar, T.R.; Jung, S.; Martin, G.D.; Hutchings, I.M. Inkjet printing of weakly elastic polymer solutions. J. Non-Newton. Fluid Mech. 2014, 205, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Derby, B.; Reis, N.; Seerden, K.A.M.; Grant, P.S.; Evans, J.R.G. Freeform fabrication of ceramics by hot-melt ink-jet printing. MRS Proc. 2000. [Google Scholar] [CrossRef]
- Duineveld, P.C.; de Kok, M.M.; Buechel, M.; Sempel, A.; Mutsaers, K.A.; van de Weijer, P.; Camps, I.G.J.; van de Biggelaar, T.; Rubingh, J.-E.J.M.; Haskal, E.I. Ink-jet printing of polymer light-emitting devices. Proc. SPIE 2002. [Google Scholar] [CrossRef]
- Stow, C.D.; Hadfield, M.G. An experimental investigation of fluid flow resulting from the impact of a water drop with an unyielding dry surface. Proc. R. Soc. Lond. A Math. Phys. Eng. Sci. 1981, 373, 419–441. [Google Scholar] [CrossRef]
- Bhola, R.; Chandra, S. Parameters controlling solidification of molten wax droplets falling on a solid surface. J. Mater. Sci. 1999, 34, 4883–4894. [Google Scholar] [CrossRef]
- Bartasun, P.; Cieslinski, H.; Bujacz, A.; Wierzbicka-Wos, A.; Kur, J. A study on the interaction of Rhodamine B with Methylthioadenosine Phosphorylase protein sourced from an antarctic soil metagenomic library. PLoS ONE 2013, 8, e55697. [Google Scholar] [CrossRef] [PubMed]
- De Paz, H.; Chemtob, A.; Croutxé-Barghorn, C.; Le Nouen, D.; Rigolet, S. Insights into Photoinduced Sol-Gel Polymerization: An in Situ Infrared Spectroscopy Study. J. Phys. Chem. B 2012, 116, 5260–5268. [Google Scholar] [CrossRef] [PubMed]
- Chemtob, A.; Ni, L.; Dietlin, C.; Croutxé-Barghorn, C.; Kitzmann, P.; Brogly, M.; Vidal, L. Spontaneous photoinduced formation of hybrid polymer films with functionalized macroporous patterns. Surf. Coat. Technol. 2012, 209, 64–72. [Google Scholar] [CrossRef]
- Keller, S.; Blagoi, G.; Lillemose, M.; Haefliger, D.; Boisen, A. Processing of thin SU-8 films. J. Micromech. Microeng. 2008, 18, 125020. [Google Scholar] [CrossRef]
- Sangermano, M.; Razza, N.; Crivello, J.V. Cationic UV-Curing: Technology and Applications. Macromol. Mater. Eng. 2014, 299, 775–793. [Google Scholar] [CrossRef]
- Nunes, P.S.; Ohlsson, P.D.; Ordeig, O.; Kutter, J.P. Cyclic olefin polymers: Emerging materials for lab-on-a-chip applications. Microfluid. Nanofluid. 2010, 9, 145–161. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alamán, J.; López-Valdeolivas, M.; Alicante, R.; Peña, J.I.; Sánchez-Somolinos, C. Digital Luminescence Patterning via Inkjet Printing of a Photoacid Catalysed Organic-Inorganic Hybrid Formulation. Polymers 2019, 11, 430. https://doi.org/10.3390/polym11030430
Alamán J, López-Valdeolivas M, Alicante R, Peña JI, Sánchez-Somolinos C. Digital Luminescence Patterning via Inkjet Printing of a Photoacid Catalysed Organic-Inorganic Hybrid Formulation. Polymers. 2019; 11(3):430. https://doi.org/10.3390/polym11030430
Chicago/Turabian StyleAlamán, Jorge, María López-Valdeolivas, Raquel Alicante, Jose Ignacio Peña, and Carlos Sánchez-Somolinos. 2019. "Digital Luminescence Patterning via Inkjet Printing of a Photoacid Catalysed Organic-Inorganic Hybrid Formulation" Polymers 11, no. 3: 430. https://doi.org/10.3390/polym11030430