Micellar Nanocarriers from Dendritic Macromolecules Containing Fluorescent Coumarin Moieties
Abstract
:1. Introduction
2. Results and Discussion
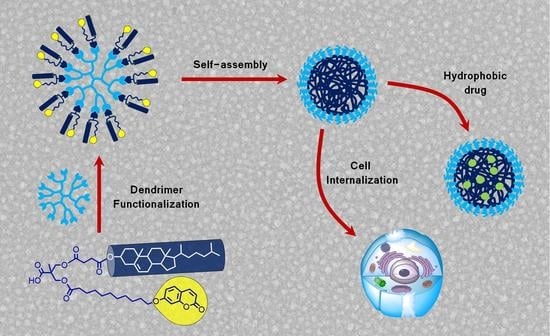
2.1. Synthesis and Characterization of Dendrimers
2.2. Synthesis and Characterization of Dendrimers
2.3. Self-Assembly of the Dendrimers in Water
2.4. Absorption and Emission Properties of the Self-Assemblies
2.5. Cytotoxicity and Celullar Uptake of the Self-Assemblies
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Yin, M. Design and development of fluorescent nanostructures for bioimaging. Prog. Polym. Sci. 2014, 39, 365–395. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Trzcinska, R.; Trzebicka, B.; Müller, A.H.E.; Dworak, A.; Tsvetanov, C.B. Loading of polymer nanocarriers: Factors, mechanisms and applications. Prog. Polym. Sci. 2014, 39, 43–86. [Google Scholar] [CrossRef]
- Smart, T.; Lomas, H.; Massignani, M.; Flores-Merino, M.V.; Perez, L.R.; Battaglia, G. Block copolymer nanostructures. Nano Today 2008, 3, 38–46. [Google Scholar] [CrossRef]
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Soc. Rev. 2012, 41, 5969–5985. [Google Scholar] [CrossRef]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design, development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Gong, J.; Chen, M.; Zheng, Y.; Wang, S.; Wang, Y. Polymeric micelles drug delivery system in oncology. J. Control. Release 2012, 159, 312–323. [Google Scholar] [CrossRef]
- Wei, H.; Zhuo, R.-X.; Zhang, X.-Z. Design and development of polymeric micelles with cleavable links for intracellular drug delivery. Prog. Polym. Sci. 2013, 38, 503–535. [Google Scholar] [CrossRef]
- Wei, T.; Chen, C.; Liu, J.; Liu, C.; Posocco, P.; Liu, X.; Cheng, Q.; Huo, S.; Liang, Z.; Fermeglia, M.; et al. Anticancer drug nanomicelles formed by self-assembling amphiphilic dendrimer to combat cancer drug resistance. Proc. Natl. Acad. Sci. USA 2015, 112, 2978–2983. [Google Scholar] [CrossRef] [Green Version]
- Sherman, S.E.; Xiao, Q.; Percec, V. Mimicking Complex Biological Membranes and Their Programmable Glycan Ligands with Dendrimersomes and Glycodendrimersomes. Chem. Rev. 2017, 117, 6538–6631. [Google Scholar] [CrossRef]
- Liko, F.; Hindré, F.; Fernández-Megía, E. Dendrimers as Innovative Radiopharmaceuticals in Cancer Radionanotherapy. Biomacromolecules 2016, 17, 3103–3114. [Google Scholar] [CrossRef] [PubMed]
- Andrén, O.C.J.; Zhang, Y.; Lundberg, P.; Hawker, C.J.; Nyström, A.M.; Malkoch, M. Therapeutic Nanocarriers via Cholesterol Directed Self-Assembly of Well-Defined Linear-Dendritic Polymeric Amphiphiles. Chem. Mater. 2017, 29, 3891–3898. [Google Scholar] [CrossRef]
- Blasco, E.; Piñol, M.; Oriol, L. Responsive linear-dendritic block copolymers. Macromol. Rapid Commun. 2014, 35, 1090–1115. [Google Scholar] [CrossRef] [PubMed]
- Lancelot, A.; Gonzalez-Pastor, R.; Concellon, A.; Sierra, T.; Martin-Duque, P.; Serrano, J.L. DNA Transfection to Mesenchymal Stem Cells Using a Novel Type of Pseudodendrimer Based on 2,2-Bis(hydroxymethyl)propionic Acid. Bioconjugate Chem. 2017, 28, 1135–1150. [Google Scholar] [CrossRef] [PubMed]
- Percec, V.; Wilson, D.A.; Leowanawat, P.; Wilson, C.J.; Hughes, A.D.; Kaucher, M.S.; Hammer, D.A.; Levine, D.H.; Kim, A.J.; Bates, F.S.; et al. Self-Assembly of Janus Dendrimers into Uniform Dendrimersomes and Other Complex Architectures. Science 2010, 328, 1009–1014. [Google Scholar] [CrossRef]
- Movellan, J.; Urbán, P.; Moles, E.; de la Fuente, J.M.; Sierra, T.; Serrano, J.L.; Fernàndez-Busquets, X. Amphiphilic dendritic derivatives as nanocarriers for the targeted delivery of antimalarial drugs. Biomaterials 2014, 35, 7940–7950. [Google Scholar] [CrossRef]
- Coma-Cros, E.M.; Lancelot, A.; Anselmo, M.S.; Borgheti-Cardoso, L.N.; Valle-Delgado, J.J.; Serrano, J.L.; Fernàndez-Busquets, X.; Sierra, T. Micelle carriers based on dendritic macromolecules containing bis-MPA and glycine for antimalarial drug delivery. Biomater. Sci. 2019, 7, 1661–1674. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Li, Z.; Loh, X.J. Recent development of unimolecular micelles as functional materials and applications. Polym. Chem. 2016, 7, 5898–5919. [Google Scholar] [CrossRef]
- Hernández-Ainsa, S.; Barberá, J.; Marcos, M.; Serrano, J.L. Nanoobjects coming from mesomorphic ionic PAMAM dendrimers. Soft Matter 2011, 7, 2560–2568. [Google Scholar] [CrossRef]
- Fedeli, E.; Hernández-Ainsa, S.; Lancelot, A.; González-Pastor, R.; Calvo, P.; Sierra, T.; Serrano, J.L. Nanoobjects formed by ionic PAMAM dendrimers: Hydrophilic/lipophilic modulation and encapsulation properties. Soft Matter 2015, 11, 6009–6017. [Google Scholar] [CrossRef]
- Concellón, A.; Liang, T.; Schenning, A.P.H.J.; Serrano, J.L.; Romero, P.; Marcos, M. Proton-conductive materials formed by coumarin photocrosslinked ionic liquid crystal dendrimers. J. Mater. Chem. C 2018, 6, 1000–1007. [Google Scholar] [CrossRef] [Green Version]
- Tolić, L.P.; Anderson, G.A.; Smith, R.D.; Brothers, H.M.; Spindler, R.; Tomalia, D.A. Electrospray ionization Fourier transform ion cyclotron resonance mass spectrometric characterization of high molecular mass Starburst™ dendrimers. Int. J. Mass Spectrom. Ion Process. 1997, 165–166, 405–418. [Google Scholar]
- Peterson, J.; Allikmaa, V.; Subbi, J.; Pehk, T.; Lopp, M. Structural deviations in poly(amidoamine) dendrimers: A MALDI-TOF MS analysis. Eur. Polym. J. 2003, 39, 33–42. [Google Scholar] [CrossRef]
- McKenna, M.D.; Barberá, J.; Marcos, M.; Serrano, J.L. Discotic Liquid Crystalline Poly(propylene imine) Dendrimers Based on Triphenylene. J. Am. Chem. Soc. 2005, 127, 619–625. [Google Scholar] [CrossRef]
- Martín-Rapún, R.; Marcos, M.; Omenat, A.; Barberá, J.; Romero, P.; Serrano, J.L. Ionic Thermotropic Liquid Crystal Dendrimers. J. Am. Chem. Soc. 2005, 127, 7397–7403. [Google Scholar] [CrossRef]
- Marcos, M.; Martín-Rapún, R.; Omenat, A.; Barberá, J.; Serrano, J.L. Ionic Liquid Crystal Dendrimers with Mono-, Di- and Trisubstituted Benzoic Acids. Chem. Mater. 2006, 18, 1206–1212. [Google Scholar] [CrossRef]
- Hernández-Ainsa, S.; Fedeli, E.; Barberá, J.; Marcos, M.; Sierra, T.; Serrano, J.L. Self-assembly modulation in ionic PAMAM derivatives. Soft Matter 2014, 10, 281–289. [Google Scholar] [CrossRef]
- Stetefeld, J.; McKenna, S.A.; Patel, T.R. Dynamic light scattering: A practical guide and applications in biomedical sciences. Biophys. Rev. 2016, 8, 409–427. [Google Scholar] [CrossRef]
- Modena, M.M.; Rühle, B.; Burg, T.P.; Wuttke, S. Nanoparticle Characterization: What to Measure? Adv. Mater. 2019, 31, 1901556. [Google Scholar] [CrossRef]
- Concellón, A.; Blasco, E.; Martínez-Felipe, A.; Martínez, J.C.; Šics, I.; Ezquerra, T.A.; Nogales, A.; Piñol, M.; Oriol, L. Light-Responsive Self-Assembled Materials by Supramolecular Post-Functionalization via Hydrogen Bonding of Amphiphilic Block Copolymers. Macromolecules 2016, 49, 7825–7836. [Google Scholar] [CrossRef]
- Concellón, A.; Clavería-Gimeno, R.; Velázquez-Campoy, A.; Abian, O.; Piñol, M.; Oriol, L. Polymeric micelles from block copolymers containing 2,6-diacylaminopyridine units for encapsulation of hydrophobic drugs. RSC Adv. 2016, 6, 24066–24075. [Google Scholar] [CrossRef] [Green Version]








| T5% a (°C) | Phase Transitions b | dobs c (Å) | h k ld | XRD Parameters | |
|---|---|---|---|---|---|
| PAMAM16-cov-ChCou | 244 | g 25 SmA 71 e I | 46.5 | 1 0 0 | d = 46.8 Å |
| 23.5 | 2 0 0 | ||||
| 4.5 (br) | |||||
| PAMAM16-ChCouf | 209 | g 21 SmA 63 e I | 46.2 | 1 0 0 | d = 46.2 Å |
| 23.1 | 2 0 0 | ||||
| 4.5 (br) | |||||
| PAMAM64-cov-ChCou | 217 | g 35 Colh 87 e I | 53.3 | 1 0 0 | a = 61.5 Å |
| 30.8 | 1 1 0 | ||||
| 4.5 (br) | |||||
| PAMAM64-ChCouf | 198 | g 29 Colh 81 e I | 49.3 | 1 0 0 | a = 56.7 Å |
| 28.2 | 1 1 0 | ||||
| 4.5 (br) |
| CAC (μg/mL) | DhTEM (nm) a | DhDLS (nm) b | |
|---|---|---|---|
| PAMAM32-ChCou | 65 | 23 ± 4 | 28 ± 8 |
| PAMAM64-ChCou | 46 | 30 ± 5 | 38 ± 10 |
| PAMAM16-cov-ChCou | 28 | 18 ± 3 | 24 ± 6 |
| PAMAM64-cov-ChCou | 19 | 40 ± 6 | 47 ± 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Concellón, A.; San Anselmo, M.; Hernández-Ainsa, S.; Romero, P.; Marcos, M.; Serrano, J.L. Micellar Nanocarriers from Dendritic Macromolecules Containing Fluorescent Coumarin Moieties. Polymers 2020, 12, 2872. https://doi.org/10.3390/polym12122872
Concellón A, San Anselmo M, Hernández-Ainsa S, Romero P, Marcos M, Serrano JL. Micellar Nanocarriers from Dendritic Macromolecules Containing Fluorescent Coumarin Moieties. Polymers. 2020; 12(12):2872. https://doi.org/10.3390/polym12122872
Chicago/Turabian StyleConcellón, Alberto, María San Anselmo, Silvia Hernández-Ainsa, Pilar Romero, Mercedes Marcos, and José Luis Serrano. 2020. "Micellar Nanocarriers from Dendritic Macromolecules Containing Fluorescent Coumarin Moieties" Polymers 12, no. 12: 2872. https://doi.org/10.3390/polym12122872