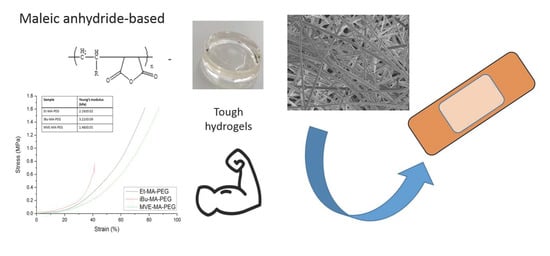
Tough Hydrogels Based on Maleic Anhydride, Bulk Properties Study and Microfiber Formation by Electrospinning
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of the Hydrogels
2.3. Hydrogel Fibers Fabrication by Electrospinning
2.4. Materials Characterization
2.4.1. Fourier-Transform Infrared Spectroscopy (FTIR)
Thermogravimetric Analyses (TGA)
2.4.2. Compressive Stress/Strain Study
2.4.3. Viscosity Measurements
2.4.4. Swelling Degree
2.4.5. Morphology of Hydrogel Nanofibers
3. Result and Discussion
3.1. Maleic Anhydride-Based Hydrogels
3.2. Swelling Ratio of Hydrogels
3.3. Thermal Stability of Hydrogels
3.4. Mechanical Properties of Hydrogels
3.5. Maleic Anhydride-Based Hydrogel Fibers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moghadam, P.N.; Azaryan, E.; Zeynizade, B. Investigation of poly(styrene-alt-maleic anhydride) copolymer for controlled drug delivery of ceftriaxone antibiotic. J. Macromol. Sci. Part. A Pure Appl. Chem. 2010, 47, 839–848. [Google Scholar] [CrossRef]
- Subramanian, B.; Rameshbabu, A.P.; Ghosh, K.; Jha, P.K.; Jha, R.; Murugesan, S.; Chattopadhyay, S.; Dhara, S.; Mondal, K.C.; Basak, P.; et al. Impact of styrene maleic anhydride (SMA) based hydrogel on rat fallopian tube as contraceptive implant with selective antimicrobial property. Mater. Sci. Eng. C 2019, 94, 94–107. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Hydrogels for tissue engineering. Chem. Rev. 2001, 101, 1869–1879. [Google Scholar] [CrossRef]
- Peak, C.W.; Wilker, J.J.; Schmidt, G. A review on tough and sticky hydrogels. Colloid Polym. Sci. 2013, 291, 2031–2047. [Google Scholar] [CrossRef]
- Costa, A.M.S.; Mano, J.F. Extremely strong and tough hydrogels as prospective candidates for tissue repair-A review. Eur. Polym. J. 2015, 72, 344–364. [Google Scholar] [CrossRef]
- Yang, J.; Han, C.R.; Duan, J.F.; Ma, M.G.; Zhang, X.M.; Xu, F.; Sun, R.C. Synthesis and characterization of mechanically flexible and tough cellulose nanocrystals-polyacrylamide nanocomposite hydrogels. Cellulose 2013, 20, 227–237. [Google Scholar] [CrossRef]
- Yu, K.; Wang, D.; Wang, Q. Tough and Self-Healable Nanocomposite Hydrogels for Repeatable Water Treatment. Polymers 2018, 10, 880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nonoyama, T.; Gong, J.P. Double-network hydrogel and its potential biomedical application: A review. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2015, 229, 853–863. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, H.; Zhu, L.; Zheng, J. Fundamentals of double network hydrogels. J. Mater. Chem. B 2015, 3, 3654–3676. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, X.; He, C.; Saricilar, S.; Wang, H. Mechanical properties of tough hydrogels synthesized with a facile simultaneous radiation polymerization and cross-linking method. Radiat. Phys. Chem. 2015, 106, 7–15. [Google Scholar] [CrossRef]
- McCarron, P.A.; Woolfson, A.D.; Donnelly, R.F.; Andrews, G.P.; Zawislak, A.; Price, J.H. Influence of plasticizer type and storage conditions on properties of poly(methyl vinyl ether-co-maleic anhydride) bioadhesive films. J. Appl. Polym. Sci. 2004, 91, 1576–1589. [Google Scholar] [CrossRef]
- Goetz, L.; Foston, M.; Mathew, A.P.; Oksman, K.; Ragauskas, A.J. Poly(methyl vinyl ether- co -maleic acid)−Polyethylene Glycol Nanocomposites Cross-Linked In Situ with Cellulose Nanowhiskers. Biomacromolecules 2010, 11, 2660–2666. [Google Scholar] [CrossRef]
- Elizondo, E.; Córdoba, A.; Sala, S.; Ventosa, N.; Veciana, J. Preparation of biodegradable poly (methyl vinyl ether-co-maleic anhydride) nanostructured microparticles by precipitation with a compressed antisolvent. J. Supercrit. Fluids 2010, 53, 108–114. [Google Scholar] [CrossRef]
- Raj Singh, T.R.; McCarron, P.A.; Woolfson, A.D.; Donnelly, R.F. Investigation of swelling and network parameters of poly(ethylene glycol)-crosslinked poly(methyl vinyl ether-co-maleic acid) hydrogels. Eur. Polym. J. 2009, 45, 1239–1249. [Google Scholar] [CrossRef]
- Calò, E.; De Barros, J.M.S.; Fernandez-Gutíerrez, M.; San Roman, J.; Ballamy, L.; Khutoryanskiy, V.V. Antimicrobial hydrogels based on autoclaved poly(vinyl alcohol) and poly(methyl vinyl ether-alt- maleic anhydride) mixtures for wound care applications. RSC Adv. 2016, 6, 55211–55219. [Google Scholar] [CrossRef] [Green Version]
- Hood, D.K.; Musa, O.M. Application of Maleic Anhydride-Based Materials; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 9783319294544. [Google Scholar]
- Pompe, T.; Zschoche, S.; Herold, N.; Salchert, K.; Gouzy, M.F.; Sperling, C.; Werner, C. Maleic anhydride copolymers-A versatile platform for molecular biosurface engineering. Biomacromolecules 2003, 4, 1072–1079. [Google Scholar] [CrossRef]
- Khil, M.-S.; Cha, D.-I.; Kim, H.-Y.; Kim, I.-S.; Bhattarai, N. Electrospun nanofibrous polyurethane membrane as wound dressing. J. Biomed. Mater. Res. 2003, 67B, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Aklog, Y.F.; Nagae, T.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Preparation of chitin nanofibers by surface esterification of chitin with maleic anhydride and mechanical treatment. Carbohydr. Polym. 2016, 153, 55–59. [Google Scholar] [CrossRef]
- Biber, E.; Gündüz, G.; Mavis, B.; Colak, U. Effects of electrospinning process parameters on nanofibers obtained from Nylon 6 and poly (ethylene-n-butyl acrylate-maleic anhydride) elastomer blends using Johnson SB statistical distribution function. Appl. Phys. A Mater. Sci. Process. 2010, 99, 477–487. [Google Scholar] [CrossRef]
- Yang, X.; Yang, D.; Zhu, X.; Nie, J.; Ma, G. Electrospun and photocrosslinked gelatin/dextran–maleic anhydride composite fibers for tissue engineering. Eur. Polym. J. 2019, 113, 142–147. [Google Scholar] [CrossRef]
- Varshosaz, J. Optimization of Poly (methyl vinyl ether-co-maleic acid) Electrospun Nanofibers as a Fast-Dissolving Drug Delivery System. Adv Biomed Res. 2018, 7, 84. [Google Scholar] [CrossRef]
- Zhao, E.; Lam, J.W.Y.; Meng, L.; Hong, Y.; Deng, H.; Bai, G.; Huang, X.; Hao, J.; Tang, B.Z. Poly[(maleic anhydride)-alt-(vinyl acetate)]: A pure oxygenic nonconjugated macromolecule with strong light emission and solvatochromic effect. Macromolecules 2015, 48, 64–71. [Google Scholar] [CrossRef]
- Yoncheva, K.; Lizarraga, E.; Irache, J.M. Pegylated nanoparticles based on poly(methyl vinyl ether-co-maleic anhydride): Preparation and evaluation of their bioadhesive properties. Eur. J. Pharm. Sci. 2005, 24, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Peng, E.; Choo, E.S.G.; Tan, C.S.H.; Tang, X.; Sheng, Y.; Xue, J. Multifunctional PEGylated nanoclusters for biomedical applications. Nanoscale 2013, 5, 5994–6005. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Yang, J.; Cui, Z.; Qin, S.; Qin, Q. The investigation of hydrophilic modification of membrane surface based on the mono-esterification between maleic anhydride and polyethylene glycol: Response surface methodology, reaction kinetics and performance analysis. J. Taiwan Inst. Chem. Eng. 2020, 112, 193–201. [Google Scholar] [CrossRef]
- Panzarasa, G.; Osypova, A.; Consolati, G.; Quasso, F.; Soliveri, G.; Ribera, J.; Schwarze, F. Preparation of a Sepia Melanin and Poly(ethylene-alt-maleic Anhydride) Hybrid Material as an Adsorbent for Water Purification. Nanomaterials 2018, 8, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, R.; Al-Haddad, S.; Al-Rughaib, M.; Salman, M. Evaluation of hydrolyzed poly(isobutylene-alt-maleic anhydride) as a polyelectrolyte draw solution for forward osmosis desalination. Desalination 2016, 394, 148–154. [Google Scholar] [CrossRef]
- Sawaguchi, T.; Seno, M. Detailed mechanism and molecular weight dependence of thermal degradation of polyisobutylene. Polymer 1996, 37, 5607–5617. [Google Scholar] [CrossRef]
- Ruiz-Rubio, L.; Vilas, J.L.; Rodríguez, M.; León, L.M. Thermal behaviour of H-bonded interpolymer complexes based on polymers with acrylamide or lactame groups and poly(acrylic acid): Influence of N-alkyl and α-methyl substitutions. Polym. Degrad. Stab. 2014, 109, 147–153. [Google Scholar] [CrossRef]
- Ruiz-rubio, L.; Laza, J.M.; Pérez, L. Polymer–polymer complexes of poly (N-isopropylacrylamide) and poly (N, N-diethylacrylamide) with poly (carboxylic acids): A comparative study. Colloid Polym. Sci. 2014, 292, 423–430. [Google Scholar] [CrossRef]
- Takahashi, R.; Ikai, T.; Kurokawa, T.; King, D.R.; Gong, J.P. Double network hydrogels based on semi-rigid polyelectrolyte physical networks. J. Mater. Chem. B 2019, 7, 6347–6354. [Google Scholar] [CrossRef]
- An, K.; Peng, S.; Yang, C.; Qing, Y.; Hu, C.; Wang, L.; Liu, C. Covalent modification of graphene oxide by 4,4′-methylenebis(phenyl isocyanate) to enhance corrosion resistance of polystyrene coating. Colloid Polym. Sci. 2019, 297, 839–848. [Google Scholar] [CrossRef]
- Simha, N.K.; Carlson, C.S.; Lewis, J.L. Evaluation of fracture toughness of cartilage by micropenetration. J. Mater. Sci. Mater. Med. 2004, 15, 631–639. [Google Scholar] [CrossRef]
- Keirouz, A.; Chung, M.; Kwon, J.; Fortunato, G.; Radacsi, N. 2D and 3D electrospinning technologies for the fabrication of nanofibrous scaffolds for skin tissue engineering: A review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2020, 12, 1–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.R.; Morsi, Y.; Zhu, T.; Ahmad, A.; Xie, X.; Yu, F.; Mo, X. Electrospinning: An emerging technology to construct polymer-based nanofibrous scaffolds for diabetic wound healing. Front. Mater. Sci. 2021, 1–26. [Google Scholar] [CrossRef]
- Zhou, N.; Ma, X.; Hu, W.; Ren, P.; Zhao, Y.; Zhang, T. Effect of RGD content in poly(ethylene glycol)-crosslinked poly(methyl vinyl ether-alt-maleic acid) hydrogels on the expansion of ovarian cancer stem-like cells. Mater. Sci. Eng. C 2021, 118, 111477. [Google Scholar] [CrossRef] [PubMed]
- Mira, A.; Mateo, C.R.; Mallavia, R.; Falco, A. Poly(methyl vinyl ether-alt-maleic acid) and ethyl monoester as building polymers for drug-loadable electrospun nanofibers. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Preparation and characterization of polyvinyl alcohol-gelatin hydrogel membranes for biomedical applications. AAPS PharmSciTech 2007, 8. [Google Scholar] [CrossRef] [PubMed]
- Oktay, B.; Baştürk, E.; Kayaman-Apohan, N.; Kahraman, M.V. Highly porous starch/poly(ethylene-alt-maleic anhydride) composite nanofiber mesh. Polym. Compos. 2013, 34, 1321–1324. [Google Scholar] [CrossRef]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bettahar, F.; Bekkar, F.; Pérez-Álvarez, L.; Ferahi, M.I.; Meghabar, R.; Vilas-Vilela, J.L.; Ruiz-Rubio, L. Tough Hydrogels Based on Maleic Anhydride, Bulk Properties Study and Microfiber Formation by Electrospinning. Polymers 2021, 13, 972. https://doi.org/10.3390/polym13060972
Bettahar F, Bekkar F, Pérez-Álvarez L, Ferahi MI, Meghabar R, Vilas-Vilela JL, Ruiz-Rubio L. Tough Hydrogels Based on Maleic Anhydride, Bulk Properties Study and Microfiber Formation by Electrospinning. Polymers. 2021; 13(6):972. https://doi.org/10.3390/polym13060972
Chicago/Turabian StyleBettahar, Faiza, Fadila Bekkar, Leyre Pérez-Álvarez, Mohammed Issam Ferahi, Rachid Meghabar, José Luis Vilas-Vilela, and Leire Ruiz-Rubio. 2021. "Tough Hydrogels Based on Maleic Anhydride, Bulk Properties Study and Microfiber Formation by Electrospinning" Polymers 13, no. 6: 972. https://doi.org/10.3390/polym13060972