Iron-Oxide-Nanoparticles-Doped Polyaniline Composite Thin Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Iron Oxide Nanoparticles
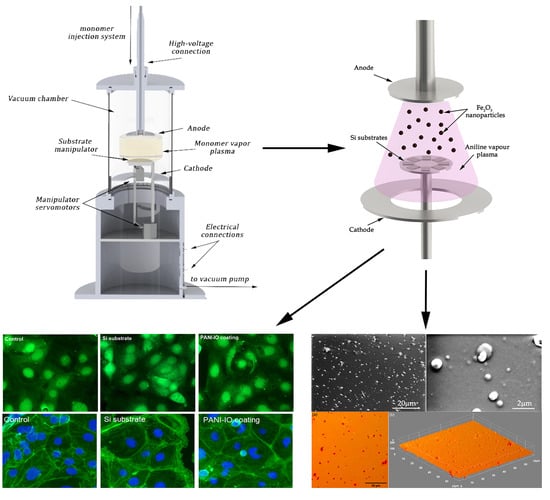
2.3. Deposition Method for Iron-Oxide-Doped Polyaniline
2.4. Characterization Methods
2.5. In Vitro Biocompatibility Assessment
2.5.1. Caco-2 Cell Line
2.5.2. Caco-2 Cell Culture on Si Substrate and PANI-IO Coating
2.5.3. MTT Cell Viability Test
2.5.4. Lactate Dehydrogenase (LDH) Assay
2.5.5. Nitric Oxide (NO) Production Measurement
2.5.6. F-Actin Cytoskeleton Labeling
2.5.7. CMFDA Staining for Intracellular Glutathione (GSH)
2.5.8. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jarach, N.; Meridor, D.; Buzhor, M.; Raichman, D.; Dodiuk, H.; Kenig, S.; Amir, E. Hybrid Antibacterial and Electro-conductive Coating for Textiles Based on Cationic Conjugated Polymer. Polymers 2020, 12, 1517. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.W.; Han, S.; Kim, B.H. Fabrication of Chromatic Electronic Textiles Synthesized by Conducting Polymer. J. Korean Phys. Soc. 2019, 74, 122–126. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Dhas, S.D.; Patil, K.T.; Moholkar, A.V.; Patil, P.S. Polyaniline (PANI)-manganese dioxide (MnO2) nanocompo-sites as efficient electrode materials for supercapacitors. Chem. Phys. Lett. 2021, 778, 138764. [Google Scholar] [CrossRef]
- Sathiyanarayanan, S.; Azim, S.S.; Venkatachari, G. Preparation of polyaniline–Fe2O3 composite and its anticorrosion performance. Synth. Met. 2007, 157, 751–757. [Google Scholar] [CrossRef]
- Elnaggar, E.M.; Kabel, K.I.; Farag, A.A.; Al-Gamal, A.G. Comparative study on doping of polyaniline with graphene and multi-walled carbon nanotubes. J. Nanostructure Chem. 2017, 7, 75–83. [Google Scholar] [CrossRef] [Green Version]
- Fuseini, M.; Zaghloul, M.M.Y.; Elkady, M.F.; El-Shazly, A.H. Evaluation of synthesized polyaniline nanofibres as corrosion protection film coating on copper substrate by electrophoretic deposition. J. Mater. Sci. 2022, 57, 6085–6101. [Google Scholar] [CrossRef]
- Butoi, B.; Groza, A.; Dinca, P.; Balan, A.; Barna, V. Morphological and Structural Analysis of Polyaniline and Poly(o-anisidine) Layers Generated in a DC Glow Discharge Plasma by Using an Oblique Angle Electrode Deposition Configuration. Polymers 2017, 9, 732. [Google Scholar] [CrossRef] [Green Version]
- Bednarczyk, K.; Matysiak, W.; Tański, T.; Janeczek, H.; Schab-Balcerzak, E.; Libera, M. Effect of polyaniline content and protonating dopants on electroconductive composites. Sci. Rep. 2021, 11, 7487. [Google Scholar] [CrossRef]
- Bagheri, N.; Lakouraj, M.M.; Hasantabar, V.; Mohseni, M. Biodegradable macro-porous CMC-polyaniline hydrogel: Synthesis, characterization and study of microbial elimination and sorption capacity of dyes from waste water. J. Hazard. Mater. 2021, 403, 123631. [Google Scholar] [CrossRef]
- Sanito, R.C.; Yeh, T.-H.; You, S.-J.; Wang, Y.-F. Novel TiO2/PANI composites as a disinfectant for the elimination of Escherichia coli and Staphylococcus aureus in aquaculture water. Environ. Technol. Innov. 2021, 22, 101502. [Google Scholar] [CrossRef]
- Rashid, M.O.M.; Islam, M.S.; Haque, M.A.; Rahman, M.A.; Hossain, M.T.; Hamid, M.A. Antibacterial activity of polyaniline coated silver nanoparticles synthesized from Piper betle leaves extract. Iranian J. Pharm. Res. 2014, 15, 591–597. [Google Scholar]
- Ong, J.-Y.; Law, Z.-J.; Pua, C.-H.; Phang, S.-W. Effect of Acid Dopants Toward Polyaniline Based Optical Sensor for Lead Detection. Polym. Sci. Ser. A 2021, 63, 485–492. [Google Scholar] [CrossRef]
- Eskandari, F.; Shabani, P.; Yousefi, R. PAni-based complementary resistive switches: The effects of Ag on physical properties and switching mechanism. Appl. Phys. A 2021, 127, 1–9. [Google Scholar] [CrossRef]
- Gómez-Quintero, T.; Arroyo-Ornelas, M.A.; López-Marín, L.M.; Castaño-Meneses, V.M.; Garcia-Contreras, R.; Acosta-Torres, L.S.; Arenas-Arrocena, M.C. Cytotoxicity of Polypyrrole and Polyaniline Matrixes for Biosensors. Acta Sci. Med. Sci. 2019, 3, 81–89. [Google Scholar]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef]
- Humpolíček, P.; Kašpárková, V.; Pacherník, J.; Stejskal, J.; Bober, P.; Capáková, Z.; Radaszkiewicz, K.A.; Junkar, I.; Lehocky, M. The biocompatibility of polyaniline and polypyrrole: A comparative study of their cytotoxicity, embryotoxicity and impurity profile. Mater. Sci. Eng. C 2018, 91, 303–310. [Google Scholar] [CrossRef]
- Vaitkuviene, A.; Ratautaite, V.; Mikoliunaite, L.; Kaseta, V.; Ramanauskaite, G.; Biziuleviciene, G.; Ramanaviciene, A.; Ramanavicius, A. Some biocompatibility aspects of conducting polymer polypyrrole evaluated with bone marrow-derived stem cells. Colloids Surfaces A Physicochem. Eng. Asp. 2014, 442, 152–156. [Google Scholar] [CrossRef]
- Vaitkuviene, A.; Kašėta, V.; Voronovic, J.; Ramanauskaite, G.; Biziuleviciene, G.; Ramanaviciene, A.; Ramanavicius, A. Evaluation of cytotoxicity of polypyrrole nanoparticles synthesized by oxidative polymerization. J. Hazard. Mater. 2013, 250–251, 167–174. [Google Scholar] [CrossRef]
- Humpolicek, P.; Kašpárková, V.; Saha, P.; Stejskal, J. Biocompatibility of polyaniline. Synth. Met. 2012, 162, 722–727. [Google Scholar] [CrossRef]
- Ramanaviciene, A.; Kausaite, A.; Tautkus, S.; Ramanavicius, A. Biocompatibility of polypyrrole particles: An in-vivo study in mice. J. Pharm. Pharmacol. 2007, 59, 311–315. [Google Scholar] [CrossRef]
- Sun, K.-H.; Liu, Z.; Liu, C.; Yu, T.; Shang, T.; Huang, C.; Zhou, M.; Liu, C.; Ran, F.; Lijia, P.; et al. Evaluation of in vitro and in vivo biocompatibility of a myo-inositol hexakisphosphate gelated polyaniline hydrogel in a rat model. Sci. Rep. 2016, 6, 23931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, M.; Dou, C.; Ma, G.; Wang, Y.; Feng, N.; Wang, W.; Fang, L. Synthesis and biocompatibility assessment of polyaniline nanomaterials. J. Bioact. Compat. Polym. 2018, 34, 16–24. [Google Scholar] [CrossRef]
- Parthibanab, E.; Kalaivasan, N.; Sudarsan, S. A study of magnetic, antibacterial and antifungal behaviour of a novel gold anchor of polyaniline/itaconic acid/Fe3O4 hybrid nanocomposite: Synthesis and characterization. Arab. J. Chem. 2020, 13, 4751–4763. [Google Scholar] [CrossRef]
- Mu, B.; Wang, A. One-pot fabrication of multifunctional superparamagnetic attapulgite/Fe3O4/polyaniline nanocomposites served as an adsorbent and catalyst support. J. Mater. Chem. A 2015, 3, 281–289. [Google Scholar] [CrossRef]
- Peymanfar, R.; Norouzi, F.; Javanshir, S. Preparation and characterization of one-pot PANi/Fe/Fe3O4/Fe2O3 nanocomposite and investigation of its microwave, magnetic and optical performance. Synth. Met. 2019, 252, 40–49. [Google Scholar] [CrossRef]
- Ayad, M.M.; Amer, W.A.; Kotp, M.G.; Minisy, I.M.; Rehab, A.F.; Kopecký, D.; Fitl, P. Synthesis of silver-anchored polyaniline–chitosan magnetic nanocomposite: A smart system for catalysis. RSC Adv. 2017, 7, 18553–18560. [Google Scholar] [CrossRef] [Green Version]
- Sriramprabha, R.; Sekar, M.; Revathi, R.; Viswanathan, C.; Wilson, J. Fe2O3/polyaniline supramolecular nanocomposite: A receptor free sensor platform for the quantitative determination of serum creatinine. Anal. Chim. Acta 2020, 1137, 103–114. [Google Scholar] [CrossRef]
- Predoi, D. A study on iron oxide nanoparticles coated with dextrin Obtained by coprecipitation. Dig. J. Nanomater. Biostructures 2007, 2, 169–173. [Google Scholar]
- Predoi, D.; Iconaru, S.L.; Predoi, M.V.; Buton, N.; Megier, C.; Motelica-Heino, M. Biocompatible Layers Obtained from Functionalized Iron Oxide Nanoparticles in Suspension. Coatings 2019, 9, 773. [Google Scholar] [CrossRef] [Green Version]
- ImageJ. Available online: http://imagej.nih.gov/ij (accessed on 30 January 2022).
- Gwyddion. Available online: http://gwyddion.net/ (accessed on 30 January 2022).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Predoi, G.; Ciobanu, C.; Iconaru, S.; Predoi, D.; Dreghici, D.; Groza, A.; Barbuceanu, F.; Cimpeanu, C.; Badea, M.-L.; Barbuceanu, S.-F.; et al. Preparation and Characterization of Dextran Coated Iron Oxide Nanoparticles Thin Layers. Polymers 2021, 13, 2351. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.K.; Ali, D.; Khan, S.H.; Gnanamoorthy, G.; Choudhary, N.; Yadav, K.K.; Thai, V.N.; Hussain, S.A.; Manhrdas, S. Synthesis and Characterization of Amorphous Iron Oxide Nanoparticles by the Sonochemical Method and Their Application for the Remediation of Heavy Metals from Wastewater. Nanomaterials 2020, 10, 1551. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., McKelvy, M.L., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Barra, G.M.O.; Leyva, M.E.; Gorelova, M.M.; Soares, B.G.; Sens, M. X-ray photoelectron spectroscopy and electrical conductivity of polyaniline doped with dodecylbenzenesulfonic acid as a function of the synthetic method. J. Appl. Polym. Sci. 2001, 80, 556–565. [Google Scholar] [CrossRef]
- Qiu, G.; Wang, Q.; Nie, M. Polyaniline/Fe3O4 magnetic nanocomposite prepared by ultrasonic irradiation. J. Appl. Polym. Sci. 2006, 102, 2107–2111. [Google Scholar] [CrossRef]
- Sreedhar, B.; Sairam, M.; Chattopadhyay, D.K.; Mitra, P.P.; Rao, D.V.M. Thermal and XPS studies on polyaniline salts prepared by inverted emulsion polymerization. J. Appl. Polym. Sci. 2006, 101, 499–508. [Google Scholar] [CrossRef]
- Kang, E.T.; Neoh, K.G.; Tan, K.L. Polyaniline: A polymer with many interesting intrinsic redox states. Prog. Polym. Sci. 1998, 23, 277. [Google Scholar] [CrossRef]
- Pascal, C.; Pascal, J.L.; Favier, F.; Moubtassim, M.L.E.; Payen, C. Electrochemical Synthesis for the Control of γ-Fe2O3 Nanoparticle Size. Morphology, Microstructure, and Magnetic Behavior. Chem. Mater. 1999, 11, 141–147. [Google Scholar] [CrossRef]
- Fujii, T.; de Groot, F.M.F.; Sawatzky, G.A.; Voogt, F.C.; Hibma, T.; Okada, K. In situ XPS analysis of various iron oxide films grown by NO2-assisted molecular-beam epitaxy. Phys. Rev. B 1999, 59, 3195–3202. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.-M.; Fu, S.-Y.; Xiao, H.-M. Synthesis of maghemite sub-microspheres by simple solvothermal reduction method. J. Solid State Chem. 2006, 179, 1554–1558. [Google Scholar] [CrossRef]
- Park, H.; Ayala, P.; Deshusses, M.A.; Mulchandani, A.; Choi, H.; Myung, N.V. Electrodeposition of maghemite (γ-Fe2O3) nanoparticles. Chem. Eng. J. 2008, 139, 208–212. [Google Scholar] [CrossRef]
- Liu, S.; Wang, J.; Zhang, D.; Zhang, P.; Ou, J.; Liu, B.; Yang, S. Investigation on cell biocompatible behaviors of polyaniline film fabricated via electroless surface polymerization. Appl. Surf. Sci. 2010, 256, 3427–3431. [Google Scholar] [CrossRef]
- Oestreicher, J.; Morgan, B. Glutathione: Subcellular distribution and membrane transport. Biochem. Cell Biol. 2019, 97, 270–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da, X.; Tang, D.; Wang, L.; Ma, J. Glutathione promoted Fenton degradation: A cocatalyst based on the –HS/–S–S– cycle with hydroxyl radicals. Environ. Sci. Water Res. Technol. 2020, 6, 515–522. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, L.; Zhao, J.; He, M.; Huang, Y.; Zhao, S. A tumor microenvironment–induced absorption red-shifted polymer nanoparticle for simultaneously activated photoacoustic imaging and photothermal therapy. Sci. Adv. 2021, 7, eabe3588. [Google Scholar] [CrossRef] [PubMed]
- Markovic, J.; Mora, N.J.; Broseta, A.M.; Gimeno, A.; de-la-Concepción, N.; Viña, J.; Pallardó, F.V. The Depletion of Nuclear Glutathione Impairs Cell Proliferation in 3t3 Fibroblasts. PLoS ONE 2009, 4, e6413. [Google Scholar] [CrossRef] [Green Version]
- Tang, B.Z.; Geng, Y.; Lam, A.J.W.Y.; Li‡, B.; Jing, X.; Wang, A.X.; Wang, F.; Pakhomov, A.B.; Zhang, X.X. Processible Nanostructured Materials with Electrical Conductivity and Magnetic Susceptibility: Preparation and Properties of Maghemite/Polyaniline Nanocomposite Films. Chem. Mater. 1999, 11, 1581–1589. [Google Scholar] [CrossRef]
- Lu, X.; Zheng, J.; Chao, D.; Chen, J.; Zhang, W.; Wei, Y. Poly (N-methylaniline)/multi-walled carbon nanotube composites—Synthesis, characterization, and electrical properties. J. Appl. Polym. Sci. 2006, 100, 2356–2361. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butoi, B.; Ciobanu, C.S.; Iconaru, S.L.; Negrilă, C.C.; Badea, M.A.; Balas, M.; Dinischiotu, A.; Predoi, G.; Bita, B.; Groza, A.; et al. Iron-Oxide-Nanoparticles-Doped Polyaniline Composite Thin Films. Polymers 2022, 14, 1821. https://doi.org/10.3390/polym14091821
Butoi B, Ciobanu CS, Iconaru SL, Negrilă CC, Badea MA, Balas M, Dinischiotu A, Predoi G, Bita B, Groza A, et al. Iron-Oxide-Nanoparticles-Doped Polyaniline Composite Thin Films. Polymers. 2022; 14(9):1821. https://doi.org/10.3390/polym14091821
Chicago/Turabian StyleButoi, Bogdan, Carmen Steluta Ciobanu, Simona Liliana Iconaru, Constantin Cătălin Negrilă, Madalina Andreea Badea, Mihaela Balas, Anca Dinischiotu, Gabriel Predoi, Bogdan Bita, Andreea Groza, and et al. 2022. "Iron-Oxide-Nanoparticles-Doped Polyaniline Composite Thin Films" Polymers 14, no. 9: 1821. https://doi.org/10.3390/polym14091821