Combining Fluorinated Polymers with Ag Nanoparticles as a Route to Enhance Optical Properties of Composite Materials
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Characterization Techniques
3. Results and Discussion
3.1. Chemical Composition of the PTFE/Ag/PTFE Nanocomposites Surface (XPS)
3.2. Morphological Characteristics
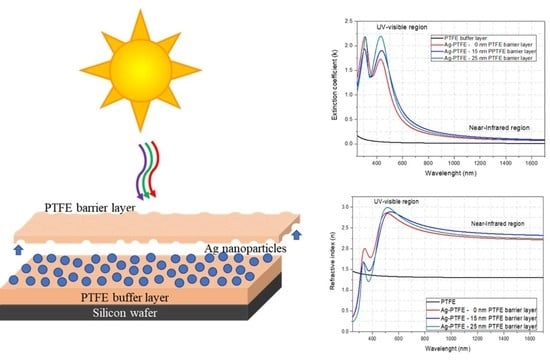
3.3. Optical Characterization of the PTFE/Ag/PTFE Nanocomposites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schürmann, U.; Hartung, W.; Takele, H.; Zaporojtchenko, V.; Faupel, F. Controlled syntheses of Ag–polytetrafluoroethylene nanocomposite thin films by co-sputtering from two magnetron sources. Nanotechnology 2005, 16, 1078–1082. [Google Scholar] [CrossRef]
- Biswas, A.; Aktas, O.C.; Schürmann, U.; Saeed, U.; Zaporojtchenko, V.; Faupel, F. Tunable multiple plasmon resonance wavelengths response from multicomponent polymer-metal nanocomposite systems. Appl. Phys. Lett. 2004, 84. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Schadler, L.S.; Braun, P.V. Nanocomposite Science and Technology; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Amjadi, M.; Pichitpajongkit, A.; Lee, S.; Ryu, S.; Park, I. Highly Stretchable and Sensitive Strain Sensor Based on Silver Nanowire–Elastomer Nanocomposite. ACS Nano 2014, 8, 5154–5163. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Bakr, O.M.; Stellacci, F. A Study of the Surface Plasmon Resonance of Silver Nanoparticles by the Discrete Dipole Approximation Method: Effect of Shape, Size, Structure, and Assembly. Plasmonics 2010, 5, 85–97. [Google Scholar] [CrossRef]
- Siegel, J.; Polívková, M.; Kasálková, N.S.; Kolská, Z.; Švorčík, V. Properties of silver nanostructure-coated PTFE and its biocompatibility. Nanoscale Res. Lett. 2013, 8, 388. [Google Scholar] [CrossRef] [Green Version]
- Zaporojtchenko, V.; Podschun, R.; Schürmann, U.; Kulkarni, A.; Faupel, F. Physico-chemical and antimicrobial properties of co-sputtered Ag–Au/PTFE nanocomposite coatings. Nanotechnology 2006, 17, 4904–4908. [Google Scholar] [CrossRef]
- Caseri, W. Nanocomposites of polymers and metals or semiconductors: Historical background and optical properties. W. Macromol. Rapid Commun. 2000, 21, 705. [Google Scholar] [CrossRef]
- Dislich, H. Plastics as optical materials. Angew. Chem. Int. Ed. Engl. 1979, 18, 49–59. [Google Scholar] [CrossRef]
- Lü, C.; Yang, B. High refractive index organic–inorganic nanocomposites: Design, synthesis and application. J. Mater. Chem. 2009, 19, 2884–2901. [Google Scholar] [CrossRef]
- Caseri, W.R. Nanocomposites of polymers and inorganic particles: Preparation, structure and properties. Mater. Sci. Technol. 2006, 22, 7. [Google Scholar] [CrossRef]
- Okutsu, R.; Ando, S.; Ueda, M. Sulfur-Containing Poly(meth)acrylates with High Refractive Indices and High Abbe’s Numbers. Chem. Mater. 2008, 20, 4017. [Google Scholar] [CrossRef]
- Nebioglu, A.; Leon, J.A.; Khudyakov, I.V. New UV-Curable High Refractive Index Oligomers. Ind. Eng. Chem. Res. 2008, 47, 2155. [Google Scholar] [CrossRef]
- Krogman, K.C.; Druffel, T.; Sunkara, M.K. Anti-reflective optical coatings incorporating nanoparticles. Nanotechnology 2005, 16, S338–S343. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.G.; Ueda, M. High refractive index polymers: Fundamental research and practical applications. J. Mater. Chem. 2009, 19, 8907–8919. [Google Scholar] [CrossRef]
- Ehrlich, P. Dielectric Properties of Teflon from Room Temperature to 314 °C and from Frequencies of 102 to 105 c/s. J. Res. Nat. Bureau Standards 1953, 51, 2449. [Google Scholar] [CrossRef]
- Yang, M.K.; French, R.H.; Tokarsky, E.W. Optical properties of Teflon® AF amorphous fluoropolymers. J. Micro/Nanolith MEMS MOEMS 2008, 7, 033010. [Google Scholar] [CrossRef]
- Son, Y.; Yeo, J.; Ha, C.W.; Lee, J.; Hong, S.; Nam, K.H.; Yang, D.-Y.; Ko, S.H. Application of the specific thermal properties of Ag nanoparticles to high-resolution metal patterning. Thermochim. Acta 2012, 542, 52–56. [Google Scholar] [CrossRef]
- Sadrolhosseini, A.R.; Noor, A.S.M.; Shameli, K.; Mamdoohi, G.; Moksin, M.; Mahdi, M.A. Laser ablation synthesis and optical properties of copper nanoparticles. J. Mat. Res. 2013, 28, 2629–2636. [Google Scholar] [CrossRef] [Green Version]
- Beyene, H.T.; Weber, J.W.; Verheijen, M.A.; van de Sanden, M.C.M.; Creatore, M. Real time in situ spectroscopic ellipsometry of the growth and plasmonic properties of au nanoparticles on SiO2. Nano Res. 2012, 5, 513–520. [Google Scholar] [CrossRef]
- Satulu, V.; Mitu, B.; Pandele, A.M.; Voicu, S.I.; Kravets, L.; Dinescu, G. Composite polyethylene terephthalate track membranes with thin teflon-like layers: Preparation and surface properties. Appl. Surf. Sci. 2019, 476, 452–459. [Google Scholar] [CrossRef]
- Matikainen, A.; Nuutinen, T.; Itkonen, T.; Heinilehto, S.; Puustinen, J.; Hiltunen, J.; Lappalainen, J.; Karioja, P.; Vahimaa, P. Atmospheric oxidation and carbon contamination of silver and its effect on surface-enhanced Raman spectroscopy (SERS). Sci. Rep. 2016, 6, 37192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffiths, A.J.V.; Walther, T. Quantification of carbon contamination under electron beam irradiation in a scanning transmission electron microscope and its suppression by plasma cleaning. J. Phys. Conf. Ser. 2010, 241, 012017. Available online: www.nature.com (accessed on 13 July 2018). [CrossRef]
- Available online: http://www.xpsfitting.com/search/label/Silver?fbclid=IwAR0iAtXoq534oqHqniKcAS-uvZbWtK-Y80FsvODJRw6_zgogGunhDD8YxnY (accessed on 13 July 2018).
- Available online: https://srdata.nist.gov/xps/main (accessed on 13 July 2018).
- Hubert, J.; Mertens, J.; Dufour, T.; Vandencasteele, N.; Reniers, F.; Viville, P.; Lazzaroni, R.; Raes, M.; Terryn, H. Synthesis and texturization processes of (super)-hydrophobic fluorinated surfaces by atmospheric plasma. J. Mater. Res. 2015, 30, 3177–3191. [Google Scholar] [CrossRef] [Green Version]
- Bodas, D.S.; Mandale, A.B.; Gangal, S.A. Deposition of PTFE thin films by RF plasma sputtering on 〈1 0 0〉 silicon substrates. Appl. Surf. Sci. 2005, 245, 202–207. [Google Scholar] [CrossRef]
- Limb, S.J.; Labelle, C.B.; Gleason, K.K.; Edell, D.J.; Gleason, E.F. Growth of fluorocarbon polymer thin films with high CF2 fractions and low dangling bond concentrations by thermal chemical vapor deposition. Appl. Phys. Lett. 1996, 68, 2810–2812. [Google Scholar] [CrossRef]
- Piwowarczyk, J.; Jedrzejewski, R.; Moszynski, D.; Kwiatkowski, K.; Niemczyk, A.; Baranowska, J. XPS and FTIR Studies of Polytetrafluoroethylene Thin Films Obtained by Physical Methods. Polymers 2019, 11, 1629. [Google Scholar] [CrossRef] [Green Version]
- Satulu, V.; Mitu, B.; Altynov, V.A.; Lizunov, N.E.; Kravets, L.; Dinescu, G. Synthesis and characterization of porous composite membranes with hydrophilic/hydrophobic sides. Thin Solid Films 2017, 630, 92–99. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Chen, C.; Huang, S. Atomic-Scale Observation of Migration and Coalescence of Au Nanoclusters on YSZ Surface by Aberration-Corrected STEM. Sci. Rep. 2014, 4, 5521. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, H. Spectroscopic Ellipsometry Principles and Applications; Wiley: Chichester, UK, 2007. [Google Scholar]
- Aziz, S.B.; Abdulwahid, R.T.; Rasheed, M.A.; Abdullah, O.G.; Ahmed, H.M. Polymer Blending as a Novel Approach for Tuning the SPR Peaks of Silver Nanoparticles. Polymers 2017, 9, 486. [Google Scholar] [CrossRef]
- Murray, W.A.; Astilean, S.; Barnes, W.L. Transition from localized surface plasmon resonance to extended surface plasmon-polariton as metallic nanoparticles merge to form a periodic hole array. Phys. Rev. B 2004, 69, 165407. [Google Scholar] [CrossRef] [Green Version]
- Hsu, D.T.; Kim, H.K.; Shi, F.G. Low and High Dielectric Constant Materials: Materials Science, Processing, and Reliability Issues: Proceedings of the Fourth International Symposium: And, Thin Film Materials for Advanced Packaging Technologies: Proceedings of the Second International Symposium. Electrochem. Soc. Proc. 2000, 99, 66. [Google Scholar]
- Radhakrishnan, A.; Murugesan, V. Calculation of the extinction cross section and lifetime of a gold nanoparticle using FDTD simulations. AIP Conf. Proc. 2014, 52, 1620. [Google Scholar] [CrossRef]
- Daghestani, H.N.; Day, B.W. Theory and Applications of Surface Plasmon Resonance, Resonant Mirror, Resonant Waveguide Grating, and Dual Polarization Interferometry Biosensors. Sensors 2010, 10, 9630–9646. [Google Scholar] [CrossRef] [PubMed]
- Duche, D.; Torchio, P.; Escoubas, L.; Monestier, F.; Simon, J.J.; Flory, F.; Mathian, G. Improving light absorption in organic solar cells by plasmonic contribution. Solar Energy Mater. Solar Cells 2009, 93, 1377–1382. [Google Scholar] [CrossRef]
- Shpacovitch, V.J. Application of Surface Plasmon Resonance (SPR) for the Detection of Single Viruses and Single Biological Nano-objects. Bacteriol. Parasitol. 2012, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Prelipceanu, M.; Tudose, O.G.; Prelipceanu, O.S.; Schrader, S.; Grytsenko, K. Study of oriented growth of oligofluorene–thiophene films onto aligned vacuum-deposited polytetrafluoroethylene layers. Mat. Sci. Semicond. Proc. 2007, 10, 24–35. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, H.; Kim, B.H.; Chang, T.; Lim, J.; Jin, H.M.; Mun, J.H.; Choi, Y.J.; Chung, K.; Shin, J.; et al. Highly tunable refractive index visible-light metasurface from block copolymer self-assembly. Nat. Commun. 2016, 7, 12911. [Google Scholar] [CrossRef] [Green Version]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satulu, V.; Mitu, B.; Ion, V.; Marascu, V.; Matei, E.; Stancu, C.; Dinescu, G. Combining Fluorinated Polymers with Ag Nanoparticles as a Route to Enhance Optical Properties of Composite Materials. Polymers 2020, 12, 1640. https://doi.org/10.3390/polym12081640
Satulu V, Mitu B, Ion V, Marascu V, Matei E, Stancu C, Dinescu G. Combining Fluorinated Polymers with Ag Nanoparticles as a Route to Enhance Optical Properties of Composite Materials. Polymers. 2020; 12(8):1640. https://doi.org/10.3390/polym12081640
Chicago/Turabian StyleSatulu, Veronica, Bogdana Mitu, Valentin Ion, Valentina Marascu, Elena Matei, Cristian Stancu, and Gheorghe Dinescu. 2020. "Combining Fluorinated Polymers with Ag Nanoparticles as a Route to Enhance Optical Properties of Composite Materials" Polymers 12, no. 8: 1640. https://doi.org/10.3390/polym12081640