Insights on the Atmospheric-Pressure Plasma-Induced Free-Radical Polymerization of Allyl Ether Cyclic Carbonate Liquid Layers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of 5-((allyloxy)methyl)-5-ethyl-1,3-dioxan-2-one: A6CC
2.2. Atmospheric-Pressure Plasma-Induced Polymerization of A6CC
2.3. Characterization
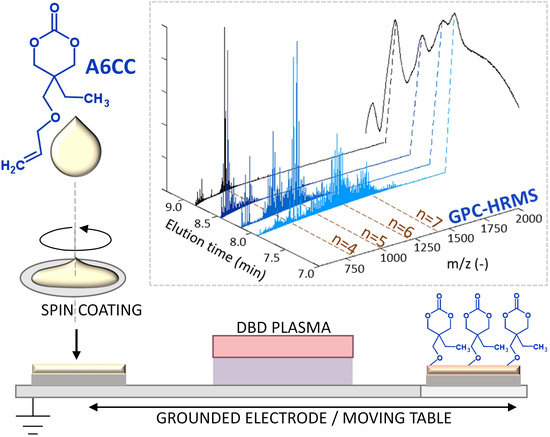
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merche, D.; Vandencasteele, N.; Reniers, F. Atmospheric plasmas for thin film deposition: A critical review. Thin Solid Film. 2012, 520, 4219–4236. [Google Scholar] [CrossRef]
- Hopfe, V.; Sheel, D.W. Atmospheric-Pressure Plasmas for Wide-Area Thin-Film Deposition and Etching. Plasma Process. Polym. 2007, 4, 253–265. [Google Scholar] [CrossRef]
- Mariotti, D.; Patel, J.; Švrček, V.; Maguire, P. Plasma-liquid interactions at atmospheric pressure for nanomaterials synthesis and surface engineering. Plasma Process Polym. 2012, 9, 1074–1085. [Google Scholar] [CrossRef]
- Bonot, S.; Mauchauffé, R.; Boscher, N.D.; Moreno-Couranjou, M.; Cauchie, H.-M.; Choquet, P. Self-Defensive Coating for Antibiotics Degradation-Atmospheric Pressure Chemical Vapor Deposition of Functional and Conformal Coatings for the Immobilization of Enzymes. Adv. Mater. Interfaces 2015, 2, 1500253. [Google Scholar] [CrossRef]
- Loyer, F.; Frache, G.; Choquet, P.; Boscher, N.D. Atmospheric Pressure Plasma-Initiated Chemical Vapor Deposition (AP-PiCVD) of Poly(alkyl acrylates): An Experimental Study. Macromolecules 2017, 50, 4351–4362. [Google Scholar] [CrossRef]
- Loyer, F.; Combrisson, A.; Omer, K.; Moreno-Couranjou, M.; Choquet, P.; Boscher, N.D. Thermoresponsive Water-Soluble Polymer Layers and Water-Stable Copolymer Layers Synthesized by Atmospheric Plasma Initiated Chemical Vapor Deposition. ACS Appl. Mater. Interfaces 2018, 11, 1335–1343. [Google Scholar] [CrossRef]
- Czuba, U.; Quintana, R.; De Pauw-Gillet, M.-C.; Bourguignon, M.; Moreno-Couranjou, M.; Alexandre, M.; Detrembleur, C.; Choquet, P. Atmospheric Plasma Deposition of Methacrylate Layers Containing Catechol/Quinone Groups: An Alternative to Polydopamine Bioconjugation for Biomedical Applications. Adv. Heal. Mater. 2018, 7, e1701059. [Google Scholar] [CrossRef]
- Manakhov, A.; Fukova, S.; Necas, D.; Michlicek, M.; Ershov, S.; Elias, M.; Visotin, M.; Popov, Z.; Zajickova, L. Analysis of epoxy functionalized layers synthesized by plasma polymerization of allyl glycidyl ether. Phys. Chem. Chem. Phys. 2018, 20, 20070–20077. [Google Scholar] [CrossRef]
- Zubov, V.P.; Kumar, M.V.; Masterova, M.N.; Kabanov, V.A. Reactivity of Allyl Monomers in Radical Polymerization. J. Macromol. Sci. Part A-Chem. 1979, 13, 111–131. [Google Scholar] [CrossRef]
- Matsumoto, A.; Kumagai, T.; Aota, H.; Kawasaki, H.; Arakawa, R. Reassessment of Free-Radical Polymerization Mechanism of Allyl Acetate Based on End-Group Determination of Resulting Oligomers by MALDI-TOF-MS Spectrometry. Polym. J. 2009, 41, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Hilt, F.; Boscher, N.D.; Duday, D.; Desbenoit, N.; Levalois-Grützmacher, J.; Choquet, P. Atmospheric pressure plasma-initiated chemical vapor deposition (AP-PiCVD) of poly(diethylallylphosphate) coating: A char-forming protective coating for cellulosic textile. ACS Appl. Mater. Interfaces 2014, 6, 18418–18422. [Google Scholar] [CrossRef]
- Kakaroglou, A.; Scheltjens, G.; Nisol, B.; De Graeve, I.; Van Assche, G.; Van Mele, B.; Willem, R.; Biesemans, M.; Reniers, F.; Terryn, H. Deposition and Characterisation of Plasma Polymerised Allyl Methacrylate Based Coatings. Plasma Process. Polym. 2012, 9, 799–807. [Google Scholar] [CrossRef]
- Watkins, L.M.; Lee, A.F.; Moir, J.W.B.; Wilson, K. Plasma-Generated Poly(allyl alcohol) Antifouling Coatings for Cellular Attachment. ACS Biomater. Sci. Eng. 2016, 3, 88–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hawker, M.J.; Pegalajar-Jurado, A.; Hicks, K.I.; Shearer, J.C.; Fisher, E.R. Allylamine and Allyl Alcohol Plasma Copolymerization: Synthesis of Customizable Biologically-Reactive Three-Dimensional Scaffolds. Plasma Process. Polym. 2015, 12, 1435–1450. [Google Scholar] [CrossRef]
- Pescarmona, P.P. Cyclic carbonates synthesised from CO2: Applications, challenges and recent research trends. Curr. Opin. Green Sustain. Chem. 2021, 29, 100457. [Google Scholar] [CrossRef]
- Alves, M.; Grignard, B.; Mereau, R.; Jerome, C.; Tassaing, T.; Detrembleur, C. Organocatalyzed coupling of carbon dioxide with epoxides for the synthesis of cyclic carbonates: Catalyst design and mechanistic studies. Catal. Sci. Technol. 2017, 7, 2651–2684. [Google Scholar] [CrossRef]
- Cornille, A.; Blain, M.; Auvergne, R.; Andrioletti, B.; Boutevin, B.; Caillol, S. A study of cyclic carbonate aminolysis at room temperature: Effect of cyclic carbonate structures and solvents on polyhydroxyurethane synthesis. Polym. Chem. 2016, 8, 592–604. [Google Scholar] [CrossRef]
- McGuire, T.M.; López-Vidal, E.M.; Gregory, G.L.; Buchard, A. Synthesis of 5- to 8-membered cyclic carbonates from diols and CO2: A one-step, atmospheric pressure and ambient temperature procedure. J. CO2 Util. 2018, 27, 283–288. [Google Scholar] [CrossRef]
- Thomas, A.W.; Kuroishi, P.K.; Pérez-Madrigal, M.M.; Whittaker, A.K.; Dove, A.P. Synthesis of aliphatic polycarbonates with a tuneable thermal response. Polym. Chem. 2017, 8, 5082–5090. [Google Scholar] [CrossRef]
- Mindemark, J.; Imholt, L.; Montero, J.; Brandell, D. Allyl ethers as combined plasticizing and crosslinkable side groups in polycarbonate-based polymer electrolytes for solid-state Li batteries. J. Polym. Sci. Part A Polym. Chem. 2016, 54, 2128–2135. [Google Scholar] [CrossRef]
- Kuschmitz, D. Makromolekulare Chemie 1989. Nachr. Chem. Tech. Lab. 1990, 38, 233–241. [Google Scholar]
- Jehanno, C.; Demarteau, J.; Mantione, D.; Arno, M.C.; Ruipérez, F.; Hedrick, J.L.; Dove, A.P.; Sardon, H. Selective Chemical Upcycling of Mixed Plastics Guided by a Thermally Stable Organocatalyst. Angew. Chem. 2020, 133, 6784–6791. [Google Scholar] [CrossRef]
- Olsén, P.; Odelius, K.; Albertsson, A.-C. Ring-Closing Depolymerization: A Powerful Tool for Synthesizing the Allyloxy-Functionalized Six-Membered Aliphatic Carbonate Monomer 2-Allyloxymethyl-2-ethyltrimethylene Carbonate. Macromolecules 2014, 47, 6189–6195. [Google Scholar] [CrossRef]
- Besse, V.; Camara, F.; Voirin, C.; Auvergne, R.; Caillol, S.; Boutevin, B. Synthesis and applications of unsaturated cyclocarbonates. Polym. Chem. 2013, 4, 4545–4561. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Karagoz, B.; Arica, M.Y. Cyclic-carbonate functionalized polymer brushes on polymeric microspheres: Immobilized laccase for degradation of endocrine disturbing compounds. J. Ind. Eng. Chem. 2018, 60, 407–417. [Google Scholar] [CrossRef]
- Desport, J.S.; Frache, G.; Patiny, L. MSPolyCalc: A web-based App for polymer mass spectrometry data interpretation. The case study of a pharmaceutical excipient. Rapid Commun. Mass Spectrom. 2020, 34, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Lu, X.; Zeng, Z.; Yang, J.; Chen, Y. Allyl ether-modified unsaturated polyesters for UV/air dual-curable coatings. I: Synthesis and characterization of the oligomers and their cured films. J. Appl. Polym. Sci. 2004, 92, 2765–2770. [Google Scholar] [CrossRef]
- He, F.; Wang, Y.-P.; Liu, G.; Jia, H.-L.; Feng, J.; Zhuo, R.-X. Synthesis, characterization and ring-opening polymerization of a novel six-membered cyclic carbonate bearing pendent allyl ether group. Polymer 2008, 49, 1185–1190. [Google Scholar] [CrossRef]
- Udayakumar, S.; Lee, M.-K.; Shim, H.-L.; Park, S.-W.; Park, D.-W. Imidazolium derivatives functionalized MCM-41 for catalytic conversion of carbon dioxide to cyclic carbonate. Catal. Commun. 2009, 10, 659–664. [Google Scholar] [CrossRef]
- Vijayalakshmi, S.; Mariadoss, A.V.A.; Ramachandran, V.; Shalini, V.; Agilan, B.; Sangeetha, C.C.; Balupillai, A.; Kotakadi, V.S.; Karthikkumar, V.; Ernest, D. Polydatin Encapsulated Poly [Lactic-co-glycolic acid] Nanoformulation Counteract the 7,12-Dimethylbenz[a] Anthracene Mediated Experimental Carcinogenesis through the Inhibition of Cell Proliferation. Antioxidants 2019, 8, 375. [Google Scholar] [CrossRef] [Green Version]
- Mindemark, J.; Tang, S.; Li, H.; Edman, L. Ion Transport beyond the Polyether Paradigm: Introducing Oligocarbonate Ion Transporters for Efficient Light-Emitting Electrochemical Cells. Adv. Funct. Mater. 2018, 28. [Google Scholar] [CrossRef]
- Rokicki, G. Aliphatic cyclic carbonates and spiroorthocarbonates as monomers. Prog. Polym. Sci. 2000, 25, 259–342. [Google Scholar] [CrossRef]
- Höcker, H.; Keul, H.; Kuhllng, S.; Hovestadt, W. Ring-opening polymerization and copolymerization of cyclic carbonates. Makromol. Chemie. Macromol. Symp. 1991, 42–43, 145–153. [Google Scholar] [CrossRef]
- Brar, A.S.; Saini, T. Optimization of atom transfer radical copolymerization of aiiyl butyl ether with acrylonitrile. Polym. J. 2007, 39, 558–567. [Google Scholar] [CrossRef] [Green Version]
- Denis, L.; Cossement, D.; Godfroid, T.; Renaux, F.; Bittencourt, C.; Snyders, R.; Hecq, M. Synthesis of Allylamine Plasma Polymer Films: Correlation between Plasma Diagnostic and Film Characteristics. Plasma Process. Polym. 2009, 6, 199–208. [Google Scholar] [CrossRef]
- Friedrich, J. The Plasma Chemistry of Polymer Surfaces: Advanced Techniques for Surface Design; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2012. [Google Scholar] [CrossRef]
- Loyer, F.; Bengasi, G.; Frache, G.; Choquet, P.; Boscher, N.D. Insights in the initiation and termination of poly(alkyl acrylates) synthesized by atmospheric pressure plasma-initiated chemical vapor deposition (AP-PiCVD). Plasma Process Polym. 2018, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Heyse, P.; Dams, R.; Paulussen, S.; Houthoofd, K.; Janssen, K.P.F.; Jacobs, P.A.; Sels, B.F. Dielectric Barrier Discharge at Atmospheric Pressure as a Tool to Deposit Versatile Organic Coatings at Moderate Power Input. Plasma Process. Polym. 2007, 4, 145–157. [Google Scholar] [CrossRef]
- Dubois, P.; Coulembier, O.; Raques, J.M. Handbook of Ring-Opening Polymerization; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niemczyk, E.M.; Gomez-Lopez, A.; Haler, J.R.N.; Frache, G.; Sardon, H.; Quintana, R. Insights on the Atmospheric-Pressure Plasma-Induced Free-Radical Polymerization of Allyl Ether Cyclic Carbonate Liquid Layers. Polymers 2021, 13, 2856. https://doi.org/10.3390/polym13172856
Niemczyk EM, Gomez-Lopez A, Haler JRN, Frache G, Sardon H, Quintana R. Insights on the Atmospheric-Pressure Plasma-Induced Free-Radical Polymerization of Allyl Ether Cyclic Carbonate Liquid Layers. Polymers. 2021; 13(17):2856. https://doi.org/10.3390/polym13172856
Chicago/Turabian StyleNiemczyk, Edyta M., Alvaro Gomez-Lopez, Jean R. N. Haler, Gilles Frache, Haritz Sardon, and Robert Quintana. 2021. "Insights on the Atmospheric-Pressure Plasma-Induced Free-Radical Polymerization of Allyl Ether Cyclic Carbonate Liquid Layers" Polymers 13, no. 17: 2856. https://doi.org/10.3390/polym13172856