Poly(ethylene-imine)-Functionalized Magnetite Nanoparticles Derivatized with Folic Acid: Heating and Targeting Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Cell Culture Assays
2.4. Hyperthermia Effects on Tested Cells
2.5. Cytotoxicity of Compounds
2.6. Clonogenic Assay
2.7. Statistical Analysis of Cell Tests
3. Results and Discussion
3.1. Particle Characterization
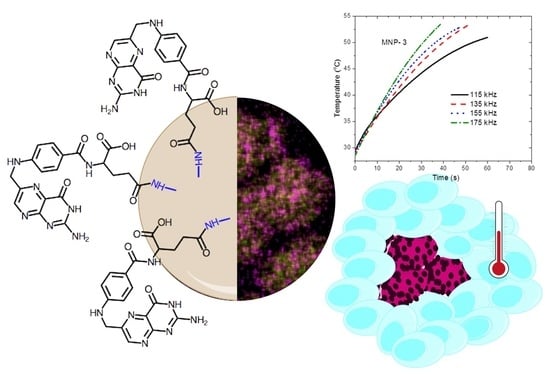
3.2. Magnetic Properties and Magnetic Field Hyperthermia (MFHT)
3.3. Functionalization of MNP-1
3.4. In Vitro Cell Viability Essays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sapareto, S.A.; Dewey, W.C. Thermal dose determination in cancer-therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef]
- Jaque, D.; Maestro, L.M.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Rodriguez, E.M.; Sole, J.G. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Fanelli, A.; Cavaliere, R.; Mondovì, B.; Moricca, G.E. Selective Heat Sensitivity of Cancer Cells; Springer Science & Business Media: Berlin, Germany, 2012; Volume 59. [Google Scholar]
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Parambi, D.G.T.; Prabhu, A.; Uddin, M.S.; Aleya, L.; Kim, H.; Mathew, B. Magnetic nanoparticles for hyperthermia in cancer treatment: An emerging tool. Environ. Sci. Pollut. Res. 2020, 27, 19214–19225. [Google Scholar] [CrossRef]
- Levine, E.M.; Robbins, E.B. Differential temperature sensitivity of normal and cancer cells in culture. J. Cell Physiol. 1970, 76, 373–379. [Google Scholar] [CrossRef]
- Roizintowle, L.; Pirro, J.P. The response of human and rodent cells to hyperthermia. Int. J. Radiat. Oncol. Biol. Phys. 1991, 20, 751–756. [Google Scholar] [CrossRef]
- Ammendola, M.; Curro, G.; Memeo, R.; Curto, L.S.; Luposella, M.; Zuccala, V.; Pessaux, P.; Navarra, G.; Gadaleta, C.D.; Ranieri, G. Targeting Stem Cells with Hyperthermia: Translational Relevance in Cancer Patients. Oncology 2020, 98, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Caccamo, A.E.; Desenzani, S.; Belloni, L.; Borghetti, A.F.; Bettuzzi, S. Nuclear clusterin accumulation during heat shock response: Implications for cell survival and thermo-tolerance induction in immortalized and prostate cancer cells. J. Cell Physiol. 2006, 207, 208–219. [Google Scholar] [CrossRef]
- Welch, A.J.; Motamedi, M.; Rastegar, S.; Lecarpentier, G.L.; Jansen, D. Laser thermal ablation. Photochem. Photobiol. 1991, 53, 815–823. [Google Scholar] [CrossRef] [Green Version]
- Wust, P.; Hildebrandt, B.; Sreenivasa, G.; Rau, B.; Gellermann, J.; Riess, H.; Felix, R.; Schlag, P.M. Hyperthermia in combined treatment of cancer. Lancet Oncol. 2002, 3, 487–497. [Google Scholar] [CrossRef]
- Dennis, C.L.; Ivkov, R. Physics of heat generation using magnetic nanoparticles for hyperthermia. Int. J. Hyperth. 2013, 29, 715–729. [Google Scholar] [CrossRef]
- Das, P.; Colombo, M.; Prosperi, D. Recent advances in magnetic fluid hyperthermia for cancer therapy. Coll. Surf. B-Biointerfaces 2019, 174, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Zhang, Y.F.; Wang, Y.Y.; Zhu, W.J.; Li, G.L.; Ma, X.W.; Zhang, Y.H.; Chen, S.Z.; Tiwari, S.; Shi, K.J.; et al. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics 2020, 10, 3793–3815. [Google Scholar] [CrossRef]
- Martinelli, C.; Pucci, C.; Ciofani, G. Nanostructured carriers as innovative tools for cancer diagnosis and therapy. Appl. Bioeng. 2019, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, S.K.; Shrivastava, N.; Rossi, F.; Tung, L.D.; Thanh, N.T.K. Nanoparticles-based magnetic and photo induced hyperthermia for cancer treatment. Nano Today 2019, 29. [Google Scholar] [CrossRef]
- Liu, S.X.; Yu, B.; Wang, S.; Shen, Y.Q.; Cong, H.L. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid Interf. Sci. 2020, 281. [Google Scholar] [CrossRef]
- Popescu, R.C.; Andronescu, E.; Vasile, B.S. Recent Advances in Magnetite Nanoparticle Functionalization for Nanomedicine. Nanomaterials 2019, 9, 1791. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Ji, H.N.; Yu, P.; Niu, J.Q.; Farooq, M.U.; Akram, M.W.; Udego, I.O.; Li, H.D.; Niu, X.B. Surface Modification of Magnetic Iron Oxide Nanoparticles. Nanomaterials 2018, 8, 810. [Google Scholar] [CrossRef] [Green Version]
- Aisida, S.O.; Akpa, P.A.; Ahmad, I.; Zhao, T.K.; Maaza, M.; Ezema, F.I. Bio-inspired encapsulation and functionalization of iron oxide nanoparticles for biomedical applications. Eur. Polym. J. 2020, 122. [Google Scholar] [CrossRef]
- Sonvico, F.; Mornet, S.; Vasseur, S.; Dubernet, C.; Jaillard, D.; Degrouard, J.; Hoebeke, J.; Duguet, E.; Colombo, P.; Couvreur, P. Folate-conjugated iron oxide nanoparticles for solid tumor targeting as potential specific magnetic hyperthermia mediators: Synthesis, physicochemical characterization, and in vitro experiments. Bioconj. Chem. 2005, 16, 1181–1188. [Google Scholar] [CrossRef]
- Mohapatra, S.; Mallick, S.K.; Maiti, T.K.; Ghosh, S.K.; Pramanik, P. Synthesis of highly stable folic acid conjugated magnetite nanoparticles for targeting cancer cells. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Hayashi, K.; Moriya, M.; Sakamoto, W.; Yogo, T. Chemoselective Synthesis of Folic Acid-Functionalized Magnetite Nanoparticles via Click Chemistry for Magnetic Hyperthermia. Chem. Mater. 2009, 21, 1318–1325. [Google Scholar] [CrossRef]
- Hayashi, K.; Nakamura, M.; Sakamoto, W.; Yogo, T.; Miki, H.; Ozaki, S.; Abe, M.; Matsumoto, T.; Ishimura, K. Superparamagnetic Nanoparticle Clusters for Cancer Theranostics Combining Magnetic Resonance Imaging and Hyperthermia Treatment. Theranostics 2013, 3, 366–376. [Google Scholar] [CrossRef] [Green Version]
- Yoo, H.; Moon, S.K.; Hwang, T.; Kim, Y.S.; Kim, J.H.; Choi, S.W. Multifunctional Magnetic Nanoparticles Modified with Polyethylenimine and Folic Acid for Biomedical Theranostics. Langmuir 2013, 29, 5962–5967. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.L.; Zheng, S.W.; Hong, R.Y.; Deng, S.M.; Guo, L.; Hu, R.L.; Gao, B.; Huang, M.; Cheng, L.F.; Liu, G.H.; et al. Folic acid-conjugated Fe3O4 magnetic nanoparticles for hyperthermia and MRI in vitro and in vivo. Appl. Surf. Sci. 2014, 307, 224–233. [Google Scholar] [CrossRef]
- Bonvin, D.; Bastiaansen, J.A.M.; Stuber, M.; Hofmann, H.; Ebersold, M.M. Folic acid on iron oxide nanoparticles: Platform with high potential for simultaneous targeting, MRI detection and hyperthermia treatment of lymph node metastases of prostate cancer. Dalton Trans. 2017, 46, 12692–12704. [Google Scholar] [CrossRef] [PubMed]
- Tudisco, C.; Cambria, M.T.; Giuffrida, A.E.; Sinatra, F.; Anfuso, C.D.; Lupo, G.; Caporarello, N.; Falanga, A.; Galdiero, S.; Oliveri, V.; et al. Comparison Between Folic Acid and gH625 Peptide-Based Functionalization of Fe3O4 Magnetic Nanoparticles for Enhanced Cell Internalization. Nanoscale Res. Lett. 2018, 13, 45. [Google Scholar] [CrossRef]
- Piazza, R.D.; Viali, W.R.; dos Santos, C.C.; Nunes, E.S.; Marques, R.F.C.; Morais, P.C.; da Silva, S.W.; Coaquira, J.A.H.; Jafelicci, M. PEGlatyon-SPION surface functionalization with folic acid for magnetic hyperthermia applications. Mater. Res. Express 2020, 7, 015078. [Google Scholar] [CrossRef]
- Li, X.J.; Wang, Z.M.; Ning, J.L.; Gao, M.M.; Jiang, W.B.; Zhou, Z.D.; Li, G.Y. Preparation and characterization of a novel polyethyleneimine cation-modified persimmon tannin bioadsorbent for anionic dye adsorption. J. Environ. Manag. 2018, 217, 305–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, F.; Li, Y.H.; Zhu, J.; Sun, J.Y.; Marshall, B.; Lee, R.J.; Teng, L.S.; Yang, Z.G.; Xie, J. Polyethyleneimine-based Formulations for Delivery fo Oligonucleotides. Curr. Med. Chem. 2019, 26, 2264–2284. [Google Scholar] [CrossRef]
- Chen, Z.H.; Lv, Z.Y.; Sun, Y.F.; Chi, Z.G.; Qing, G.Y. Recent advancements in polyethyleneimine-based materials and their biomedical, biotechnology, and biomaterial applications. J. Mater. Chem. B 2020, 8, 2951–2973. [Google Scholar] [CrossRef]
- Goon, I.Y.; Lai, L.M.H.; Lim, M.; Munroe, P.; Gooding, J.J.; Amal, R. Fabrication and Dispersion of Gold-Shell-Protected Magnetite Nanoparticles: Systematic Control Using Polyethyleneimine. Chem. Mater. 2009, 21, 673–681. [Google Scholar] [CrossRef]
- Xie, H.Y.; Zhen, R.; Wang, B.; Feng, Y.J.; Chen, P.; Hao, J. Fe3O4/Au Core/Shell Nanoparticles Modified with Ni2+-Nitrilotriacetic Acid Specific to Histidine-Tagged Proteins. J. Phys. Chem. C 2010, 114, 4825–4830. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, W.L.; Wang, Y.; Kuang, Q.; Shi, Y.F.; Zhong, L.B.; Zhang, Q.Q. Fabrication of Cluster/Shell Fe3O4/Au Nanoparticles and Application in Protein Detection via a SERS Method. J. Phys. Chem. C 2010, 114, 19607–19613. [Google Scholar] [CrossRef]
- Wang, Y.F.; Xu, F.; Zhang, L.; Wei, X.L. One-pot solvothermal synthesis of Fe3O4-PEI composite and its further modification with Au nanoparticles. J. Nannopart. Res. 2013, 15. [Google Scholar] [CrossRef]
- Felix, L.L.; Sanz, B.; Sebastian, V.; Torres, T.E.; Sousa, M.H.; Coaquira, J.A.H.; Ibarra, M.R.; Goya, G.F. Gold-decorated magnetic nanoparticles design for hyperthermia applications and as a potential platform for their surface-functionalization. Sci. Rep. 2019, 9, 4185. [Google Scholar] [CrossRef] [Green Version]
- Ping, T.; Wang, Q.Y.; Zhou, Y.; Nie, J. Reducing oxygen inhibition by Fe3O4@PEI nanoparticles co-initiator. J. Photochem. Photobiol. A-Chem. 2019, 373, 171–175. [Google Scholar] [CrossRef]
- Hanafy, N.A.; Ferraro, N.M.; Gaballo, A.; Dini, L.; Tasco, V.; Nobile, C.; De Giorgi, M.L.; Carallo, S.; Rinaldi, R.; Leporatti, S. Fabrication and characterization of ALK1fc-loaded fluoro-magnetic nanoparticles for inhibiting TGF beta 1 in hepatocellular carcinoma. RSC Adv. 2016, 6, 48834–48842. [Google Scholar] [CrossRef]
- Guaragna, A.; Chiaviello, A.; Paolella, C.; D’Alonzo, D.; Palumbo, G. Synthesis and Evaluation of Folate-Based Chlorambucil Delivery Systems for Tumor-Targeted Chemotherapy. Bioconj. Chem. 2012, 23, 84–96. [Google Scholar] [CrossRef]
- Ohshima, H. Electrostatic interaction of soft particles. Adv. Colloid Interface Sci. 2015, 226, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Pankhurst, Q.A.; Thanh, N.T.K.; Jones, S.K.; Dobson, J. Progress in applications of magnetic nanoparticles in biomedicine. J. Phys. D-Appl. Phys. 2009, 42. [Google Scholar] [CrossRef] [Green Version]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A. On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J. Phys. D Appl. Phys. 2014, 47. [Google Scholar] [CrossRef]
- Abdi, H.; Williams, L.J. Tukey’s Honestly Significant Difference (HSD) Test. In Encyclopedia or Research Design; Salkind, N., Ed.; Sage: Thousand Oaks, CA, USA, 2010; pp. 1–5. [Google Scholar] [CrossRef]
- Wang, J.D.; Guan, H.Y.; Han, Q.; Tan, S.Y.; Liang, Q.L.; Ding, M.Y. Fabrication of Yb3+-Immobilized Hydrophilic Phytic-Acid-Coated Magnetic Nanocomposites for the Selective Separation of Bovine Hemoglobin from Bovine Serum. ACS Biomater. Sci. Eng. 2019, 5, 2740–2749. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Li, X.L.; Peng, Q.; Wang, X.; Chen, J.P.; Li, Y.D. Monodisperse magnetic single-crystal ferrite microspheres. Angew. Chem. Int. Ed. 2005, 44, 2782–2785. [Google Scholar] [CrossRef]
- Radu, T.; Iacovita, C.; Benea, D.; Turcu, R. X-Ray Photoelectron Spectroscopic Characterization of Iron Oxide Nanoparticles. Appl. Surf. Sci. 2017, 405, 337–343. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interf. Anal. 2004, 36. [Google Scholar] [CrossRef]
- Graf, N.; Yegen, E.; Gross, T.; Lippitz, A.; Weigel, W.; Krakert, S.; Terfort, A.; Unger, W.E.S. XPS and NEXAFS studies of aliphatic and aromatic amine species on functionalized surfaces. Surf. Sci. 2009, 603, 2849–2860. [Google Scholar] [CrossRef]
- Mohapatra, J.; Xing, M.Y.; Beatty, J.; Elkins, J.; Seda, T.; Mishra, S.R.; Liu, J.P. Enhancing the magnetic and inductive heating properties of Fe3O4 nanoparticles via morphology control. Nanotechnology 2020, 31. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Lanier, O.L.; Korotych, O.I.; Monsalve, A.G.; Wable, D.; Savliwala, S.; Grooms, N.W.F.; Nacea, C.; Tuitt, O.R.; Dobson, J. Evaluation of magnetic nanoparticles for magnetic fluid hyperthermia. Int. J. Hyperth. 2019, 36, 687–701. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S. Magnetic particle hyperthermia-biophysical limitations of a visionary tumour therapy. J. Magn. Magn. Mater. 2007, 311, 187–192. [Google Scholar] [CrossRef]
- Spirou, S.V.; Lima, S.A.C.; Bouziotis, P.; Vranjes-Djuric, S.; Efthimiadou, E.K.; Laurenzana, A.; Barbosa, A.I.; Garcia-Alonso, I.; Jones, C.; Jankovic, D.; et al. Recommendations for In Vitro and In Vivo Testing of Magnetic Nanoparticle Hyperthermia Combined with Radiation Therapy. Nanomaterials 2018, 8, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latunde-Dada, G.O. Ferroptosis: Role of lipid peroxidation, iron and ferritinophagy. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 1893–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kajarabille, N.; Latunde-Dada, G.O. Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators. Int. J. Mol. Sci. 2019, 20, 4968. [Google Scholar] [CrossRef] [Green Version]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J. Neuro-Oncol. 2011, 103, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.; Lim, M.; Goos, J.; Qiao, R.R.; Ng, Y.Y.; Mansfeld, F.M.; Jackson, M.; Davis, T.P.; Kavallaris, M. Biologically Targeted Magnetic Hyperthermia: Potential and Limitations. Front. Phamacol. 2018, 9, 831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.C.; Shi, W.; Chen, W.; Li, X.H.; Ma, H.M. Fluorescent carbon nanodots conjugated with folic acid for distinguishing folate-receptor-positive cancer cells from normal cells. J. Mater. Chem. 2012, 22, 12568–12573. [Google Scholar] [CrossRef]
- Nour, A.M.A.; Ringot, D.; Gueant, J.L.; Chango, A. Folate receptor and human reduced folate carrier expression in HepG2 cell line exposed to fumonisin B-1 and folate deficiency. Carcinogenesis 2007, 28, 2291–2297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bannunah, A.M.; Vllasaliu, D.; Lord, J.; Stolnik, S. Mechanisms of Nanoparticle Internalization and Transport Across an Intestinal Epithelial Cell Model: Effect of Size and Surface Charge. Mol. Pharmaceut. 2014, 11, 4363–4373. [Google Scholar] [CrossRef]
- Ankamwar, B.; Lai, T.C.; Huang, J.H.; Liu, R.S.; Hsia, M.; Chen, C.H.; Hwu, Y.K. Biocompatibility of Fe3O4 nanoparticles evaluated by in vitro cytotoxicity assays using normal, glia and breast cancer cells. Nanotechnology 2010, 21, 075102. [Google Scholar] [CrossRef]
- Remya, N.S.; Syama, S.; Sabareeswaran, A.; Mohanan, P.V. Toxicity, toxicokinetics and biodistribution of dextran stabilized Iron oxide Nanoparticles for biomedical applications. Int. J. Pharm. 2016, 511, 586–598. [Google Scholar] [CrossRef]
- Nosrati, F.; Hamzehei, H.; Afroogh, S.; Ashabi, S.F.; Attari, E.; Manjili, H.K. Phenyl alanine & Tyrosine Amino acids Coated Magnetic Nanoparticles: Preparation and Toxicity study. Drug Res. 2019, 69, 277–283. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, D. Carboxymethyl group activation of dextran cross-linked superparamagnetic iron oxide nanoparticles. J. Korean Ceram. Soc. 2020, 58, 106–115. [Google Scholar] [CrossRef]








| Sample | Frequency | dT/dt (°C/s) | SAR (W/g) |
|---|---|---|---|
| MNP-1 | 115 | 0.48 ± 0.03 | 200 ± 12 |
| 135 | 0.56 ± 0.04 | 233 ± 17 | |
| 155 | 0.67 ± 0.03 | 279 ± 13 | |
| 175 | 0.80 ±0.04 | 330 ± 17 | |
| MNP-2 | 115 | 0.38 ± 0.03 | 157 ± 12 |
| 135 | 0.448 ± 0.026 | 186 ± 11 | |
| 155 | 0.48 ± 0.04 | 200 ± 17 | |
| 175 | 0.58 ± 0.04 | 239 ± 16 | |
| MNP-3 | 115 | 0.45 ± 0.03 | 187 ± 12 |
| 135 | 0.58 ± 0.04 | 242 ± 17 | |
| 155 | 0.63 ± 0.04 | 261 ± 17 | |
| 175 | 0.72 ± 0.04 | 299 ± 17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Muñoz, M.; Plesselova, S.; Delgado, A.V.; Santoyo-Gonzalez, F.; Salto-Gonzalez, R.; Giron-Gonzalez, M.D.; Iglesias, G.R.; López-Jaramillo, F.J. Poly(ethylene-imine)-Functionalized Magnetite Nanoparticles Derivatized with Folic Acid: Heating and Targeting Properties. Polymers 2021, 13, 1599. https://doi.org/10.3390/polym13101599
Ortega-Muñoz M, Plesselova S, Delgado AV, Santoyo-Gonzalez F, Salto-Gonzalez R, Giron-Gonzalez MD, Iglesias GR, López-Jaramillo FJ. Poly(ethylene-imine)-Functionalized Magnetite Nanoparticles Derivatized with Folic Acid: Heating and Targeting Properties. Polymers. 2021; 13(10):1599. https://doi.org/10.3390/polym13101599
Chicago/Turabian StyleOrtega-Muñoz, Mariano, Simona Plesselova, Angel V. Delgado, Francisco Santoyo-Gonzalez, Rafael Salto-Gonzalez, Maria Dolores Giron-Gonzalez, Guillermo R. Iglesias, and Francisco Javier López-Jaramillo. 2021. "Poly(ethylene-imine)-Functionalized Magnetite Nanoparticles Derivatized with Folic Acid: Heating and Targeting Properties" Polymers 13, no. 10: 1599. https://doi.org/10.3390/polym13101599