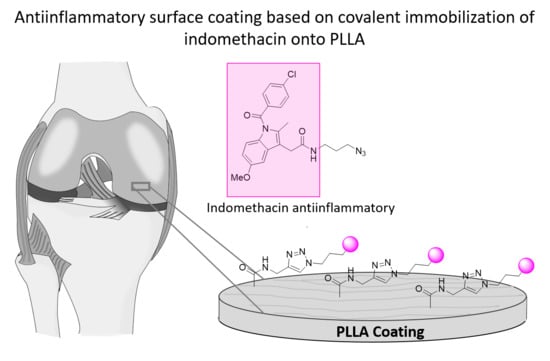
Poly(l-lactide)-Based Anti-Inflammatory Responsive Surfaces for Surgical Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. General Procedure for the Synthesis of 3-Azidopropan-1-amine
2.3. Synthesis of Dansyl Derivatives
2.4. Derivatization of Indomethacin: Synthesis of 3a
2.5. Preparation of PLLA Films
2.6. Hydrolysis of PLLA Films
2.7. Amidation of PLLA Films
2.8. CuAAC Reaction on PLLA Films
2.9. Characterization of the Samples
3. Results and Discussion
3.1. Surface Hydrolysis and Amidation
3.2. Synthesis Fluorophore Derivative
3.3. Derivatization of Indomethacin
3.4. Surface Chemistry and Morphology with Immobilized Indomethacin Derivative 3a
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arciola, C.R.; Campoccia, D. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16. [Google Scholar] [CrossRef] [PubMed]
- Campoccia, D.; Montanaro, L.; Renata, C. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, Z.; Brooks, P.J.; Barzilay, O.; Fine, N.; Glogauer, M. Macrophages, foreign body giant cells and their response to implantable biomaterials. Materials 2015, 8, 5671–5701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M. Biological Interactions on Materials Surfaces-Understanding and Controlling Protein, Cell and Tissue Responses; Puleo, D.A., Bizios, R., Eds.; Springer: Heidelberg, Germany; London, UK, 2009; ISBN 9780387981604. [Google Scholar]
- Zhou, G.; Groth, T. Host Responses to Biomaterials and Anti-Inflammatory Design—A Brief Review. Macromol. Biosci. 2018, 18, 1–15. [Google Scholar] [CrossRef]
- Bridges, A.W. Anti-Inflammatory Polymeric Coatings for Implantable Biomaterials and Devices. J. Diabetes Sci. Technol. 2008, 2, 984–994. [Google Scholar] [CrossRef]
- AlKhoury, H.; Hautmann, A.; Erdmann, F.; Zhou, G.; Stojanović, S.; Najman, S.; Groth, T. Study on the potential mechanism of anti-inflammatory activity of covalently immobilized hyaluronan and heparin. J. Biomed. Mater. Res. Part A 2020, 108, 1099–1111. [Google Scholar] [CrossRef]
- Al-Khoury, H.; Espinosa-Cano, E.; Aguilar, M.R.; Román, J.S.; Syrowatka, F.; Schmidt, G.; Groth, T. Anti-inflammatory Surface Coatings Based on Polyelectrolyte Multilayers of Heparin and Polycationic Nanoparticles of Naproxen-Bearing Polymeric Drugs. Biomacromolecules 2019, 20, 4015–4025. [Google Scholar] [CrossRef]
- Yang, P.; Leng, Y.X.; Zhao, A.S.; Zhou, H.F.; Xu, L.X.; Hong, S.; Huang, N. Blood compatibility improvement of titanium oxide film modified by phosphorus ion implantation. Nucl. Instruments Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2006, 242, 15–17. [Google Scholar] [CrossRef]
- Xiong, D.; Gao, Z.; Jin, Z. Friction and wear properties of UHMWPE against ion implanted titanium alloy. Surf. Coat. Technol. 2007, 201, 6847–6850. [Google Scholar] [CrossRef]
- Jingrun, R.; Jin, W.; Hong, S.; Nan, H. Surface modification of polyethylene terephthalate with albumin and gelatin for improvement of anticoagulation and endothelialization Ren. Appl. Surf. Sci. 2008, 255, 263–266. [Google Scholar] [CrossRef]
- Jing, F.J.; Wang, L.; Fu, R.K.Y.; Leng, Y.X.; Chen, J.Y.; Huang, N.; Chu, P.K. Behavior of endothelial cells on micro-patterned titanium oxide fabricated by plasma immersion ion implantation and deposition and plasma etching. Surf. Coat. Technol. 2007, 201, 6874–6877. [Google Scholar] [CrossRef]
- Jaffer, I.H.; Weitz, J.I. Acta Biomaterialia The blood compatibility challenge. Part 1: Blood-contacting medical devices: The scope of the problem. Acta Biomater. 2019, 94, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Rechtenbach, A.; Hao, J.; Jandt, K.D. Polysaccharide-protein surface modification of titanium via a layer-by-layer technique: Characterization and cell behaviour aspects. Biomaterials 2005, 26, 5960–5971. [Google Scholar] [CrossRef] [PubMed]
- Al-mayouf, A.M.; Al-swayih, A.A.; Al-mobarak, N.A.; Al-jabab, A.S. Corrosion behavior of a new titanium alloy for dental implant applications in fluoride media. Materials Chem. Phys. 2004, 86, 320–329. [Google Scholar] [CrossRef]
- Hwang, A.A.M.; Lim, J.K. In vitro bioactivity of titanium implants coated with bicomponent hybrid biodegradable polymers. J. Sol-Gel Sci. Technol. 2012, 756–764. [Google Scholar] [CrossRef]
- Kim, S.; Kang, M.; Kim, H.; Lim, H.; Byun, S.; Lee, J.; Lee, S. Innovative micro-textured hydroxyapatite and poly (l-lactic )-acid polymer composite fi lm as a fl exible, corrosion resistant, biocompatible, and bioactive coating for Mg implants. Mater. Sci. Eng. C 2017, 81, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (Lactic Acid)-Based Biomaterials for Orthopaedic Regenerative Engineering. Adv. Drug Deliv. Rev. 2017, 247–276. [Google Scholar] [CrossRef]
- Lichter, J.A.; Van Vliet, K.J.; Rubner, M.F. Design of Antibacterial Surfaces and Interfaces: Polyelectrolyte Multilayers as a Multifunctional Platform. Macromolecules 2009, 8573–8586. [Google Scholar] [CrossRef]
- Mukherjee, T.; Kao, N. PLA Based Biopolymer Reinforced with Natural Fibre: A Review. J. Polym. Environ. 2011, 19, 714. [Google Scholar] [CrossRef]
- Bergström, J.S.; Hayman, D. An Overview of Mechanical Properties and Material Modeling of Polylactide (PLA) for Medical Applications. Ann. Biomed. Eng. 2016, 44, 330–340. [Google Scholar] [CrossRef] [PubMed]
- Rebelo, R.; Fernandes, M.; Fangueiro, R. ScienceDirect ScienceDirect Biopolymers in Medical Implants: A Brief Review. Procedia Eng. 2017, 200, 236–243. [Google Scholar] [CrossRef]
- da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, biodegradation and excretion of polylactic acid (PLA) in medical implants and theranostic systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Baran, E.; Erbil, H. Surface Modification of 3D Printed PLA Objects by Fused Deposition Modeling: A Review. Colloids Interfaces 2019, 3, 43. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Wan, Y.; Tu, C.; Cai, Q.; Bei, J.; Wang, S. Enhancing the cell affinity of macroporous poly(l-lactide) cell scaffold by a convenient surface modification method. Polym. Int. 2003, 52, 1892–1899. [Google Scholar] [CrossRef]
- Guo, C.; Xiang, M.; Dong, Y. Surface modification of poly (lactic acid) with an improved alkali-acid hydrolysis method. Mater. Lett. 2015, 140, 144–147. [Google Scholar] [CrossRef]
- Immobilization, P.F.; Ozaltin, K.; Lehocky, M.; Humpolicek, P.; Pelkova, J.; Di Martino, A.; Karakurt, I.; Saha, P. Anticoagulant Polyethylene Terephthalate Surface by Plasma-Mediated Fucoidan Immobilization. Polymers 2019, 11, 750. [Google Scholar]
- Wilson, C.J.; Clegg, R.E.; Leavesley, D.I.; Pearcy, M.J. Mediation of Biomaterial-Cell Interactions by Adsorbed Proteins: A Review. Tissue Eng. 2005, 11, 1–18. [Google Scholar] [CrossRef]
- Xu, L.; Siedlecki, C.A. Effects of surface wettability and contact time on protein adhesion to biomaterial surfaces. Biomaterials 2007, 28, 3273–3283. [Google Scholar] [CrossRef] [Green Version]
- Hadjesfandiari, N.; Yu, K.; Mei, Y.; Kizhakkedathu, J.N. Polymer brush-based approaches for the development of infection-resistant surfaces. J. Mater. Chem. B 2014, 2, 4968–4978. [Google Scholar] [CrossRef]
- Oyarbide, J.; Azpiroz, P.; Briz, N.; Ipin, E.; Fratila, R.M.; Aizpurua, J.M. “Plasma-Click” Based Strategy for Obtaining Antibacterial Surfaces on Implants. Plasma Process. Polym. 2013, 34, 328–335. [Google Scholar] [CrossRef]
- Sun, E.Y.; Josephson, L.; Weissleder, R. “Clickable” Nanoparticles for Targeted Imaging. Mol. Imaging 2006, 5, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Lucas, S. The Pharmacology of Indomethacin. J. Head Face Pain 2016, 56, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T. HPLC of Amino Acids as Dansyl and Dabsyl Derivatives. J. Chromatogr. Libr. 2005, 70, 229–241. [Google Scholar]
- Li, L.; Wang, Y.; Chen, G.; Dong, K. Pre-column derivatization method for determining phenylephrine in human plasma and its application in a pharmacokinetic study. Biomed. Chromatogr. 2020, 34, e4843. [Google Scholar] [CrossRef]
- Muhammad, I.; Muhammad, T.; Hoji, A.; Yang, X. Determination of mercury (II) in water samples by fluorescence using a dansyl chloride immobilized glass slide. Instrum. Sci. Technol. 2020, 0, 1–14. [Google Scholar] [CrossRef]
- Oguz, M.; Ali, A.; Yilmaz, M. Surface coating of magnetite nanoparticles with fluorescence derivative for the detection of mercury in water environments. Mater. Lett. 2020, 267, 127548. [Google Scholar] [CrossRef]
- Zeng, D.; Kuang, G.; Wang, S.; Peng, W.; Lin, S.; Zhang, Q.; Su, X.; Hu, M.; Wang, H.; Tan, J.; et al. Discovery of Novel 11-Triazole Substituted Benzofuro[3,2-b]quinolone Derivatives as c-myc G-Quadruplex Specific Stabilizers via Click Chemistry. J. Med. Chem. 2017, 60, 5407–5423. [Google Scholar] [CrossRef]
- Olmo, J.A.; Leyre, P. Food Hydrocolloids Antibacterial chitosan electrostatic/covalent coating onto biodegradable poly (l-lactic acid). Food Hydrocoll. 2020, 105. [Google Scholar] [CrossRef]
- Lao, L.; Tan, H.; Wang, Y.; Gao, C. Chitosan modified poly (l-lactide ) microspheres as cell microcarriers for cartilage tissue engineering. Colloids Surf. B Biointerfaces 2008, 66, 218–225. [Google Scholar] [CrossRef]
- Chollet, C.; Chanseau, C.; Remy, M.; Guignandon, A.; Bareille, R.; Bordenave, L.; Durrieu, M.; Labruge, C.; Cedex, F.-B.; Bordeaux, U.; et al. The effect of RGD density on osteoblast and endothelial cell behavior on RGD-grafted polyethylene terephthalate surfaces. Biomaterials 2009, 30, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Uchida, E.; Uyama, Y.; Ikada, Y. Sorption of Low-Molecular-Weight Anions into Thin Polycation Layers Grafted onto a Film. Langmuir 1993, 9, 1121–1124. [Google Scholar] [CrossRef]
- Tellitu, I.; Serna, S.; Domínguez, E. Application of the PIFA—Mediated alkyne amidation reaction to the formal synthesis of (±)-clausenamide. Arch. Org. Chem. 2010, 3, 7–14. [Google Scholar]
- Alberti, G.; Desimone, A. Wetting of rough surfaces: A homogenization approach. R. Soc. Lond. Proc. Ser. A 2005, 461, 79–97. [Google Scholar] [CrossRef]
- Kantheti, S.; Narayan, R.; Raju, K.V.S.N. The impact of 1,2,3-triazoles in the design of functional coatings. RSC Adv. 2015, 5, 3687–3708. [Google Scholar] [CrossRef]










| Entry | 3-Azido-1-propyl-amine (eq.) | Base (eq.) | Active Agent (eq.) | Solvent | T (°) | t (h) | Yield (%) |
|---|---|---|---|---|---|---|---|
| 9 | (1.5) | Et3N (1.5) | EDC·HCl (1.5)/HOBT (1.4) | CH2Cl2 | Reflux | 48 | - [a] |
| 10 | (1.5) | Et3N (1.5) | EDC·HCl (1.5)/NHS (1.4) | CH2Cl2 | Reflux | 48 | - [a] |
| 11 | 2 | - | DCC (2.1) | CH2Cl2 | Reflux | 48 | - [a] |
| 12 | 4 | - | DCC (4.1) | CH2Cl2 | Reflux | 48 | - [a] |
| 13 | 5 | - | DCC (5.1) | CH2Cl2 | Reflux | 48 | - [a] |
| 14 | 2 | - | DCC (2.1) | Toluene | Reflux | 48 | - [b] |
| 15 | 4 | - | DCC (4.1) | Toluene | Reflux | 48 | - [b] |
| 16 | 4 | - | DCC (4.1) | Toluene | 80 | 48 | Traces [c] |
| 17 | 1.5 | DIPEA (1.5) | EDC·HCl (1.5)/NHS (1.4) | Toluene | 80 | 48 | Traces [c] |
| 18 | 4 | - | DCC (4.1) | DMF | 85 | 48 | - [b] |
| 19 | 4 | Et3N (4) | DCC (4.1) | DMF | 85 | 48 | - [b] |
| 20 | 1 | - | DCC (1)/DMAP (0.05) | CH2Cl2 | r.t | 24 | Traces [c] |
| 21 | 1 | - | SOCl2(1.1) | CH2Cl2 | Reflux/r.t | 2+72 | 71 |
| Composition (%) | ||||||
|---|---|---|---|---|---|---|
| Sample type | C | O | N | S | Cl | Si |
| Hyrolyzed PLLA | 38.7 | 46.4 | - | - | - | 14.9 |
| Dansylated 2a PLLA (PA) | 61.2 | 25.7 | 8.8 | 0.6 | - | 1.7 |
| Dansylated 2b PLLA (PB) | 62.5 | 37.4 | 0.06 | - | - | - |
| Indomethacin-PLLA | 59.4 | 36.6 | 2.8 | - | 0.3 | 0.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Bodón, J.; Ruiz-Rubio, L.; Hernáez-Laviña, E.; Vilas-Vilela, J.L.; Moreno-Benítez, M.I. Poly(l-lactide)-Based Anti-Inflammatory Responsive Surfaces for Surgical Implants. Polymers 2021, 13, 34. https://doi.org/10.3390/polym13010034
Sánchez-Bodón J, Ruiz-Rubio L, Hernáez-Laviña E, Vilas-Vilela JL, Moreno-Benítez MI. Poly(l-lactide)-Based Anti-Inflammatory Responsive Surfaces for Surgical Implants. Polymers. 2021; 13(1):34. https://doi.org/10.3390/polym13010034
Chicago/Turabian StyleSánchez-Bodón, Julia, Leire Ruiz-Rubio, Estíbaliz Hernáez-Laviña, José Luis Vilas-Vilela, and Mª Isabel Moreno-Benítez. 2021. "Poly(l-lactide)-Based Anti-Inflammatory Responsive Surfaces for Surgical Implants" Polymers 13, no. 1: 34. https://doi.org/10.3390/polym13010034