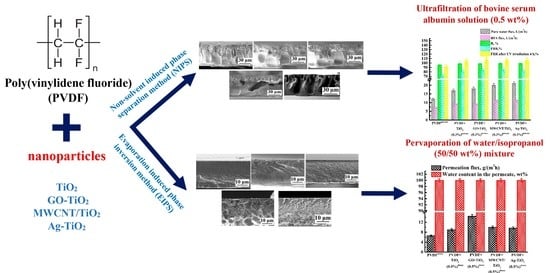
Novel Mixed Matrix Membranes Based on Poly(vinylidene fluoride): Development, Characterization, Modeling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Modification of TiO2 Particles
2.2.1. Preparation of GO–TiO2 Composites
2.2.2. Preparation of Ag–TiO2 Composites
2.3. Porous Membranes Preparation
2.4. Dense Membranes Preparation
2.5. Fourier Transform Infrared Spectroscopy
2.6. Atomic Force Microscopy
2.7. Scanning Electron Microscopy
2.8. Thermogravimetric Analysis
2.9. Contact Angle Measurements
2.10. Critical Surface Tension
2.11. Pervaporation Experiment
2.12. Ultrafiltration Experiment
2.13. Atomistic Molecular Dynamics Simulations
3. Results and Discussion
3.1. Development and Investigation of Porous PVDF and PVDF/Nanoparticle Membranes
3.1.1. Ultrafiltration Performance of Porous PVDF and PVDF/Nanoparticle Membranes
3.1.2. Structure and Physicochemical Properties of Porous PVDF and PVDF/Nanoparticle Membranes
3.2. Development and Investigation of Dense PVDF and PVDF/Nanoparticle Membranes
3.2.1. Pervaporation Performance of Dense PVDF and PVDF/Nanoparticle Membranes
3.2.2. Structure and Physicochemical Properties of Dense PVDF and PVDF/Nanoparticle Membranes
3.3. Comparison of the Performance with Membranes
3.4. Molecular Dynamics Simulation of PVDF and TiO2 System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Symbols and Abbreviations
| AFM | atomic force microscopy |
| BSA | bovine serum albumin |
| CL | coolant lubricant |
| DMA | N,N′-dimethylacetamide |
| EIPS | evaporation induced phase inversion method |
| FTIR | Fourier transform infrared spectroscopy |
| GO | graphene oxide |
| i-PrOH | isopropanol |
| LSPR | localized surface plasmon resonance |
| MD | molecular dynamics |
| MWCNT | multi-walled nanotubes |
| NIPS | non-solvent induced phase separation |
| PA | poly(m-phenylene isophtalamide) |
| PAA | polyacrylic acid |
| PAIU | polyamidoimideurea |
| PAN | polyacrylonitrile |
| PES | polyethersulfone |
| PSF | polysulfone |
| PVDF | poly(vinylidene fluoride) |
| PVP K-30 | polyvinylpyrrolidone K-30 |
| SEM | scanning electron microscopy |
| TGA | thermogravimetric analysis |
| TiO2 | titanium dioxide |
| UV | ultraviolet |
| FRR | flux recovery ratio, % |
| J | flux, L/(m2h) |
| Q | permeation flux, kg/(m2h) |
| R | rejection coefficient, % |
References
- El-Kholy, R.A.; Zaghlool, E.; Isawi, H.; Soliman, E.A.; Khalil, M.M.H.; El-Aassar, A.M.; Said, M.M. Groundwater quality assessment using water quality index and multivariate statistical analysis case study: East Matrouh, Northwestern coast, Egypt. Environ. Sci. Pollut. Res. 2022, 29, 65699–65722. [Google Scholar] [CrossRef] [PubMed]
- Plisko, T.V.; Bildyukevich, A.V.; Burts, K.S.; Ermakov, S.S.; Penkova, A.V.; Kuzminova, A.I.; Dmitrenko, M.E.; Hliavitskaya, T.A.; Ulbricht, M. One-Step Preparation of Antifouling Polysulfone Ultrafiltration Membranes via Modification by a Cationic Polyelectrolyte Based on Polyacrylamide. Polymers 2020, 12, 1017. [Google Scholar] [CrossRef] [PubMed]
- Polotskaya, G.A.; Penkova, A.V.; Pientka, Z.; Toikka, A.M. Polymer membranes modified by fullerene C60 for pervaporation of organic mixtures. Desalination Water Treat. 2010, 14, 83–88. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Atta, R.; Zolotarev, A.; Kuzminova, A.; Ermakov, S.; Penkova, A. Development of Novel Membranes Based on Polyvinyl Alcohol Modified by Pluronic F127 for Pervaporation Dehydration of Isopropanol. Sustainability 2022, 14, 3561. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Ermakov, S.; Roizard, D.; Penkova, A. Enhanced pervaporation properties of PVA-based membranes modified with polyelectrolytes. application to IPA dehydration. Polymers 2020, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Penkova, A.V.; Kuzminova, A.I.; Dmitrenko, M.E.; Surkova, V.A.; Liamin, V.P.; Markelov, D.A.; Komolkin, A.V.; Poloneeva, D.Y.; Laptenkova, A.V.; Selyutin, A.A.; et al. Novel pervaporation mixed matrix membranes based on polyphenylene isophtalamide modified by metal–organic framework UiO-66(NH2)-EDTA for highly efficient methanol isolation. Sep. Purif. Technol. 2021, 263, 118370. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Liamin, V.; Kuzminova, A.; Mazur, A.; Lahderanta, E.; Ermakov, S.; Penkova, A. Novel Mixed Matrix Sodium Alginate–Fullerenol Membranes: Development, Characterization, and Study in Pervaporation Dehydration of Isopropanol. Polymers 2020, 12, 864. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Zolotarev, A.; Liamin, V.; Kuzminova, A.; Mazur, A.; Semenov, K.; Ermakov, S.; Penkova, A. Novel Membranes Based on Hydroxyethyl Cellulose/Sodium Alginate for Pervaporation Dehydration of Isopropanol. Polymers 2021, 13, 674. [Google Scholar] [CrossRef]
- Zeng, Z.; Yu, D.; He, Z.; Liu, J.; Xiao, F.-X.; Zhang, Y.; Wang, R.; Bhattacharyya, D.; Tan, T.T.Y. Graphene Oxide Quantum Dots Covalently Functionalized PVDF Membrane with Significantly-Enhanced Bactericidal and Antibiofouling Performances. Sci. Rep. 2016, 6, 20142. [Google Scholar] [CrossRef] [Green Version]
- Jang, W.; Yun, J.; Jeon, K.; Byun, H. PVdF/graphene oxide hybrid membranes via electrospinning for water treatment applications. RSC Adv. 2015, 5, 46711–46717. [Google Scholar] [CrossRef]
- Otvagina, K.V.; Penkova, A.V.; Dmitrenko, M.E.; Kuzminova, A.I.; Sazanova, T.S.; Vorotyntsev, A.V.; Vorotyntsev, I.V. Novel composite membranes based on chitosan copolymers with polyacrylonitrile and polystyrene: Physicochemical properties and application for pervaporation dehydration of tetrahydrofuran. Membranes 2019, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Dhand, V.; Hong, S.K.; Li, L.; Kim, J.-M.; Kim, S.H.; Rhee, K.Y.; Lee, H.W. Fabrication of robust, ultrathin and light weight, hydrophilic, PVDF-CNT membrane composite for salt rejection. Compos. Part B Eng. 2019, 160, 632–643. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, Y.; Zhang, B.; Liu, Z.; Ma, X.; Chen, C. The preparation of a modified PVDF hollow fiber membrane by coating with multiwalled carbon nanotubes for high antifouling performance. RSC Adv. 2020, 10, 1848–1857. [Google Scholar] [CrossRef] [Green Version]
- Rajeswari, A.; Jackcina Stobel Christy, E.; Pius, A. New insight of hybrid membrane to degrade Congo red and Reactive yellow under sunlight. J. Photochem. Photobiol. B Biol. 2018, 179, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, A.; Vismaiya, S.; Pius, A. Preparation, characterization of nano ZnO-blended cellulose acetate-polyurethane membrane for photocatalytic degradation of dyes from water. Chem. Eng. J. 2017, 313, 928–937. [Google Scholar] [CrossRef]
- Méricq, J.-P.; Mendret, J.; Brosillon, S.; Faur, C. High performance PVDF-TiO2 membranes for water treatment. Chem. Eng. Sci. 2015, 123, 283–291. [Google Scholar] [CrossRef]
- Tran, D.-T.; Mendret, J.; Méricq, J.-P.; Faur, C.; Brosillon, S. Study of permeate flux behavior during photo-filtration using photocatalytic composite membranes. Chem. Eng. Process.-Process Intensif. 2020, 148, 107781. [Google Scholar] [CrossRef]
- Chen, Q.; Yu, Z.; Pan, Y.; Zeng, G.; Shi, H.; Yang, X.; Li, F.; Yang, S.; He, Y. Enhancing the photocatalytic and antibacterial property of polyvinylidene fluoride membrane by blending Ag–TiO2 nanocomposites. J. Mater. Sci. Mater. Electron. 2017, 28, 3865–3874. [Google Scholar] [CrossRef]
- Lee, J.-P.; Choi, S.; Cho, S.; Song, W.-J.; Park, S. Fabrication of Carbon Nanofibers Decorated with Various Kinds of Metal Oxides for Battery Applications. Energies 2021, 14, 1353. [Google Scholar] [CrossRef]
- Das, R. (Ed.) Polymeric Materials for Clean Water; Springer Series on Polymer and Composite Materials; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-00742-3. [Google Scholar]
- Isawi, H.; Abdelaziz, M.O.; Abo Zeed, D.; El-Kholy, R.A.; El-Noss, M.; Said, M.M.; El-Aassar, A.M.; Shawky, H.A. Semi industrial continuous flow photoreactor for wastewater purification in some polluted areas: Design, Manufacture, and Socio-economic impacts. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100544. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Markelov, D.; Komolkin, A.; Loginova, E.; Plisko, T.; Burts, K.; Bildyukevich, A.; Penkova, A. Modification strategies of polyacrylonitrile ultrafiltration membrane using TiO<inf>2</inf> for enhanced antifouling performance in water treatment. Sep. Purif. Technol. 2022, 286, 120500. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Kuzminova, A.; Zolotarev, A.; Liamin, V.; Plisko, T.; Burts, K.; Bildyukevich, A.; Ermakov, S.; Penkova, A. Novel High Flux Poly(m-phenylene isophtalamide)/TiO2 Membranes for Ultrafiltration with Enhanced Antifouling Performance. Polymers 2021, 13, 2804. [Google Scholar] [CrossRef] [PubMed]
- Jyothi, M.S.; Nayak, V.; Padaki, M.; Balakrishna, R.G.; Soontarapa, K. Eco-friendly membrane process and product development for complete elimination of chromium toxicity in wastewater. J. Hazard. Mater. 2017, 332, 112–123. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H.; Wang, P.; Zheng, Q.; Li, J. The influence of nano-sized TiO2 fillers on the morphologies and properties of PSF UF membrane. J. Memb. Sci. 2007, 288, 231–238. [Google Scholar] [CrossRef]
- Sotto, A.; Boromand, A.; Zhang, R.; Luis, P.; Arsuaga, J.M.; Kim, J.; Van der Bruggen, B. Effect of nanoparticle aggregation at low concentrations of TiO2 on the hydrophilicity, morphology, and fouling resistance of PES–TiO2 membranes. J. Colloid Interface Sci. 2011, 363, 540–550. [Google Scholar] [CrossRef] [PubMed]
- Damodar, R.A.; You, S.-J.; Chou, H.-H. Study the self cleaning, antibacterial and photocatalytic properties of TiO2 entrapped PVDF membranes. J. Hazard. Mater. 2009, 172, 1321–1328. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, C.; Zhang, G.; Hu, L.; Wang, P. Photocatalytic Fe-doped TiO2/PSF composite UF membranes: Characterization and performance on BPA removal under visible-light irradiation. Chem. Eng. J. 2017, 319, 39–47. [Google Scholar] [CrossRef]
- Kuvarega, A.T.; Khumalo, N.; Dlamini, D.; Mamba, B.B. Polysulfone/N,Pd co-doped TiO2 composite membranes for photocatalytic dye degradation. Sep. Purif. Technol. 2018, 191, 122–133. [Google Scholar] [CrossRef]
- Salazar, H.; Martins, P.M.; Santos, B.; Fernandes, M.M.; Reizabal, A.; Sebastián, V.; Botelho, G.; Tavares, C.J.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Photocatalytic and antimicrobial multifunctional nanocomposite membranes for emerging pollutants water treatment applications. Chemosphere 2020, 250, 126299. [Google Scholar] [CrossRef]
- Hoseini, S.N.; Pirzaman, A.K.; Aroon, M.A.; Pirbazari, A.E. Photocatalytic degradation of 2,4-dichlorophenol by Co-doped TiO2 (Co/TiO2) nanoparticles and Co/TiO2 containing mixed matrix membranes. J. Water Process Eng. 2017, 17, 124–134. [Google Scholar] [CrossRef]
- Safarpour, M.; Vatanpour, V.; Khataee, A. Preparation and characterization of graphene oxide/TiO2 blended PES nanofiltration membrane with improved antifouling and separation performance. Desalination 2016, 393, 65–78. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, T.; Shi, J.; Teng, K.; Wang, W.; Ma, M.; Li, J.; Qian, X.; Li, C.; Fan, J. Photocatalytic antifouling PVDF ultrafiltration membranes based on synergy of graphene oxide and TiO2 for water treatment. J. Memb. Sci. 2016, 520, 281–293. [Google Scholar] [CrossRef]
- Almeida, N.A.; Martins, P.M.; Teixeira, S.; Lopes da Silva, J.A.; Sencadas, V.; Kühn, K.; Cuniberti, G.; Lanceros-Mendez, S.; Marques, P.A. TiO2/graphene oxide immobilized in P(VDF-TrFE) electrospun membranes with enhanced visible-light-induced photocatalytic performance. J. Mater. Sci. 2016, 51, 6974–6986. [Google Scholar] [CrossRef]
- El-Aassar, A.M.; Isawi, H.; El-Noss, M.; El-Kholy, R.A.; Said, M.M.; Shawky, H.A. Design and fabrication of continuous flow photoreactor using semiconductor oxides for degradation of organic pollutants. J. Water Process Eng. 2019, 32, 100922. [Google Scholar] [CrossRef]
- Yar, A.; Haspulat, B.; Üstün, T.; Eskizeybek, V.; Avcı, A.; Kamış, H.; Achour, S. Electrospun TiO2/ZnO/PAN hybrid nanofiber membranes with efficient photocatalytic activity. RSC Adv. 2017, 7, 29806–29814. [Google Scholar] [CrossRef] [Green Version]
- Muhulet, A.; Tuncel, C.; Miculescu, F.; Pandele, A.M.; Bobirica, C.; Orbeci, C.; Bobirica, L.; Palla-Papavlu, A.; Voicu, S.I. Synthesis and characterization of polysulfone–TiO2 decorated MWCNT composite membranes by sonochemical method. Appl. Phys. A 2020, 126, 233. [Google Scholar] [CrossRef]
- Cheng, J.; Pu, H. A facile method to prepare polyvinylidene fluoride composite nanofibers with high photocatalytic activity via nanolayer coextrusion. Eur. Polym. J. 2018, 99, 361–367. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, D.; Cho, M.; Lee, S.S.; Zhang, F.; Biswas, P.; Fortner, J.D. In Situ Photocatalytic Synthesis of Ag Nanoparticles (nAg) by Crumpled Graphene Oxide Composite Membranes for Filtration and Disinfection Applications. Environ. Sci. Technol. 2016, 50, 2514–2521. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Yu, Z.; Li, F.; Yang, Y.; Pan, Y.; Peng, Y.; Yang, X.; Zeng, G. A novel photocatalytic membrane decorated with RGO-Ag-TiO2 for dye degradation and oil–water emulsion separation. J. Chem. Technol. Biotechnol. 2018, 93, 761–775. [Google Scholar] [CrossRef]
- Isawi, H. Evaluating the performance of different nano-enhanced ultrafiltration membranes for the removal of organic pollutants from wastewater. J. Water Process Eng. 2019, 31, 100833. [Google Scholar] [CrossRef]
- Kang, G.; Cao, Y. Application and modification of poly(vinylidene fluoride) (PVDF) membranes—A review. J. Memb. Sci. 2014, 463, 145–165. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Z.; Shan, M.; Zhou, B.; Li, Y.; Li, B.; Niu, J.; Qian, X. Synergetic effects of oxidized carbon nanotubes and graphene oxide on fouling control and anti-fouling mechanism of polyvinylidene fluoride ultrafiltration membranes. J. Memb. Sci. 2013, 448, 81–92. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, S.; Zhang, Y.; Zhao, S. Comparing the antifouling effects of activated carbon and TiO2 in ultrafiltration membrane development. J. Colloid Interface Sci. 2018, 515, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Sakarkar, S.; Muthukumaran, S.; Jegatheesan, V. Polyvinylidene Fluoride and Titanium Dioxide Ultrafiltration Photocatalytic Membrane: Fabrication, Morphology, and Its Application in Textile Wastewater Treatment. J. Environ. Eng. 2020, 146, 04020053. [Google Scholar] [CrossRef]
- Benhabiles, O.; Galiano, F.; Marino, T.; Mahmoudi, H.; Lounici, H.; Figoli, A. Preparation and Characterization of TiO2-PVDF/PMMA Blend Membranes Using an Alternative Non-Toxic Solvent for UF/MF and Photocatalytic Application. Molecules 2019, 24, 724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Qu, F.; Jia, R.; Sun, S.; Li, G.; Liang, H. Preliminary Study on the Removal of Steroidal Estrogens Using TiO2-Doped PVDF Ultrafiltration Membranes. Water 2016, 8, 134. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Liu, X.D.; Wang, D.X.; Lin, Y.K.; Han, Y.P.; Wang, X.L. Feasibility of using an innovative PVDF MF membrane prior to RO for reuse of a secondary municipal effluent. Desalination 2013, 311, 16–23. [Google Scholar] [CrossRef]
- Park, C.; Hong, S.-W.; Chung, T.H.; Choi, Y.-S. Performance evaluation of pretreatment processes in integrated membrane system for wastewater reuse. Desalination 2010, 250, 673–676. [Google Scholar] [CrossRef]
- Tomaszewska, M. Preparation and properties of flat-sheet membranes from poly(vinylidene fluoride) for membrane distillation. Desalination 1996, 104, 1–11. [Google Scholar] [CrossRef]
- Hou, D.; Dai, G.; Wang, J.; Fan, H.; Zhang, L.; Luan, Z. Preparation and characterization of PVDF/nonwoven fabric flat-sheet composite membranes for desalination through direct contact membrane distillation. Sep. Purif. Technol. 2012, 101, 1–10. [Google Scholar] [CrossRef]
- Fan, H.; Peng, Y. Application of PVDF membranes in desalination and comparison of the VMD and DCMD processes. Chem. Eng. Sci. 2012, 79, 94–102. [Google Scholar] [CrossRef]
- Ong, Y.K.; Widjojo, N.; Chung, T.-S. Fundamentals of semi-crystalline poly(vinylidene fluoride) membrane formation and its prospects for biofuel (ethanol and acetone) separation via pervaporation. J. Memb. Sci. 2011, 378, 149–162. [Google Scholar] [CrossRef]
- Jian, K.; Pintauro, P. Integral asymmetric poly(vinylidene fluoride) (PVDF) pervaporation membranes. J. Memb. Sci. 1993, 85, 301–309. [Google Scholar] [CrossRef]
- Rashid, A.H.; Hatem, M.D.I.; Ahmad, M.S.; Othman, M.H.D. A Morphological Study of Poly (Vinylidene Fluoride) Pvdf Membranes: In Perspective of Membrane Pervaporation Process. ASEAN J. Chem. Eng. 2015, 14, 38. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wang, R.; Zhang, S.; Li, G.; Zhang, Y. Treatment of wastewater containing oil using phosphorylated silica nanotubes (PSNTs)/polyvinylidene fluoride (PVDF) composite membrane. Desalination 2014, 332, 109–116. [Google Scholar] [CrossRef]
- Huang, X.; Wang, W.; Liu, Y.; Wang, H.; Zhang, Z.; Fan, W.; Li, L. Treatment of oily waste water by PVP grafted PVDF ultrafiltration membranes. Chem. Eng. J. 2015, 273, 421–429. [Google Scholar] [CrossRef]
- Jian, K.; Pintauro, P.N.; Ponangi, R. Separation of dilute organic/water mixtures with asymmetric poly(vinylidene fluoride) membranes. J. Memb. Sci. 1996, 117, 117–133. [Google Scholar] [CrossRef]
- Romay, M.; Diban, N.; Rivero, M.J.; Urtiaga, A.; Ortiz, I. Critical Issues and Guidelines to Improve the Performance of Photocatalytic Polymeric Membranes. Catalysts 2020, 10, 570. [Google Scholar] [CrossRef]
- Liu, F.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Memb. Sci. 2011, 375, 1–27. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Yu, B.; Browne, C.; Garnier, G. Reversible pH Responsive Bovine Serum Albumin Hydrogel Sponge Nanolayer. Front. Bioeng. Biotechnol. 2020, 8, 573. [Google Scholar] [CrossRef]
- Fehér, B.; Lyngsø, J.; Bartók, B.; Mihály, J.; Varga, Z.; Mészáros, R.; Pedersen, J.S.; Bóta, A.; Varga, I. Effect of pH on the conformation of bovine serume albumin-gold bioconjugates. J. Mol. Liq. 2020, 309, 113065. [Google Scholar] [CrossRef]
- SADLER, P.J.; TUCKER, A. pH-induced structural transitions of bovine serum albumin. Histidine pKa values and unfolding of the N-terminus during the N to F transition. Eur. J. Biochem. 1993, 212, 811–817. [Google Scholar] [CrossRef]
- Rufino, A.F.C.S.; Almeida, M.R.; Sharma, M.; Coutinho, J.A.P.; Freire, M.G. Separation of Albumin from Bovine Serum Applying Ionic-Liquid-Based Aqueous Biphasic Systems. Appl. Sci. 2022, 12, 707. [Google Scholar] [CrossRef]
- Tang, P.; Si, S.; Liu, L. Analysis of Bovine Serum Albumin Ligands from Puerariae flos Using Ultrafiltration Combined with HPLC-MS. J. Chem. 2015, 2015, 648361. [Google Scholar] [CrossRef] [Green Version]
- Nora’aini, A.; Sofiah, H.; Asmadi, A.; Suriyani, A.R. Fabrication and Characterisation of Asymmetric Ultrafiltration Membrane for BSA Separation: Effect of Shear Rates. J. Appl. Sci. 2010, 10, 1083–1089. [Google Scholar] [CrossRef] [Green Version]
- Kuzminova, A.I.; Dmitrenko, M.E.; Poloneeva, D.Y.; Selyutin, A.A.; Mazur, A.S.; Emeline, A.V.; Mikhailovskii, V.Y.; Solovyev, N.D.; Ermakov, S.S.; Penkova, A.V. Sustainable composite pervaporation membranes based on sodium alginate modified by metal organic frameworks for dehydration of isopropanol. J. Memb. Sci. 2021, 626, 119194. [Google Scholar] [CrossRef]
- Baker, R.W. Membrane Technology and Applications; McGraw-Hill: New York, NY, USA, 2000. [Google Scholar]
- Daraei, P.; Madaeni, S.S.; Ghaemi, N.; Khadivi, M.A.; Rajabi, L.; Derakhshan, A.A.; Seyedpour, F. PAA grafting onto new acrylate-alumoxane/PES mixed matrix nano-enhanced membrane: Preparation, characterization and performance in dye removal. Chem. Eng. J. 2013, 221, 111–123. [Google Scholar] [CrossRef]
- Ma, T.; Su, Y.; Li, Y.; Zhang, R.; Liu, Y.; He, M.; Li, Y.; Dong, N.; Wu, H.; Jiang, Z. Fabrication of electro-neutral nanofiltration membranes at neutral pH with antifouling surface via interfacial polymerization from a novel zwitterionic amine monomer. J. Memb. Sci. 2016, 503, 101–109. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. Potential energy functions for atomic-level simulations of water and organic and biomolecular systems. Proc. Natl. Acad. Sci. USA 2005, 102, 6665–6670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, M.; Akaogi, M. Molecular Dynamics Simulation of the Structural and Physical Properties of the Four Polymorphs of TiO2. Mol. Simul. 1991, 6, 239–244. [Google Scholar] [CrossRef]
- Berendsen, H.J.C.; Grigera, J.R.; Straatsma, T.P. The missing term in effective pair potentials. J. Phys. Chem. 1987, 91, 6269–6271. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindah, E. Gromacs: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Berendsen, H.J.C.; Postma, J.P.M.; van Gunsteren, W.F.; DiNola, A.; Haak, J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984, 81, 3684–3690. [Google Scholar] [CrossRef] [Green Version]
- Bussi, G.; Donadio, D.; Parrinello, M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007, 126, 014101. [Google Scholar] [CrossRef] [Green Version]
- Ravidhas, C.; Anitha, B.; Arivukarasan, D.; Venkatesh, R.; Christy, A.J.; Jothivenkatachalam, K.; Nithya, A.; Moses Ezhil Raj, A.; Ravichandran, K.; Sanjeeviraja, C. Tunable morphology with selective faceted growth of visible light active TiO2 thin films by facile hydrothermal method: Structural, optical and photocatalytic properties. J. Mater. Sci. Mater. Electron. 2016, 27, 5020–5032. [Google Scholar] [CrossRef]
- Tian, Y.; Tatsuma, T. Mechanisms and Applications of Plasmon-Induced Charge Separation at TiO2 Films Loaded with Gold Nanoparticles. J. Am. Chem. Soc. 2005, 127, 7632–7637. [Google Scholar] [CrossRef]
- Awazu, K.; Fujimaki, M.; Rockstuhl, C.; Tominaga, J.; Murakami, H.; Ohki, Y.; Yoshida, N.; Watanabe, T. A Plasmonic Photocatalyst Consisting of Silver Nanoparticles Embedded in Titanium Dioxide. J. Am. Chem. Soc. 2008, 130, 1676–1680. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, H.; Farahani, M.H.D.A.; Vatanpour, V. Preparation and characterization of emulsion poly(vinyl chloride) (EPVC)/TiO2 nanocomposite ultrafiltration membrane. J. Memb. Sci. 2014, 472, 185–193. [Google Scholar] [CrossRef]
- Cai, X.; Lei, T.; Sun, D.; Lin, L. A critical analysis of the α, β and γ phases in poly(vinylidene fluoride) using FTIR. RSC Adv. 2017, 7, 15382–15389. [Google Scholar] [CrossRef] [Green Version]
- Dmitrenko, M.; Chepeleva, A.; Liamin, V.; Kuzminova, A.; Mazur, A.; Semenov, K.; Penkova, A. Novel PDMS-b-PPO Membranes Modified with Graphene Oxide for Efficient Pervaporation Ethanol Dehydration. Membranes 2022, 12, 832. [Google Scholar] [CrossRef]
- Dmitrenko, M.; Chepeleva, A.; Liamin, V.; Mazur, A.; Semenov, K.; Solovyev, N.; Penkova, A. Novel Mixed Matrix Membranes Based on Polyphenylene Oxide Modified with Graphene Oxide for Enhanced Pervaporation Dehydration of Ethylene Glycol. Polymers 2022, 14, 691. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Roso, M.; Boaretti, C.; Lorenzetti, A.; Martucci, A.; Modesti, M. PVDF-TiO2 core-shell fibrous membranes by microwave-hydrothermal method: Preparation, characterization, and photocatalytic activity. J. Environ. Chem. Eng. 2021, 9, 106250. [Google Scholar] [CrossRef]
- de Jesus Silva, A.J.; Contreras, M.M.; Nascimento, C.R.; da Costa, M.F. Kinetics of thermal degradation and lifetime study of poly(vinylidene fluoride) (PVDF) subjected to bioethanol fuel accelerated aging. Heliyon 2020, 6, e04573. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Xu, X.; Chen, J.; Yang, F. Effect of graphene oxide concentration on the morphologies and antifouling properties of PVDF ultrafiltration membranes. J. Environ. Chem. Eng. 2013, 1, 349–354. [Google Scholar] [CrossRef]
- Moghadam, M.T.; Lesage, G.; Mohammadi, T.; Mericq, J.-P.; Mendret, J.; Heran, M.; Faur, C.; Brosillon, S.; Hemmati, M.; Naeimpoor, F. Improved antifouling properties of TiO2/PVDF nanocomposite membranes in UV-coupled ultrafiltration. J. Appl. Polym. Sci. 2015, 132, 41731. [Google Scholar] [CrossRef]
- Cheng, L.; Zhou, Z.; Li, L.; Xiao, P.; Ma, Y.; Liu, F.; Li, J. PVDF/MOFs mixed matrix ultrafiltration membrane for efficient water treatment. Front. Chem. 2022, 10, 985750. [Google Scholar] [CrossRef]
- Zhao, X.; Xuan, H.; Qin, A.; Liu, D.; He, C. Improved antifouling property of PVDF ultrafiltration membrane with plasma treated PVDF powder. RSC Adv. 2015, 5, 64526–64533. [Google Scholar] [CrossRef]


















| Membrane | Type of Membrane | Type of Nanoparticles | Content of Nanoparticles, wt% |
|---|---|---|---|
| PDVFporous | porous | - | - |
| PDVF+TiO2(0.1)porous | porous | TiO2 | 0.1 |
| PDVF+TiO2(0.3)porous | porous | TiO2 | 0.3 |
| PDVF+TiO2(0.5)porous | porous | TiO2 | 0.5 |
| PDVF+TiO2(0.75)porous | porous | TiO2 | 0.75 |
| PDVF+TiO2(1)porous | porous | TiO2 | 1 |
| PVDF+GO-TiO2(0.3%)porous | porous | GO-TiO2 | 0.3 |
| PVDF+MWCNT/TiO2(0.3%)porous | porous | MWCNT/TiO2 | 0.3 |
| PVDF+Ag-TiO2(0.3%)porous | porous | Ag-TiO2 | 0.3 |
| PDVFdense | dense | - | - |
| PDVF+TiO2(0.3)dense | dense | TiO2 | 0.3 |
| PDVF+TiO2(0.5)dense | dense | TiO2 | 0.5 |
| PDVF+TiO2(1)dense | dense | TiO2 | 1 |
| PVDF+GO-TiO2(0.5%)dense | dense | GO-TiO2 | 0.5 |
| PVDF+MWCNT/TiO2(0.5%)dense | dense | MWCNT/TiO2 | 0.5 |
| PVDF+Ag-TiO2(0.5%)dense | dense | Ag-TiO2 | 0.5 |
| Membrane | Ra, nm | Rq, nm | Contact Angle, ° |
|---|---|---|---|
| PVDF porous | 5.7 | 7.2 | 27 |
| PVDF+TiO2(0.3%)porous | 6.0 | 7.5 | 24 |
| PVDF+GO-TiO2(0.3%)porous | 6.8 | 8.5 | 20 |
| PVDF+MWCNT/TiO2(0.3%)porous | 7.5 | 9.5 | 20 |
| PVDF+Ag-TiO2(0.3%)porous | 6.2 | 8.0 | 23 |
| Membrane | Ra, nm | Rq, nm |
|---|---|---|
| PVDFdense | 21.1 | 27.0 |
| PVDF+TiO2(0.5%)dense | 52.0 | 65.1 |
| PVDF+GO-TiO2(0.5%)dense | 76.8 | 99.1 |
| PVDF+MWCNT/TiO2(0.5%)dense | 74.2 | 96.3 |
| PVDF+Ag-TiO2(0.5%)dense | 64.9 | 87.2 |
| Membrane | Contact Angle, ° | Critical Surface Tension | |||
|---|---|---|---|---|---|
| Water | Glycerol | ||||
| PVDFdense | 82 | 81 | 4.66 | 19.32 | 23.98 |
| PVDF+TiO2(0.5%)dense | 74 | 83 | 0.00 | 42.46 | 42.46 |
| PVDF+GO-TiO2(0.5%)dense | 73 | 79 | 0.55 | 37.24 | 37.79 |
| PVDF+MWCNT/TiO2(0.5%)dense | 76 | 83 | 0.16 | 36.86 | 37.01 |
| PVDF+Ag-TiO2(0.5%)dense | 75 | 83 | 0.03 | 39.62 | 39.65 |
| Membranes | Pure Water Flux, L/(m2h) | BSA Flux, L/(m2h) | FRR, % | FRR after UV-Irradiation, % | Rejection Coefficient, % | References |
|---|---|---|---|---|---|---|
| PVDF+MWCNT/TiO2(0.3%)porous | 20 | 11 | 84 | 105 | 98.6 | This study |
| PVDF+Ag-TiO2(0.3%)porous | 21 | 11 | 81 | 106 | 98.4 | This study |
| PVDF+Ag-TiO2(0.06%) | ~90 | ~10 | - | ~100 | 89.8 | [18] |
| PVDF+TiO2(30%) | ~90 | 29 | 103 | 112 | - | [16] |
| PVDF+GO(2%) | 27 | 11 | 87 | - | - | [86] |
| PVDF+GO/TiO2 | 488 | ~325 | 71 | 82 | 92.5 | [33] |
| PVDF+TiO2(20%) | ~100 | ~50 | 60.2 | 97 | 85.6 | [87] |
| PVDF+MIL-53(Al)(5%) | 44 | ~25 | 89 | - | 80.3 | [88] |
| PVDF-g-PAA (polyvinylidene fluoride-g-polyacrylic acid) | ~160 | ~60 | ~80 | - | ~83 | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzminova, A.; Dmitrenko, M.; Zolotarev, A.; Markelov, D.; Komolkin, A.; Dubovenko, R.; Selyutin, A.; Wu, J.; Su, R.; Penkova, A. Novel Mixed Matrix Membranes Based on Poly(vinylidene fluoride): Development, Characterization, Modeling. Polymers 2023, 15, 1222. https://doi.org/10.3390/polym15051222
Kuzminova A, Dmitrenko M, Zolotarev A, Markelov D, Komolkin A, Dubovenko R, Selyutin A, Wu J, Su R, Penkova A. Novel Mixed Matrix Membranes Based on Poly(vinylidene fluoride): Development, Characterization, Modeling. Polymers. 2023; 15(5):1222. https://doi.org/10.3390/polym15051222
Chicago/Turabian StyleKuzminova, Anna, Mariia Dmitrenko, Andrey Zolotarev, Denis Markelov, Andrei Komolkin, Roman Dubovenko, Artem Selyutin, Jiangjiexing Wu, Rongxin Su, and Anastasia Penkova. 2023. "Novel Mixed Matrix Membranes Based on Poly(vinylidene fluoride): Development, Characterization, Modeling" Polymers 15, no. 5: 1222. https://doi.org/10.3390/polym15051222