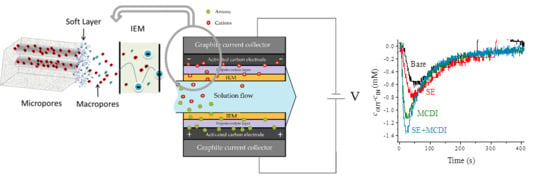
Assembly of Soft Electrodes and Ion Exchange Membranes for Capacitive Deionization
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Setup
3. Results and Discussion
3.1. Effect of the Applied Field Strength
- Bare activated carbon electrodes (Bare)
- Polyelectrolyte-coated electrodes, or soft electrodes (SEs)
- Ion exchange membranes in contact with the electrodes (MCDI)
- Combination of polyelectrolyte coating with the use of membranes (SE + MCDI)
3.2. Zero-Voltage versus Reverse-Voltage Desorption
3.3. Comparison of Different Saline Concentration Solutions
3.4. Salt Adsorption, Efficiency and Consumed Energy
4. Theoretical Predictions
4.1. Mass Balance Equation
4.1.1. Spacer Channel
4.1.2. Membranes
4.1.3. Electrodes
4.2. Boundary Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blair, J.W.; Murphy, G.W. Electrochemical Demineralization of Water with Porous Electrodes of Large Surface Area. In Saline Water Conversion; ACS: Washington, DC, USA, 1960; pp. 206–223. [Google Scholar]
- Porada, S.; Zhao, R.; van der Wal, A.; Presser, V.; Biesheuvel, P.M. Review on the science and technology of water desalination by capacitive deionization. Prog. in Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef] [Green Version]
- Jia, B.P.; Zou, L.D. Graphene nanosheets reduced by a multi-step process as high-performance electrode material for capacitive deionisation. Carbon 2012, 50, 2315–2321. [Google Scholar] [CrossRef]
- Rasines, G.; Lavela, P.; Macias, C.; Zafra, M.C.; Tirado, J.L.; Parra, J.B.; Ania, C.O. N-doped monolithic carbon aerogel electrodes with optimized features for the electrosorption of ions. Carbon 2015, 83, 262–274. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Wang, L.; Yu, F. Water-enhanced performance in capcitive deionization for desalination based on graphene gel as electrode material. Electrochim. Acta 2018, 263, 40–46. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Chen, C.; Yu, F.; Ma, J. A Comparison of graphene hydrogels modified with single-walled/multi-walled carbon nanotubes as electrode materials for capacitive deionization. J. Colloid Interface Sci. 2018, 518, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Biesheuvel, P.M.; van der Wal, A. Membrane capacitive deionization. J. Membr. Sci. 2010, 346, 256–262. [Google Scholar] [CrossRef]
- Lee, J.Y.; Seo, S.J.; Yun, S.H.; Moon, S.H. Preparation of ion exchanger layered electrodes for advanced membrane capacitive deionization (MCDI). Water Res. 2011, 45, 5375–5380. [Google Scholar] [CrossRef]
- Hassanvand, A.; Wei, K.J.; Talebi, S.; Chen, G.Q.; Kentish, S.E. The Role of Ion Exchange Membranes in Membrane Capacitive Deionisation. Membranes 2017, 7, 54. [Google Scholar] [CrossRef]
- Ahualli, S.; Jimenez, M.L.; Fernandez, M.M.; Iglesias, G.; Brogioli, D.; Delgado, A.V. Polyelectrolyte-coated carbons used in the generation of blue energy from salinity differences. Phys. Chem. Chem. Phys. 2014, 16, 25241–25246. [Google Scholar] [CrossRef] [Green Version]
- Ahualli, S.; Iglesias, G.R.; Fernandez, M.M.; Jimenez, M.L.; Delgado, A.V. Use of Soft Electrodes in Capacitive Deionization of Solutions. Environ. Sci. Technol. 2017, 51, 5326–5333. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, J.H. Improvement of desalination efficiency in capacitive deionization using a carbon electrode coated with an ion-exchange polymer. Water Res. 2010, 44, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.M.; Wagterveld, R.M.; Ahualli, S.; Liu, F.; Delgado, A.V.; Hamelers, H.V.M. Polyelectrolyte-versus membrane-coated electrodes for energy production by capmix salinity exchange methods. J. Power Sources 2016, 302, 387–393. [Google Scholar] [CrossRef]
- Gao, X.; Omosebi, A.; Landon, J.; Liu, K.L. Surface charge enhanced carbon electrodes for stable and efficient capacitive deionization using inverted adsorption-desorption behavior. Energy Environ. Sci. 2015, 8, 897–909. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Zhao, R.; Porada, S.; van der Wal, A. Theory of membrane capacitive deionization including the effect of the electrode pore space. J. Colloid Interface Sci. 2011, 360, 239–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suss, M.E.; Porada, S.; Sun, X.; Biesheuvel, P.M.; Yoon, J.; Presser, V. Water desalination via capacitive deionization: what is it and what can we expect from it? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Gao, Y.; Pan, L.; Zhang, Y.; Chen, Y.; Sun, Z. Electrosorptive desalination by carbon nanotubes and nanofibres electrodes and ion-exchange membranes. Water Res. 2008, 42, 4923–4928. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; van Soestbergen, M.; Rijnaarts, H.H.M.; van der Wal, A.; Bazant, M.Z.; Biesheuvel, P.M. Time-dependent ion selectivity in capacitive charging of porous electrodes. J. Colloid Interface Sci. 2012, 384, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Choi, J.-H. Enhanced desalination efficiency in capacitive deionization with an ion-selective membrane. Sep. Purif. Technol. 2010, 71, 70–75. [Google Scholar] [CrossRef]
- Porada, S.; Borchardt, L.; Oschatz, M.; Bryjak, M.; Atchison, J.S.; Keesman, K.J.; Kaskel, S.; Biesheuvel, P.M.; Presser, V. Direct prediction of the desalination performance of porous carbon electrodes for capacitive deionization. Energy Environ. Sci. 2013, 6, 3700–3712. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.W.; He, D.; Zhang, C.Y.; Kovalsky, P.; Waite, T.D. Comparison of Faradaic reactions in capacitive deionization (CDI) and membrane capacitive deionization (MCDI) water treatment processes. Water Res. 2017, 120, 229–237. [Google Scholar] [CrossRef]
- Hemmatifar, A.; Palko, J.W.; Stadermann, M.; Santiago, J.G. Energy breakdown in capacitive deionization. Water Res. 2016, 104, 303–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawks, S.A.; Ramachandran, A.; Porada, S.; Campbell, P.G.; Suss, M.E.; Biesheuvel, P.M.; Santiago, J.G.; Stadermann, M. Performance metrics for the objective assessment of capacitive deionization systems. Water Res. 2019, 152, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Biesheuvel, P.M.; Miedema, H.; Bruning, H.; van der Wal, A. Charge Efficiency: A Functional Tool to Probe the Double-Layer Structure Inside of Porous Electrodes and Application in the Modeling of Capacitive Deionization. J. Phys. Chem. Lett. 2010, 1, 205–210. [Google Scholar] [CrossRef]
- Tang, W.; Kovalsky, P.; He, D.; Waite, T.D. Fluoride and nitrate removal from brackish groundwaters by batch-mode capacitive deionization. Water Res. 2015, 84, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Biesheuvel, P.M.; van Limpt, B.; van der Wal, A. Dynamic Adsorption/Desorption Process Model for Capacitive Deionization. J. Phys. Chem. C 2009, 113, 5636–5640. [Google Scholar] [CrossRef]
- Ahualli, S.; Delgado, A.V. Charge and Energy Storage in Electrical Double Layers, 1st ed.; Academic Press-Elsevier: London, UK, 2018. [Google Scholar]
- Ohshima, H.; Kondo, T. Membrane-potential and donnan potential. Biophys. Chem. 1988, 29, 277–281. [Google Scholar] [CrossRef]
- Makino, K.; Ohshima, H.; Kondo, T. Surface-potential of an ion-penetrable charged membrane. J. Theor. Biol. 1987, 125, 367–368. [Google Scholar] [CrossRef]
- Biesheuvel, P.M.; Bazant, M.Z. Nonlinear dynamics of capacitive charging and desalination by porous electrodes. Phys. Rev. E 2010, 81, 031502. [Google Scholar] [CrossRef] [Green Version]
- Biesheuvel, P.M.; Fu, Y.Q.; Bazant, M.Z. Diffuse charge and Faradaic reactions in porous electrodes. Phys. Rev. E 2011, 83, 061507. [Google Scholar] [CrossRef] [Green Version]









© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahualli, S.; Orozco-Barrera, S.; Fernández, M.d.M.; Delgado, Á.V.; Iglesias, G.R. Assembly of Soft Electrodes and Ion Exchange Membranes for Capacitive Deionization. Polymers 2019, 11, 1556. https://doi.org/10.3390/polym11101556
Ahualli S, Orozco-Barrera S, Fernández MdM, Delgado ÁV, Iglesias GR. Assembly of Soft Electrodes and Ion Exchange Membranes for Capacitive Deionization. Polymers. 2019; 11(10):1556. https://doi.org/10.3390/polym11101556
Chicago/Turabian StyleAhualli, Silvia, Sergio Orozco-Barrera, María del Mar Fernández, Ángel V. Delgado, and Guillermo R. Iglesias. 2019. "Assembly of Soft Electrodes and Ion Exchange Membranes for Capacitive Deionization" Polymers 11, no. 10: 1556. https://doi.org/10.3390/polym11101556