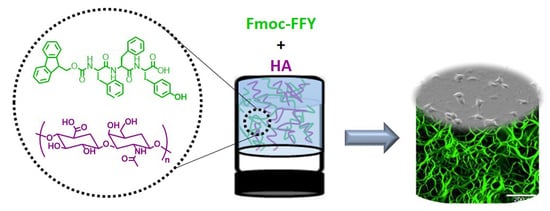
Localized Enzyme-Assisted Self-Assembly in the Presence of Hyaluronic Acid for Hybrid Supramolecular Hydrogel Coating
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Precursor Peptide Fmoc-FFpY and HA
2.1.2. Buffers and Substrates
2.2. Methods
2.2.1. Upside down Test Vials
2.2.2. Confocal Microscopy
2.2.3. Rheological Measurements
2.2.4. Multilayer Film Buildup and Directed Hydrogelation
2.2.5. Electronic Microscopy Analyses (TEM, SEM and cryo-SEM)
2.2.6. Atomic Force Microscopy (AFM)
2.2.7. Cell Viability Test
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Draper, E.R.; Adams, D.J. Controlling the assembly and properties of low-molecular-waight-hydrogelators. Langmuir 2019, 35, 6506–6521. [Google Scholar]
- Roy, S.; Ulijn, R.V. Exploiting biocatalysis in the synthesis of supramolecular polymers. In Enzymatic Polymerization; Palmans, A.R.A., Heise, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 127–145. [Google Scholar]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular hydrogelators and hydrogels: From soft matter to molecular biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef] [PubMed]
- Baillet, J.; Gaubert, A.; Bassani, D.M.; Verget, J.; Latxague, L.; Barthelemy, P. Supramolecular gels derived from nucleoside based bolaamphiphiles as a light-sensitive soft material. Chem. Commun. 2020, 56, 3397–3400. [Google Scholar] [CrossRef]
- Clemente, M.J.; Romero, P.; Serrano, J.L.; Fitremann, J.; Oriol, L. Supramolecular hydrogels based on glycoamphiphiles: Effect of the disaccharide polar head. Chem. Mater. 2012, 24, 3847–3858. [Google Scholar] [CrossRef] [Green Version]
- McCloskey, A.P.; Gilmore, S.M.; Zhou, J.; Draper, E.R.; Porter, S.; Gilmore, B.F.; Xu, B.; Laverty, G. Self-assembling ultrashort NSAID-peptide nanosponges: Multifunctional antimicrobial and anti-inflammatory materials. RSC Adv. 2016, 6, 114738–114749. [Google Scholar] [CrossRef] [Green Version]
- Webber, M.J.; Appel, E.A.; Meijer, E.W.; Langer, R. Supramolecular biomaterials. Nat. Mater. 2016, 15, 13–26. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Iqbal, M.H.; Carvalho, A.; Schmutz, M.; Jierry, L.; Schaaf, P.; Boulmedais, F. Surface triggered self-assembly of Fmoc-tripeptide as an antibacterial coating. Front. Bioeng. Biotechnol. 2020, 8, 938. [Google Scholar] [CrossRef]
- Yang, Z.M.; Gu, H.W.; Fu, D.G.; Gao, P.; Lam, J.K.; Xu, B. Enzymatic formation of supramolecular hydrogels. Adv. Mater. 2004, 16, 1440–1444. [Google Scholar] [CrossRef]
- Williams, R.J.; Smith, A.M.; Collins, R.; Hodson, N.; Das, A.K.; Ulijn, R.V. Enzyme-assisted self-assembly under thermodynamic control. Nat. Nanotechnol. 2009, 4, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Vigier-Carriere, C.; Garnier, T.; Wagner, D.; Lavalle, P.; Rabineau, M.; Hemmerle, J.; Senger, B.; Schaaf, P.; Boulmedais, F.; Jierry, L. Bioactive seed layer for surface-confined self-assembly of peptides. Angew. Chem. Int. Ed. 2015, 54, 10198–10201. [Google Scholar] [CrossRef]
- Vigier-Carriere, C.; Wagner, D.; Chaumont, A.; Durr, B.; Lupattelli, P.; Lambour, C.; Schmutz, M.; Hemmerle, J.; Senger, B.; Schaaf, P.; et al. Control of surface-localized enzyme-assisted self-assembly of peptides through catalyzed oligomerization. Langmuir 2017, 33, 8267–8276. [Google Scholar] [CrossRef]
- Rodon Fores, J.; Mendez, M.L.M.; Mao, X.Y.; Wagner, D.; Schmutz, M.; Rabineau, M.; Lavalle, P.; Schaaf, P.; Boulmedais, F.; Jierry, L. Localized supramolecular peptide self-assembly directed by enzyme-induced proton gradients. Angew. Chem. Int. Ed. 2017, 56, 15984–15988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodon Fores, J.; Criado-Gonzalez, M.; Chaumont, A.; Carvalho, A.; Blanck, C.; Schmutz, M.; Serra, C.A.; Boulmedais, F.; Schaaf, P.; Jierry, L. Supported catalytically active supramolecular hydrogels for continuous flow chemistry. Angew. Chem. Int. Ed. 2019, 58, 18817–18822. [Google Scholar] [CrossRef] [Green Version]
- Rodon Fores, J.; Criado-Gonzalez, M.; Chaumont, A.; Carvalho, A.; Blanck, C.; Schmutz, M.; Boulmedais, F.; Schaaf, P.; Jierry, L. Autonomous growth of a spatially localized supramolecular hydrogel with autocatalytic ability. Angew. Chem. Int. Ed. 2020, 59, 14558–14563. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Rodon Fores, J.; Carvalho, A.; Blanck, C.; Schmutz, M.; Kocgozlu, L.; Schaaf, P.; Jierry, L.; Boulmedais, F. Phase separation in supramolecular hydrogels based on peptide self-Assembly from enzyme-coated nanoparticles. Langmuir 2019, 35, 10838–10845. [Google Scholar] [CrossRef]
- Criado-Gonzalez, M.; Rodon Fores, J.; Wagner, D.; Schroder, A.P.; Carvalho, A.; Schmutz, M.; Harth, E.; Schaaf, P.; Jierry, L.; Boulmedais, F. Enzyme-assisted self-assembly within a hydrogel induced by peptide diffusion. Chem. Commun. 2019, 55, 1156–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Criado-Gonzalez, M.; Loftin, B.; Rodon Fores, J.; Vautier, D.; Kocgozlu, L.; Jierry, L.; Schaaf, P.; Boulmedais, F.; Harth, E. Enzyme assisted peptide self-assemblies trigger cell adhesion in high density oxime based host gels. J. Mater. Chem. B 2020, 8, 4419–4427. [Google Scholar] [CrossRef] [PubMed]
- Vigier-Carriere, C.; Boulmedais, F.; Schaaf, P.; Jierry, L. Surface-assisted self-assembly strategies leading to supramolecular hydrogels. Angew. Chem. Int. Ed. 2018, 57, 1448–1456. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Controlled patterning of aligned self-assembled peptide nanotubes. Nat. Nanotechnol. 2006, 1, 195–200. [Google Scholar] [CrossRef]
- Jayawarna, V.; Ali, M.; Jowitt, T.A.; Miller, A.E.; Saiani, A.; Gough, J.E.; Ulijn, R.V. Nanostructured hydrogels for three-dimensional cell culture through self-assembly of fluorenylmethoxycarbonyl-dipeptides. Adv. Mater. 2006, 18, 611–614. [Google Scholar] [CrossRef]
- Yang, Z.M.; Liang, G.L.; Xu, B. Enzymatic control of the self-assembly of small molecules: A new way to generate supramolecular hydrogels. Soft Matter 2007, 3, 515–520. [Google Scholar] [CrossRef]
- Liebmann, T.; Rydholm, S.; Akpe, V.; Brismar, H. Self-assembling Fmoc dipeptide hydrogel for in situ 3D cell culturing. BMC Biotechnol. 2007, 7, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayawarna, V.; Richardson, S.M.; Hirst, A.R.; Hodson, N.W.; Saiani, A.; Gough, J.E.; Ulijn, R.V. Introducing chemical functionality in Fmoc-peptide gels for cell culture. Acta Biomater. 2009, 5, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Jayawarna, V.; Smith, A.; Gough, J.E.; Ulijn, R.V. Three-dimensional cell culture of chondrocytes on modified di-phenylalanine scaffolds. Biochem. Soc. Trans. 2007, 35, 535–577. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Kuang, Y.; Gao, Y.A.; Xu, B. Versatile small-molecule motifs for self-assembly in water and the formation of biofunctional supramolecular hydrogels. Langmuir 2011, 27, 529–537. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Smith, A.M.; Das, A.K.; Hodson, N.W.; Collins, R.F.; Ulijn, R.V.; Gough, J.E. Self-assembled peptide-based hydrogels as scaffolds for anchorage-dependent cells. Biomaterials 2009, 30, 2523–2530. [Google Scholar] [CrossRef]
- Cheng, G.; Castelletto, V.; Jones, R.R.; Connon, C.J.; Hamley, I.W. Hydrogelation of self-assembling RGD-based peptides. Soft Matter 2011, 7, 1326–1333. [Google Scholar] [CrossRef]
- Jung, J.P.; Nagaraj, A.K.; Fox, E.K.; Rudra, J.S.; Devgun, J.M.; Collier, J.H. Co-assembling peptides as defined matrices for endothelial cells. Biomaterials 2009, 30, 2400–2410. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.F.; Devgun, J.M.; Collier, J.H. Fibrillized peptide microgels for cell encapsulation and 3D cell culture. Soft Matter 2011, 7, 6005–6011. [Google Scholar] [CrossRef] [PubMed]
- Silva, G.A.; Czeisler, C.; Niece, K.L.; Beniash, E.; Harrington, D.A.; Kessler, J.A.; Stupp, S.I. Selective differentiation of neural progenitor cells by high-epitope density nanofibers. Science 2004, 303, 1352–1355. [Google Scholar] [CrossRef] [Green Version]
- Matson, J.B.; Stupp, S.I. Self-assembling peptide scaffolds for regenerative medicine. Chem. Commun. 2012, 48, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.L.; Zheng, Q.X.; Guo, X.D.; Zheng, J.F. Cytocompatibility of self-assembled hydrogel from IKVAV-containing peptide amphiphile with neural stem cells. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 24, 753–756. [Google Scholar] [CrossRef]
- Song, Y.L.; Zheng, Q.X.; Wu, Y.C.; Guo, X.D. Two-dimensional effectsof hydrogel self-organizedfrom IKVAV-containing peptides on growth and differentiation NSCs. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 24, 186–192. [Google Scholar] [CrossRef]
- Song, Y.L.; Li, Y.X.; Zheng, Q.X.; Wu, K.; Guo, X.D.; Wu, Y.C.; Yin, M.; Wu, Q.; Fu, X.L. Neural progenitor cells survival and neuronal differentiation in peptide-based hydrogels. J. Biomater. Sci. Polym. Ed. 2011, 22, 475–487. [Google Scholar] [CrossRef]
- Sur, S.; Matson, J.B.; Webber, M.J.; Newcomb, C.J.; Stupp, S.I. Photodynamic control of bioactivity in a nanofiber matrix. ACS Nano 2012, 6, 10776–10785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muraoka, T.; Koh, C.Y.; Cui, H.G.; Stupp, S.I. Light-triggered bioactivity in three dimensions. Angew. Chem. Int. Ed. 2009, 48, 5946–5949. [Google Scholar] [CrossRef]
- Capito, R.M.; Azevedo, H.S.; Velichko, Y.S.; Mata, A.; Stupp, S.I. Self-assembly of large and small molecules into hierarchically ordered sacs and membranes. Science 2008, 319, 1812–1816. [Google Scholar] [CrossRef] [PubMed]
- Mata, A.; Geng, Y.B.; Henrikson, K.J.; Aparicio, C.; Stock, S.R.; Satcher, R.L.; Stupp, S.I. Bone regeneration mediated by biomimetic mineralization of a nanofiber matrix. Biomaterials 2010, 31, 6004–6012. [Google Scholar] [CrossRef] [Green Version]
- Mendes, A.C.; Smith, K.H.; Tejeda-Montes, E.; Engel, E.; Reis, R.L.; Azevedo, H.S.; Mata, A. Co-assembled and microfabricated bioactive membranes. Adv. Funct. Mater. 2013, 23, 430–438. [Google Scholar] [CrossRef]
- Ribeiro, S.; Radvar, E.; Shi, Y.; Borges, J.; Pirraco, R.P.; Leonor, I.B.; Mano, J.F.; Reis, R.L.; Mata, A.; Azevedo, H.S. Nanostructured interfacial self-assembled peptide-polymer membranes for enhanced mineralization and cell adhesion. Nanoscale 2017, 9, 13670–13682. [Google Scholar] [CrossRef]
- Aviv, M.; Halperin-Sternfeld, M.; Grigoriants, I.; Buzhansky, L.; Mironi-Harpaz, I.; Seliktar, D.; Einav, S.; Nevo, Z.; Adler-Abramovich, L. Improving the mechanical rigidity of hyaluronic acid by integration of a supramolecular peptide matrix. ACS Appl. Mater. Interfaces 2018, 10, 41883–41891. [Google Scholar] [CrossRef] [PubMed]
- Nadernezhad, A.; Forster, L.; Netti, F.; Adler-Abramovich, L.; Tessmar, J.; Groll, J. Rheological analysis of the interplay between the molecular weight and concentration of hyaluronic acid in formulations of supramolecular HA/FmocFF hybrid hydrogels. Polym. J. 2020, 52, 1007–1012. [Google Scholar] [CrossRef]
- Adhikari, B.; Nanda, J.; Banerjee, A. Multicomponent hydrogels from enantiomeric amino acid derivatives: Helical nanofibers, handedness and self-sorting. Soft Matter 2011, 7, 8913–8922. [Google Scholar] [CrossRef]
- Yang, B.; Adams, D.J.; Marlow, M.; Zelzer, M. Surface-mediated supramolecular self-assembly of protein, peptide, and nucleoside derivatives: From surface design to the underlying mechanism and tailored functions. Langmuir 2018, 34, 15109–15125. [Google Scholar] [CrossRef] [Green Version]
- Decher, G. Fuzzy nanoassemblies: Toward layered polymeric multicomposites. Science 1977, 277, 1232–1237. [Google Scholar] [CrossRef]
- Olive, A.G.L.; Abdullah, N.H.; Ziemecka, I.; Mendes, E.; Eelkema, R.; van Esh, J.H. Spatial and directional control over self-assembly using catalytic micropatterned surfaces. Angew. Chem. Int. Ed. 2014, 53, 4132–4136. [Google Scholar] [CrossRef]
- Rodon Fores, J.; Criado-Gonzalez, M.; Schmutz, M.; Blanck, C.; Schaaf, P.; Boulmedais, F.; Jierry, L. Protein-induced low-molecular weight hydrogelator self-assembly through a self-sustaining process. Chem. Sci. 2019, 10, 4761–4766. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodon Fores, J.; Bigo-Simon, A.; Wagner, D.; Payrastre, M.; Damestoy, C.; Blandin, L.; Boulmedais, F.; Kelber, J.; Schmutz, M.; Rabineau, M.; et al. Localized Enzyme-Assisted Self-Assembly in the Presence of Hyaluronic Acid for Hybrid Supramolecular Hydrogel Coating. Polymers 2021, 13, 1793. https://doi.org/10.3390/polym13111793
Rodon Fores J, Bigo-Simon A, Wagner D, Payrastre M, Damestoy C, Blandin L, Boulmedais F, Kelber J, Schmutz M, Rabineau M, et al. Localized Enzyme-Assisted Self-Assembly in the Presence of Hyaluronic Acid for Hybrid Supramolecular Hydrogel Coating. Polymers. 2021; 13(11):1793. https://doi.org/10.3390/polym13111793
Chicago/Turabian StyleRodon Fores, Jennifer, Alexis Bigo-Simon, Déborah Wagner, Mathilde Payrastre, Camille Damestoy, Lucille Blandin, Fouzia Boulmedais, Julien Kelber, Marc Schmutz, Morgane Rabineau, and et al. 2021. "Localized Enzyme-Assisted Self-Assembly in the Presence of Hyaluronic Acid for Hybrid Supramolecular Hydrogel Coating" Polymers 13, no. 11: 1793. https://doi.org/10.3390/polym13111793