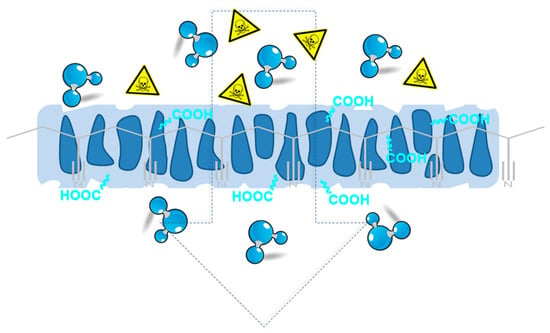
Characterization and Optimization of the Alkaline Hydrolysis of Polyacrylonitrile Membranes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PAN Membrane Preparation
2.3. Characterization Techniques
2.3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
2.3.2. UV–Vis Spectroscopy
2.3.3. X-Ray Photoelectron Spectroscopy (XPS)
2.3.4. Contact Angle
2.3.5. Scanning Electron Microscopy (SEM)
2.3.6. Mechanical Properties
2.3.7. Thermogravimetric Analyses (TGA)
3. Results
3.1. Modification of Surface Composition
3.2. Properties of Modified Membranes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aubert, J.H.; Sylwester, A.P. Morphological characterization of microcellular carbon foams. J. Mater. Sci. 1991, 26, 5741–5752. [Google Scholar] [CrossRef]
- Jain, S.; Chattopadhyay, S.; Jackeray, R.; Singh, H. Surface modification of polyacrylonitrile fiber for immobilization of antibodies and detection of analyte. Anal. Chim. Acta 2009, 654, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Akdag, A.; Zhu, C.; Kou, L.; Worley, S.D.; Huang, T.S. Electrospun polyacrylonitrile nanofibrous biomaterials. J. Biomed. Mater. Res. Part A 2009, 91, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Phadke, M.A.; Kulkarni, S.S.; Karode, S.K.; Musale, D.A. Poly(acrylonitrile) ultrafiltration membranes. II. membrane morphology and permeation characteristics. J. Polym. Sci. Part B 2005, 43, 2074–2085. [Google Scholar] [CrossRef]
- Huang, Y.H.; Huang, S.H.; Chao, W.C.; Li, C.L.; Hsieh, Y.Y.; Hung, W.S.; Liaw, D.J.; Hu, C.C.; Lee, K.R.; Lai, J.Y. A study on the characteristics and pervaporation performance of polyamide thin-film composite membranes with modified polyacrylonitrile as substrate for bioethanol dehydration. Polym. Int. 2014, 63, 1478–1486. [Google Scholar] [CrossRef]
- Wang, Z.G.; Wan, L.S.; Xu, Z.K. Surface engineerings of polyacrylonitrile-based asymmetric membranes towards biomedical applications: An overview. J. Membr. Sci. 2007, 304, 8–23. [Google Scholar] [CrossRef]
- Yang, M.C.; Lin, W.C.J. Surface modification and blood compatibility of polyacrylonitrile membrane with immobilized chitosan-heparin conjugate. Polym. Res. 2002, 9, 201–206. [Google Scholar] [CrossRef]
- Tran, T.D.; Mori, S.; Suzuki, M. Plasma modification of polyacrylonitrile ultrafiltration membrane. Thin Solid Films 2007, 515, 4148–4152. [Google Scholar] [CrossRef]
- Zhang, G.; Meng, H.; Ji, S. Hydrolysis differences of polyacrylonitrile support membrane and its influences on polyacrylonitrile-based membrane performance. Desalination 2009, 242, 313–324. [Google Scholar] [CrossRef]
- Litmanovich, A.D.; Plate, N.A. Alkaline hydrolysis of polyacrylonitrile. On the reaction mechanism. Macromol. Chem. Phys. 2000, 201, 2176–2180. [Google Scholar] [CrossRef]
- Gupta, M.L.; Gupta, B.; Oppermann, W.; Hardtmann, G. Surface modification of polyacrylonitrile staple fibers via alkaline hydrolysis for superabsorbent applications. J. Appl. Polym. Sci. 2004, 91, 3127–3133. [Google Scholar] [CrossRef]
- Michels, G.; Sackmann, G.; Struss, K. Process for the Preparation of Superabsorbent Polymers from Polyacrylonitrile Precipitation Polymers. U.S. Patent 6,573,358 B2, 3 June 2003. [Google Scholar]
- Cornelissen, E.R.; Van den Boomgaard, T.; Strathmann, H. Physicochemical aspects of polymer selection for ultrafiltration and microfiltration membranes. Colloids Surf. A 1998, 138, 283–289. [Google Scholar] [CrossRef]
- Liu, T.Y.; Lin, W.C.; Huang, L.Y.; Chen, S.Y.; Yang, M.C. Hemocompatibility and anaphylatoxin formation of protein-immobilizing polyacrylonitrile hemodialysis membrane. Biomaterials 2005, 26, 437–1444. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhang, M.; Ding, J.; Pan, F.; Jiang, Z.; Li, Y.; Zhao, J. Pervaporation dehydration of ethanol by hyaluronic acid/sodium alginate two-active-layer composite membranes. Carbohydr. Polym. 2014, 99, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yan, H.; Ji, S.; Liu, Z. Self-assembly of polyelectrolyte multilayer pervaporation membranes by a dynamic layer-by-layer technique on a hydrolyzed polyacrylonitrile ultrafiltration membrane. J. Membr. Sci. 2007, 292, 1–8. [Google Scholar] [CrossRef]
- Dyatlov, V.A.; Grebeneva, T.A.; Rustamov, I.R.; Koledenkov, A.A.; Kolotilova, N.V.; Kireev, V.V.; Prudskov, B.M. Hydrolysis of polyacrylonitrile in aqueous solution of sodium carbonate. Polym. Sci. Ser. B 2012, 54, 161–166. [Google Scholar] [CrossRef]
- Sanli, O. Homogeneous hydrolysis of polyacrylonitrile by potassium hydroxide. Eur. Polym. J. 1990, 26, 9–13. [Google Scholar] [CrossRef]
- Krentsel, L.B.; Kudryavtsev, Y.V.; Rebrov, A.I.; Litmanovich, A.D.; Platé, N.A. Acidic Hydrolysis of Polyacrylonitrile: Effect of Neighboring Group. Macromolecules 2001, 34, 5607–5610. [Google Scholar] [CrossRef]
- Wei, J.; Qiu, C.; Tang, C.Y.; Wang, R.; Fane, A.G. Synthesis and characterization of flat-sheet thin film composite forward osmosis membranes. J. Memb. Sci. 2011, 372, 292–302. [Google Scholar] [CrossRef]
- Liu, Y.; He, T.; Gao, C. Surface modification of poly(ethylene terephthalate) via hydrolysis and layer-by-layer assembly of chitosan and chondroitin sulfate to construct cytocompatible layer for human endothelial cells. Colloids Surf. B 2005, 46, 117–126. [Google Scholar] [CrossRef]
- Xu, B.; Wang, X.; Lu, Y. Surface modification of polyacrylonitrile-based carbon fiber and its interaction with imide. Appl. Surf. Sci. 2006, 253, 2695–2701. [Google Scholar] [CrossRef]
- Bao, W.; Xu, Z.; Yang, H. Electrokinetic and permeation characterization of hydrolyzed polyacrylonitrile (PAN) hollow fiber ultrafiltration membrane. Sci. China Ser. B Chem. 2009, 52, 683–689. [Google Scholar] [CrossRef]
- Deng, S.; Bai, R.; Chen, J.P. Behaviors and mechanisms of copper adsorption on hydrolyzed polyacrylonitrile fibers. J. Colloid Interface Sci. 2003, 260, 265–272. [Google Scholar] [CrossRef]
- Pirlot, B.C.; Willems, I.; Fonseca, A.; Nagy, J.B.; Delhalle, J. Preparation and Characterization of Carbon Nanotube/Polyacrylonitrile Composites. J. Adv. Eng. Mater. 2002, 4, 1–11. [Google Scholar] [CrossRef]
- Tiraferri, A.; Elimelech, M. Direct quantification of negatively charged functional groups on membrane surfaces. J. Membr. Sci. 2012, 389, 499–508. [Google Scholar] [CrossRef]
- Rosmaninho, M.G.; Jardim, E.; Moura, F.C.C.; Ferreira, G.L.; Thom, V.; Yoshida, M.I.; Araujo, M.H.; Lago, R.M. Surface hydrolysis of postconsumer polyethylene terephthalate to produce adsorbents for cationic contaminants. J. Appl. Polym. Sci. 2006, 102, 5284–5291. [Google Scholar] [CrossRef]
- Saren, Q.; Qiu, C.Q.; Tang, C.Y. Synthesis and characterization of novel forward osmosis membranes based on layer-by-layer assembly. Environ. Sci. Technol. 2011, 45, 5201–5208. [Google Scholar] [CrossRef]
- Mirbaha, H.; Arbab, S.; Zeinolebadi, A.; Nourpanah, P. An investigation on actuation behavior of polyacrylonitrile gel fibers as a function of microstructure and stabilization temperature. Smart Mater. Struct. 2013, 22, 1–12. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Álvarez, L.; Ruiz-Rubio, L.; Moreno, I.; Vilas-Vilela, J.L. Characterization and Optimization of the Alkaline Hydrolysis of Polyacrylonitrile Membranes. Polymers 2019, 11, 1843. https://doi.org/10.3390/polym11111843
Pérez-Álvarez L, Ruiz-Rubio L, Moreno I, Vilas-Vilela JL. Characterization and Optimization of the Alkaline Hydrolysis of Polyacrylonitrile Membranes. Polymers. 2019; 11(11):1843. https://doi.org/10.3390/polym11111843
Chicago/Turabian StylePérez-Álvarez, Leyre, Leire Ruiz-Rubio, Isabel Moreno, and José Luis Vilas-Vilela. 2019. "Characterization and Optimization of the Alkaline Hydrolysis of Polyacrylonitrile Membranes" Polymers 11, no. 11: 1843. https://doi.org/10.3390/polym11111843