Metal-Organic Frameworks Applications in Synergistic Cancer Photo-Immunotherapy
Abstract
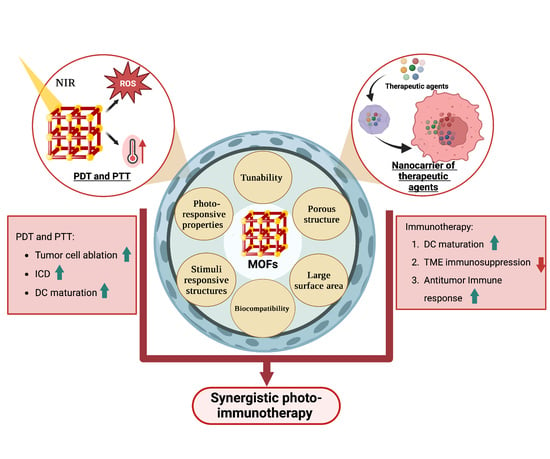
:1. Introduction
2. Metal-Organic Frameworks (MOFs)
2.1. Structure and Properties
2.2. MOFs in Phototherapy
3. Synergistic Photo-Immunotherapy
3.1. Synergistic Strategies of PDT and Immunotherapy
3.2. Synergistic Strategies of PTT and Immunotherapy
3.3. Synergetic Dual-Photo-Immunotherapeutic Strategies
4. Challenges and Opportunities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deo, S.V.S.; Sharma, J.; Kumar, S. GLOBOCAN 2020 Report on Global Cancer Burden: Challenges and Opportunities for Surgical Oncologists. Ann. Surg. Oncol. 2022, 29, 6497–6500. [Google Scholar] [CrossRef]
- Wang, X.; Yang, L.; Chen, Z.; Shin, D.M. Application of Nanotechnology in Cancer Therapy and Imaging. CA Cancer J. Clin. 2008, 58, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Murugan, C.; Sharma, V.; Murugan, R.K.; Malaimegu, G.; Sundaramurthy, A. Two-Dimensional Cancer Theranostic Nanomaterials: Synthesis, Surface Functionalization and Applications in Photothermal Therapy. J. Control. Release 2019, 299, 1–20. [Google Scholar] [CrossRef]
- Jiachen, L.U.; Ding, J.; Zhaoxia, L.I.U.; Chen, T. Retrospective Analysis of the Preparation and Application of Immunotherapy in Cancer Treatment (Review). Int. J. Oncol. 2022, 60, 12. [Google Scholar] [CrossRef]
- Hak, A.; Ravasaheb Shinde, V.; Rengan, A.K. A Review of Advanced Nanoformulations in Phototherapy for Cancer Therapeutics. Photodiagnosis Photodyn. 2021, 33, 102205. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, L.; Gu, Z.; Dai, L. Carbon Nanomaterials for Phototherapy. Nanophotonics 2022, 11, 4955–4976. [Google Scholar] [CrossRef]
- Zheng, Q.; Liu, X.; Zheng, Y.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Li, Z.; Zhu, S.; Wang, X.; Wu, S. The Recent Progress on Metal-Organic Frameworks for Phototherapy. Chem. Soc. Rev. 2021, 50, 5086–5125. [Google Scholar] [CrossRef]
- Dias, L.D.; Mfouo-Tynga, I.S. Learning from Nature: Bioinspired Chlorin-Based Photosensitizers Immobilized on Carbon Materials for Combined Photodynamic and Photothermal Therapy. Biomimetics 2020, 5, 53. [Google Scholar] [CrossRef]
- dos Santos, A.F.; de Almeida, D.R.Q.; Terra, L.F.; Baptista, M.S.; Labriola, L. Photodynamic Therapy in Cancer Treatment—An Update Review. J. Cancer Metastasis Treat. 2019, 5, 25. [Google Scholar] [CrossRef] [Green Version]
- Robertson, C.A.; Evans, D.H.; Abrahamse, H. Photodynamic Therapy (PDT): A Short Review on Cellular Mechanisms and Cancer Research Applications for PDT. J. Photochem. Photobiol. B 2009, 96, 1–8. [Google Scholar] [CrossRef]
- Lismont, M.; Dreesen, L.; Wuttke, S. Metal-Organic Framework Nanoparticles in Photodynamic Therapy: Current Status and Perspectives. Adv. Funct. Mater. 2017, 27, 1606314. [Google Scholar] [CrossRef]
- Chouikrat, R.; Seve, A.; Vanderesse, R.; Benachour, H.; Barberi-Heyob, M.; Richeter, S.; Raehm, L.; Durand, J.-O.; Verelst, M.; Frochot, C. Non Polymeric Nanoparticles for Photodynamic Therapy Applications: Recent Developments. Curr. Med. Chem. 2012, 19, 781–792. [Google Scholar] [CrossRef]
- Kadkhoda, J.; Tarighatnia, A.; Tohidkia, M.R.; Nader, N.D.; Aghanejad, A. Photothermal Therapy-Mediated Autophagy in Breast Cancer Treatment: Progress and Trends. Life Sci. 2022, 298, 120499. [Google Scholar] [CrossRef]
- Dias, L.D.; Buzzá, H.H.; Stringasci, M.D.; Bagnato, V.S. Recent Advances in Combined Photothermal and Photodynamic Therapies against Cancer Using Carbon Nanomaterial Platforms for In Vivo Studies. Photochem 2021, 1, 434–450. [Google Scholar] [CrossRef]
- Liu, J.; Lu, K.; Gao, F.; Zhao, L.; Li, H.; Jiang, Y. Multifunctional MoS2 Composite Nanomaterials for Drug Delivery and Synergistic Photothermal Therapy in Cancer Treatment. Ceram. Int. 2022, 48, 22419–22427. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Feng, C.; Ren, J.; Bao, L.; Zhao, Y.; Tao, W.; Zhao, Y.; Yang, X. Tailoring Aggregation Extent of Photosensitizers to Boost Phototherapy Potency for Eliciting Systemic Antitumor Immunity. Adv. Mater. 2022, 34, 2106390. [Google Scholar] [CrossRef]
- Alzeibak, R.; Mishchenko, T.A.; Shilyagina, N.Y.; Balalaeva, I.v.; Vedunova, M.v.; Krysko, D.v. Targeting Immunogenic Cancer Cell Death by Photodynamic Therapy: Past, Present and Future. J Immunother. Cancer 2021, 9, e001926. [Google Scholar] [CrossRef]
- Galluzzi, L.; Bravo-San Pedro, J.M.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Alnemri, E.S.; Altucci, L.; Andrews, D.; Annicchiarico-Petruzzelli, M.; et al. Essential versus Accessory Aspects of Cell Death: Recommendations of the NCCD 2015. Cell Death Differ. 2015, 22, 58–73. [Google Scholar] [CrossRef] [Green Version]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunogenic Cell Death in Cancer and Infectious Disease. Nat. Rev. Immunol. 2017, 17, 97–111. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Pan, W.; Li, N.; Tang, B. Photothermal Therapy-Induced Immunogenic Cell Death Based on Natural Melanin Nanoparticles against Breast Cancer. Chem. Commun. 2020, 56, 1389–1392. [Google Scholar] [CrossRef]
- Wei, B.; Pan, J.; Yuan, R.; Shao, B.; Wang, Y.; Guo, X.; Zhou, S. Polarization of Tumor-Associated Macrophages by Nanoparticle-Loaded Escherichia Coli Combined with Immunogenic Cell Death for Cancer Immunotherapy. Nano Lett. 2021, 21, 4231–4240. [Google Scholar] [CrossRef]
- Garg, A.D.; Dudek-Peric, A.M.; Romano, E.; Agostinis, P. Immunogenic Cell Death. Int. J. Dev. Biol. 2015, 59, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Kimura, A.; Kishimoto, T. IL-6: Regulator of Treg/Th17 Balance. Eur. J. Immunol. 2010, 40, 1830–1835. [Google Scholar] [CrossRef]
- Sun, R.; Gao, D.S.; Shoush, J.; Lu, B. The IL-1 Family in Tumorigenesis and Antitumor Immunity. Semin. Cancer Biol. 2022, 86, 280–295. [Google Scholar] [CrossRef]
- Nath, S.; Obaid, G.; Hasan, T. The Course of Immune Stimulation by Photodynamic Therapy: Bridging Fundamentals of Photochemically Induced Immunogenic Cell Death to the Enrichment of T-Cell Repertoire. Photochem. Photobiol. 2019, 95, 1288–1305. [Google Scholar] [CrossRef] [Green Version]
- Duan, Q.; Zhang, H.; Zheng, J.; Zhang, L. Turning Cold into Hot: Firing up the Tumor Microenvironment. Trends Cancer 2020, 6, 605–618. [Google Scholar] [CrossRef]
- Li, W.; Yang, J.; Luo, L.; Jiang, M.; Qin, B.; Yin, H.; Zhu, C.; Yuan, X.; Zhang, J.; Luo, Z.; et al. Targeting Photodynamic and Photothermal Therapy to the Endoplasmic Reticulum Enhances Immunogenic Cancer Cell Death. Nat. Commun. 2019, 10, 3349. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Feng, X.; Wan, C.; Lovell, J.F.; Jin, H.; Ding, J. Role of Nanoparticle-Mediated Immunogenic Cell Death in Cancer Immunotherapy. Asian J. Pharm. Sci. 2021, 16, 129–132. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Kong, Z.; Sun, X.; He, Z.; Sun, B.; Luo, C.; Sun, J. Emerging Photodynamic Nanotherapeutics for Inducing Immunogenic Cell Death and Potentiating Cancer Immunotherapy. Biomaterials 2022, 282, 121433. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer Immunotherapy Comes of Age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Behl, T.; Islam, M.R.; Alam, M.N.; Islam, M.M.; Albarrati, A.; Albratty, M.; Meraya, A.M.; Bungau, S.G. Emerging Management Approach for the Adverse Events of Immunotherapy of Cancer. Molecules 2022, 27, 3798. [Google Scholar] [CrossRef]
- Taefehshokr, S.; Parhizkar, A.; Hayati, S.; Mousapour, M.; Mahmoudpour, A.; Eleid, L.; Rahmanpour, D.; Fattahi, S.; Shabani, H.; Taefehshokr, N. Cancer Immunotherapy: Challenges and Limitations. Pathol. Res. Pract. 2022, 229, 153723. [Google Scholar] [CrossRef]
- Bird, B.H.; Nally, K.; Ronan, K.; Clarke, G.; Amu, S.; Almeida, A.S.; Flavin, R.; Finn, S. Cancer Immunotherapy with Immune Checkpoint Inhibitors-Biomarkers of Response and Toxicity; Current Limitations and Future Promise. Diagnostics 2022, 12, 124. [Google Scholar] [CrossRef]
- Miao, L.; Zhang, Z.; Ren, Z.; Tang, F.; Li, Y. Obstacles and Coping Strategies of CAR-T Cell Immunotherapy in Solid Tumors. Front. Immunol. 2021, 12, 687822. [Google Scholar] [CrossRef]
- Sengupta, S. Cancer Nanomedicine: Lessons for Immuno-Oncology. Trends Cancer 2017, 3, 551–560. [Google Scholar] [CrossRef]
- Liu, Q.; Duo, Y.; Fu, J.; Qiu, M.; Sun, Z.; Adah, D.; Kang, J.; Xie, Z.; Fan, T.; Bao, S.; et al. Nano-Immunotherapy: Unique Mechanisms of Nanomaterials in Synergizing Cancer Immunotherapy. Nano Today 2021, 36, 101023. [Google Scholar] [CrossRef]
- Ma, Y.; Huang, J.; Song, S.; Chen, H.; Zhang, Z. Cancer-Targeted Nanotheranostics: Recent Advances and Perspectives. Small 2016, 12, 4936–4954. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, X.; Chen, F.; Yang, L.; Yuan, M.; Fu, D.Y.; Wang, W.; Yu, H. Stimuli-Activatable Nanomaterials for Phototherapy of Cancer. Biomed. Mater. 2021, 16, 042008. [Google Scholar] [CrossRef]
- Guo, R.; Wang, S.; Zhao, L.; Zong, Q.; Li, T.; Ling, G.; Zhang, P. Engineered Nanomaterials for Synergistic Photo-Immunotherapy. Biomaterials 2022, 282, 121425. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.; Ju, E.; Liu, Z.; Chen, Z.; Ren, J.; Qu, X. Metal-Organic-Framework-Based Vaccine Platforms for Enhanced Systemic Immune and Memory Response. Adv. Funct. Mater. 2016, 26, 6454–6461. [Google Scholar] [CrossRef]
- Gao, P.; Chen, Y.; Pan, W.; Li, N.; Liu, Z.; Tang, B. Antitumor Agents Based on Metal–Organic Frameworks. Angew. Chem.–Int. Ed. 2021, 60, 16763–16776. [Google Scholar] [CrossRef] [PubMed]
- Ni, K.; Luo, T.; Nash, G.T.; Lin, W. Nanoscale Metal-Organic Frameworks for Cancer Immunotherapy. Acc. Chem. Res. 2020, 53, 1739–1748. [Google Scholar] [CrossRef]
- Zeng, J.Y.; Wang, X.S.; Song, W.F.; Cheng, H.; Zhang, X.Z. Metal-Organic Framework Mediated Multifunctional Nanoplatforms for Cancer Therapy. Adv. Ther. 2019, 2, 1800100. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Zhang, Y.; Jiang, W. Immunogenicity-Boosted Cancer Immunotherapy Based on Nanoscale Metal-Organic Frameworks. J. Control. Release 2022, 347, 183–198. [Google Scholar] [CrossRef]
- Du, Y.; Jia, X.; Zhong, L.; Jiao, Y.; Zhang, Z.; Wang, Z.; Feng, Y.; Bilal, M.; Cui, J.; Jia, S. Metal-Organic Frameworks with Different Dimensionalities: An Ideal Host Platform for Enzyme@MOF Composites. Coord. Chem. Rev. 2022, 454, 214327. [Google Scholar] [CrossRef]
- Ockwig, N.W.; Delgado-Friedrichs, O.; O’Keeffe, M.; Yaghi, O.M. Reticular Chemistry: Occurrence and Taxonomy of Nets and Grammar for the Design of Frameworks. Acc. Chem. Res. 2005, 38, 176–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moumen, E.; Bazzi, L.; el Hankari, S. Metal-Organic Frameworks and Their Composites for the Adsorption and Sensing of Phosphate. Coord. Chem. Rev. 2022, 455, 214376. [Google Scholar] [CrossRef]
- Tsivadze, A.Y.; Aksyutin, O.E.; Ishkov, A.G.; Knyazeva, M.K.; Solovtsova, O.v.; Men’shchikov, I.E.; Fomkin, A.A.; Shkolin, A.v.; Khozina, E.v.; Grachev, V.A. Metal-Organic Framework Structures: Adsorbents for Natural Gas Storage. Russ. Chem. Rev. 2019, 88, 925–978. [Google Scholar] [CrossRef]
- Jeyaseelan, C.; Jain, P.; Soin, D.; Gupta, D. Metal Organic Frameworks: An Effective Application in Drug Delivery Systems. Inorg. Nano-Met. Chem. 2022, 52, 1463–1475. [Google Scholar] [CrossRef]
- Jiao, L.; Seow, J.Y.R.; Skinner, W.S.; Wang, Z.U.; Jiang, H.L. Metal–Organic Frameworks: Structures and Functional Applications. Mater. Today 2019, 27, 43–68. [Google Scholar] [CrossRef]
- Natarajan, S.; Mahata, P. Metal–Organic Framework Structures—How Closely Are They Related to Classical Inorganic Structures? Chem. Soc. Rev. 2009, 38, 2304–2318. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Reboul, J.; Diring, S.; Sumida, K.; Kitagawa, S. Structuring of Metal-Organic Frameworks at the Mesoscopic/Macroscopic Scale. Chem. Soc. Rev. 2014, 43, 5700–5734. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, J.; Murugan, P.; Ganapathy, D.; Nallaswamy, D.; Atchudan, R.; Arya, S.; Khosla, A.; Barathi, S.; Sundramoorthy, A.K. Synthesis of Various Dimensional Metal Organic Frameworks (MOFs) and Their Hybrid Composites for Emerging Applications—A Review. Chemosphere 2022, 298, 134184. [Google Scholar] [CrossRef]
- Bai, Y.; Liu, C.; Shan, Y.; Chen, T.; Zhao, Y.; Yu, C.; Pang, H. Metal-Organic Frameworks Nanocomposites with Different Dimensionalities for Energy Conversion and Storage. Adv. Energy Mater 2022, 12, 2100346. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y.W. Metal–Organic Frameworks for Biomedical Applications. Small 2020, 16, 1906846. [Google Scholar] [CrossRef]
- Gu, C.; Guo, C.; Li, Z.; Wang, M.; Zhou, N.; He, L.; Zhang, Z.; Du, M. Bimetallic ZrHf-Based Metal-Organic Framework Embedded with Carbon Dots: Ultra-Sensitive Platform for Early Diagnosis of HER2 and HER2-Overexpressed Living Cancer Cells. Biosens. Bioelectron. 2019, 134, 8–15. [Google Scholar] [CrossRef]
- Liao, L.G.; Ke, D.; Li, G.C.; Zhang, S.; Li, B.J. Cyclodextrin Metal-Organic Framework as a Broad-Spectrum Potential Delivery Vehicle for the Gasotransmitters. Molecules 2023, 28, 852. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh Harzand, F.; Mousavi Nejad, S.N.; Babapoor, A.; Mousavi, S.M.; Hashemi, S.A.; Gholami, A.; Chiang, W.-H.; Buonomenna, M.G.; Lai, C.W. Recent Advances in Metal-Organic Framework (MOF) Asymmetric Membranes/Composites for Biomedical Applications. Symmetry 2023, 15, 403. [Google Scholar] [CrossRef]
- Moribe, S.; Takeda, Y.; Umehara, M.; Kikuta, H.; Ito, J.; Ma, J.; Yamada, Y.; Hirano, M. Spike Current Induction by Photogenerated Charge Accumulation at the Surface Sites of Porous Porphyrinic Zirconium Metal-Organic Framework Electrodes in Photoelectrochemical Cells. Bull. Chem. Soc. Jpn. 2023. [Google Scholar] [CrossRef]
- Alabyadh, T.; Albadri, R.; Es-haghi, A.; Yazdi, M.E.T.; Ajalli, N.; Rahdar, A.; Thakur, V.K. ZnO/CeO2 Nanocomposites: Metal-Organic Framework-Mediated Synthesis, Characterization, and Estimation of Cellular Toxicity toward Liver Cancer Cells. J. Funct. Biomater. 2022, 13, 139. [Google Scholar] [CrossRef]
- Li, M.; Yin, S.; Lin, M.; Chen, X.; Pan, Y.; Peng, Y.; Sun, J.; Kumar, A.; Liu, J. Current Status and Prospects of Metal-Organic Frameworks for Bone Therapy and Bone Repair. J. Mater. Chem. B 2022, 10, 5105–5128. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cheng, F.; Luo, D.; Huang, J.; Ouyang, J.; Nezamzadeh-Ejhieh, A.; Khan, M.S.; Liu, J.; Peng, Y. Recent Advances in Ti-Based MOFs in Biomedical Applications. Dalton Trans. 2022, 51, 14817–14832. [Google Scholar] [CrossRef] [PubMed]
- Burtch, N.C.; Heinen, J.; Bennett, T.D.; Dubbeldam, D.; Allendorf, M.D. Mechanical Properties in Metal–Organic Frameworks: Emerging Opportunities and Challenges for Device Functionality and Technological Applications. Adv. Mater. 2018, 30, 1704124. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, W.; Zhu, C.; Liu, Y.; Cui, Y. Mesoporous Metal–Organic Framework Materials. Chem. Soc. Rev. 2012, 41, 1677–1695. [Google Scholar] [CrossRef]
- Nezhad-Mokhtari, P.; Arsalani, N.; Javanbakht, S.; Shaabani, A. Development of Gelatin Microsphere Encapsulated Cu-Based Metal-Organic Framework Nanohybrid for the Methotrexate Delivery. J. Drug Deliv. Sci. Technol. 2019, 50, 174–180. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, J.; Wen, N.; Xiong, H.; Cai, S.; He, Q.; Hu, Y.; Peng, D.; Liu, Z.; Liu, Y. Metal-Organic Frameworks for Stimuli-Responsive Drug Delivery. Biomaterials 2020, 230, 119619. [Google Scholar] [CrossRef]
- Oroojalian, F.; Karimzadeh, S.; Javanbakht, S.; Hejazi, M.; Baradaran, B.; Webster, T.J.; Mokhtarzadeh, A.; Varma, R.S.; Kesharwani, P.; Sahebkar, A. Current Trends in Stimuli-Responsive Nanotheranostics Based on Metal–Organic Frameworks for Cancer Therapy. Mater. Today 2022, 57, 192–224. [Google Scholar] [CrossRef]
- Cai, W.; Wang, J.; Chu, C.; Chen, W.; Wu, C.; Liu, G. Metal–Organic Framework-Based Stimuli-Responsive Systems for Drug Delivery. Adv. Sci. 2019, 6, 1801526. [Google Scholar] [CrossRef] [Green Version]
- Lan, G.; Ni, K.; Lin, W. Nanoscale Metal–Organic Frameworks for Phototherapy of Cancer. Coord. Chem. Rev. 2019, 379, 65–81. [Google Scholar] [CrossRef]
- Saeb, M.R.; Rabiee, N.; Mozafari, M.; Mostafavi, E. Metal-Organic Frameworks-Based Nanomaterials for Drug Delivery. Materials 2021, 14, 3652. [Google Scholar] [CrossRef]
- Qin, L.; Liang, F.; Li, Y.; Wu, J.; Guan, S.; Wu, M.; Xie, S.; Luo, M.; Ma, D. A 2D Porous Zinc-Organic Framework Platform for Loading of 5-Fluorouracil. Inorganics 2022, 10, 202. [Google Scholar] [CrossRef]
- Feng, L.; Wang, K.Y.; Day, G.S.; Ryder, M.R.; Zhou, H.C. Destruction of Metal-Organic Frameworks: Positive and Negative Aspects of Stability and Lability. Chem. Rev. 2020, 120, 13087–13133. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal–Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, 1704303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howarth, A.J.; Liu, Y.; Li, P.; Li, Z.; Wang, T.C.; Hupp, J.T.; Farha, O.K. Chemical, Thermal and Mechanical Stabilities of Metal-Organic Frameworks. Nat. Rev. Mater. 2016, 1, 15018. [Google Scholar] [CrossRef]
- Bosch, M.; Zhang, M.; Zhou, H.-C. Increasing the Stability of Metal-Organic Frameworks. Adv. Chem. 2014, 2014, 1155. [Google Scholar] [CrossRef]
- Moosavi, S.M.; Boyd, P.G.; Sarkisov, L.; Smit, B. Improving the Mechanical Stability of Metal-Organic Frameworks Using Chemical Caryatids. ACS Cent. Sci. 2018, 4, 832–839. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Goh, L.; Otake, K.; Goh, S.S.; Loh, X.J.; Kitagawa, S. Biomedically-Relevant Metal Organic Framework-Hydrogel Composites. Biomater. Sci. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Li, D.; Lee, C.; Xie, J. Nanoparticle Phototherapy in the Era of Cancer Immunotherapy. Trends Chem. 2020, 2, 1082–1095. [Google Scholar] [CrossRef]
- Mendes, R.F.; Figueira, F.; Leite, J.P.; Gales, L.; Almeida Paz, F.A. Metal-Organic Frameworks: A Future Toolbox for Biomedicine? Chem. Soc. Rev. 2020, 49, 9121–9153. [Google Scholar] [CrossRef]
- Bao, Z.; Li, K.; Hou, P.; Xiao, R.; Yuan, Y.; Sun, Z. Nanoscale Metal-Organic Framework Composites for Phototherapy and Synergistic Therapy of Cancer. Mater. Chem. Front. 2021, 5, 1632–1654. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, J.; Shi, W.; Zhang, J.; Tao, C. Recent Advances in Metal-Organic Frameworks and Their Composites for the Phototherapy of Skin Wounds. J. Mater. Chem. B 2022, 10, 4695–4713. [Google Scholar] [CrossRef] [PubMed]
- Rajora, M.A.; Lou, J.W.H.; Zheng, G. Advancing Porphyrin’s Biomedical Utility: Via Supramolecular Chemistry. Chem. Soc. Rev. 2017, 46, 6433–6469. [Google Scholar] [CrossRef] [PubMed]
- DeRosa, M.; Crutchley, R. Photosensitized Singlet Oxygen and Its Applications. Coord. Chem. Rev. 2002, 233–234, 351–371. [Google Scholar] [CrossRef]
- Lu, K.; He, C.; Lin, W. Nanoscale Metal-Organic Framework for Highly Effective Photodynamic Therapy of Resistant Head and Neck Cancer. J. Am. Chem. Soc. 2014, 136, 16712–16715. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Zhao, Y.; Sun, Y.; Cao, J. Recent Progress of Metal-Organic Framework-Based Photodynamic Therapy for Cancer Treatment. Int. J. Nanomed. 2022, 17, 2367–2395. [Google Scholar] [CrossRef]
- Liu, T.F.; Feng, D.; Chen, Y.P.; Zou, L.; Bosch, M.; Yuan, S.; Wei, Z.; Fordham, S.; Wang, K.; Zhou, H.C. Topology-Guided Design and Syntheses of Highly Stable Mesoporous Porphyrinic Zirconium Metal-Organic Frameworks with High Surface Area. J. Am. Chem. Soc. 2015, 137, 413–419. [Google Scholar] [CrossRef] [Green Version]
- Matlou, G.G.; Abrahamse, H. Nanoscale Metal–Organic Frameworks as Photosensitizers and Nanocarriers in Photodynamic Therapy. Front. Chem. 2022, 10, 971747. [Google Scholar] [CrossRef]
- Park, J.; Jiang, Q.; Feng, D.; Mao, L.; Zhou, H.C. Size-Controlled Synthesis of Porphyrinic Metal-Organic Framework and Functionalization for Targeted Photodynamic Therapy. J. Am. Chem. Soc. 2016, 138, 3518–3525. [Google Scholar] [CrossRef]
- Jia, J.; Zhang, Y.; Zheng, M.; Shan, C.; Yan, H.; Wu, W.; Gao, X.; Cheng, B.; Liu, W.; Tang, Y. Functionalized Eu(III)-Based Nanoscale Metal-Organic Framework to Achieve Near-IR-Triggered and -Targeted Two-Photon Absorption Photodynamic Therapy. Inorg. Chem. 2018, 57, 300–310. [Google Scholar] [CrossRef]
- Cai, W.; Gao, H.; Chu, C.; Wang, X.; Wang, J.; Zhang, P.; Lin, G.; Li, W.; Liu, G.; Chen, X. Engineering Phototheranostic Nanoscale Metal-Organic Frameworks for Multimodal Imaging-Guided Cancer Therapy. ACS Appl. Mater. Interfaces 2017, 9, 2040–2051. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xing, J.; Akakuru, O.U.; Luo, L.; Sun, S.; Zou, R.; Yu, Z.; Fang, Q.; Wu, A. Nanozymes-Engineered Metal-Organic Frameworks for Catalytic Cascades-Enhanced Synergistic Cancer Therapy. Nano Lett. 2019, 19, 5674–5682. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zheng, P.; Li, Z.; Feng, X.; Yan, W.; Chen, S.; Guo, W.; Liu, D.; Yang, X.; Wang, S.; et al. Biomimetic O2-Evolving Metal-Organic Framework Nanoplatform for Highly Efficient Photodynamic Therapy against Hypoxic Tumor. Biomaterials 2018, 178, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Wang, J.; Qi, Y.; Zhang, Y.; Han, X.; Xu, Y.; Xu, J.; Li, Y.; Chen, L.; Cheng, K.; et al. Biomimetic Metal–Organic Framework Nanoparticles for Cooperative Combination of Antiangiogenesis and Photodynamic Therapy for Enhanced Efficacy. Adv. Mater. 2019, 31, 1808200. [Google Scholar] [CrossRef]
- Illes, B.; Hirschle, P.; Barnert, S.; Cauda, V.; Wuttke, S.; Engelke, H. Exosome-Coated Metal-Organic Framework Nanoparticles: An Efficient Drug Delivery Platform. Chem. Mater. 2017, 29, 8042–8046. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, Q.; Li, C.; Zeng, X.; Zhang, X.Z. Near Infrared Light-Triggered Metal Ion and Photodynamic Therapy Based on AgNPs/Porphyrinic MOFs for Tumors and Pathogens Elimination. Biomaterials 2020, 248, 120029. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, Y.; Peng, F.; Meng, F.; Zha, J.; Ma, L.; Du, Y.; Peng, N.; Ma, L.; Zhang, Q.; et al. Intercalation-Activated Layered MoO3 Nanobelts as Biodegradable Nanozymes for Tumor-Specific Photo-Enhanced Catalytic Therapy. Angew. Chem.–Int. Ed. 2022, 61, e202115939. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Eshaghi, M.M.; Ostovar, S.; Shamsabadipour, A.; Safakhah, S.; Mousavi, M.S.; Rahdar, A.; Pandey, S. UiO-66 Metal-Organic Framework Nanoparticles as Gifted MOFs to the Biomedical Application: A Comprehensive Review. J. Drug Deliv. Sci. Technol. 2022, 76, 103758. [Google Scholar] [CrossRef]
- Liang, W.; Ricco, R.; Maddigan, N.K.; Dickinson, R.P.; Xu, H.; Li, Q.; Sumby, C.J.; Bell, S.G.; Falcaro, P.; Doonan, C.J. Control of Structure Topology and Spatial Distribution of Biomacromolecules in Protein@ZIF-8 Biocomposites. Chem. Mater. 2018, 30, 1069–1077. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Gao, Y.; Sun, S.; Li, Z.; Wu, A.; Zeng, L. PH-Responsive Metal-Organic Framework Encapsulated Gold Nanoclusters with Modulated Release to Enhance Photodynamic Therapy/Chemotherapy in Breast Cancer. J. Mater. Chem. B 2020, 8, 1739–1747. [Google Scholar] [CrossRef]
- Zorainy, M.Y.; Gar Alalm, M.; Kaliaguine, S.; Boffito, D.C. Revisiting the MIL-101 Metal-Organic Framework: Design, Synthesis, Modifications, Advances, and Recent Applications. J. Mater. Chem. A Mater 2021, 9, 22159–22217. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surblé, S.; Margiolaki, I. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Lei, J.; Shen, H.; Ju, H. Multifunctional Metal-Organic Framework Nanoprobe for Cathepsin B-Activated Cancer Cell Imaging and Chemo-Photodynamic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 2150–2158. [Google Scholar] [CrossRef]
- Ding, Q.; Xu, Z.; Zhou, L.; Rao, C.; Li, W.; Muddassir, M.; Sakiyama, H.; Li, B.; Ouyang, Q.; Liu, J. A Multimodal Metal-Organic Framework Based on Unsaturated Metal Site for Enhancing Antitumor Cytotoxicity through Chemo-Photodynamic Therapy. J. Colloid Interface Sci. 2022, 621, 180–194. [Google Scholar] [CrossRef]
- Wang, P.; Chen, B.; Zhan, Y.; Wang, L.; Luo, J.; Xu, J.; Zhan, L.; Li, Z.; Liu, Y.; Wei, J. Enhancing the Efficiency of Mild-Temperature Photothermal Therapy for Cancer Assisting with Various Strategies. Pharmaceutics 2022, 14, 2279. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, J.; Liang, C.; Feng, L.; Fu, T.; Dong, Z.; Chao, Y.; Li, Y.; Lu, G.; Chen, M.; et al. Nanoscale Metal-Organic Particles with Rapid Clearance for Magnetic Resonance Imaging-Guided Photothermal Therapy. ACS Nano 2016, 10, 2774–2781. [Google Scholar] [CrossRef]
- Deng, Z.; Fang, C.; Ma, X.; Li, X.; Zeng, Y.J.; Peng, X. One Stone Two Birds: Zr-Fc Metal-Organic Framework Nanosheet for Synergistic Photothermal and Chemodynamic Cancer Therapy. ACS Appl. Mater. Interfaces 2020, 12, 20321–20330. [Google Scholar] [CrossRef]
- Mei, X.; Han, Y.; Xi, J.; Liu, J.; Xu, L.; Yuan, J.; Wang, S.; Li, X.; Si, W.; Li, J. Preparation of Hollow Mesoporous Prussian Blue Coated with Mesoporous Silica Shell Nanocubes for Photothermal Therapy and Drug Carrier. Mater. Lett. 2022, 312, 131697. [Google Scholar] [CrossRef]
- Qin, Z.; Li, Y.; Gu, N. Progress in Applications of Prussian Blue Nanoparticles in Biomedicine. Adv. Healthc. Mater. 2018, 7, 1800347. [Google Scholar] [CrossRef]
- Luo, Y.; Li, J.; Liu, X.; Tan, L.; Cui, Z.; Feng, X.; Yang, X.; Liang, Y.; Li, Z.; Zhu, S.; et al. Dual Metal-Organic Framework Heterointerface. ACS Cent. Sci. 2019, 5, 1591–1601. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zeng, K.; Liu, H.; Ouyang, J.; Wang, L.; Liu, Y.; Wang, H.; Deng, L.; Liu, Y.N. Cell Membrane Camouflaged Hollow Prussian Blue Nanoparticles for Synergistic Photothermal-/Chemotherapy of Cancer. Adv. Funct. Mater. 2017, 27, 1605795. [Google Scholar] [CrossRef]
- Peng, X.; Wang, R.; Wang, T.; Yang, W.; Wang, H.; Gu, W.; Ye, L. Carbon Dots/Prussian Blue Satellite/Core Nanocomposites for Optical Imaging and Photothermal Therapy. ACS Appl. Mater. Interfaces 2018, 10, 1084–1092. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Yao, X.; Ma, K.; Niu, X.; Grothe, J.; Xu, Q.; Liu, L.; Kaskel, S.; Zhu, Y. Metal-Organic Framework/Graphene Quantum Dot Nanoparticles Used for Synergistic Chemo- and Photothermal Therapy. ACS Omega 2017, 2, 1249–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.d.; Chen, S.P.; Zhao, H.; Yang, Y.; Chen, X.Q.; Sun, J.; Fan, H.S.; Zhang, X.D. PPy@MIL-100 Nanoparticles as a PH- and Near-IR-Irradiation-Responsive Drug Carrier for Simultaneous Photothermal Therapy and Chemotherapy of Cancer Cells. ACS Appl. Mater. Interfaces 2016, 8, 34209–34217. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Li, L.; Yang, Z.; Chen, X. Phototherapy Meets Immunotherapy: A Win-Win Strategy to Fight against Cancer. Nanophotonics 2021, 10, 3229–3245. [Google Scholar] [CrossRef]
- Yue, J.; Mei, Q.; Wang, P.; Miao, P.; Dong, W.F.; Li, L. Light-Triggered Multifunctional Nanoplatform for Efficient Cancer Photo-Immunotherapy. J Nanobiotechnol. 2022, 20, 181. [Google Scholar] [CrossRef]
- Xie, Z.; Fan, T.; An, J.; Choi, W.; Duo, Y.; Ge, Y.; Zhang, B.; Nie, G.; Xie, N.; Zheng, T.; et al. Emerging Combination Strategies with Phototherapy in Cancer Nanomedicine. Chem. Soc. Rev. 2020, 49, 8065–8087. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Rao, J.; Wang, M.; Li, X.; Liu, K.; Naylor, M.F.; Nordquist, R.E.; Chen, W.R.; Zhou, F. Cancer Photo-Immunotherapy: From Bench to Bedside. Theranostics 2021, 11, 2218–2231. [Google Scholar] [CrossRef]
- Maranescu, B.; Visa, A. Applications of Metal-Organic Frameworks as Drug Delivery Systems. Int. J. Mol. Sci. 2022, 23, 4458. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Li, Q.; Song, A.; Tian, H.; Wang, J.; Li, Z.; Luan, Y. Site-Specific MOF-Based Immunotherapeutic Nanoplatforms via Synergistic Tumor Cells-Targeted Treatment and Dendritic Cells-Targeted Immunomodulation. Biomaterials 2020, 245, 119983. [Google Scholar] [CrossRef]
- Yang, J.C.; Shang, Y.; Li, Y.H.; Cui, Y.; Yin, X.B. An “All-in-One” Antitumor and Anti-Recurrence/Metastasis Nanomedicine with Multi-Drug Co-Loading and Burst Drug Release for Multi-Modality Therapy. Chem. Sci. 2018, 9, 7210–7217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Sun, J.; Liu, F.; Yu, S.; Hu, J.; Zhao, Y.; Wang, X.; Liu, X. Treating Immunologically Cold Tumors by Precise Cancer Photoimmunotherapy with an Extendable Nanoplatform. ACS Appl. Mater. Interfaces 2020, 12, 40002–40012. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, W.; Qi, Y.; Wang, G.; Li, L.; Jin, Z.; Tian, J.; Du, Y. PD-L1 Aptamer-Functionalized Metal–Organic Framework Nanoparticles for Robust Photo-Immunotherapy Against Cancer with Enhanced Safety. Angew. Chem. 2023, 135, e202214750. [Google Scholar] [CrossRef]
- Bai, X.-F.; Chen, Y.; Zou, M.-Z.; Li, C.-X.; Zhang, Y.; Li, M.-J.; Cheng, S.-X.; Zhang, X.-Z. Homotypic Targeted Photosensitive Nanointerferer for Tumor Cell Cycle Arrest to Boost Tumor Photoimmunotherapy. ACS Nano 2022, 16, 18555–18567. [Google Scholar] [CrossRef]
- Sun, Q.; Yang, J.; Shen, W.; Lu, H.; Hou, X.; Liu, Y.; Xu, Y.; Wu, Q.; Xuan, Z.; Yang, Y.; et al. Engineering Mitochondrial Uncoupler Synergistic Photodynamic Nanoplatform to Harness Immunostimulatory Pro-Death Autophagy/Mitophagy. Biomaterials 2022, 289, 121796. [Google Scholar] [CrossRef]
- Liu, W.L.; Zou, M.Z.; Liu, T.; Zeng, J.Y.; Li, X.; Yu, W.Y.; Li, C.X.; Ye, J.J.; Song, W.; Feng, J.; et al. Expandable Immunotherapeutic Nanoplatforms Engineered from Cytomembranes of Hybrid Cells Derived from Cancer and Dendritic Cells. Adv. Mater. 2019, 31, 1900499. [Google Scholar] [CrossRef]
- Cai, Z.; Xin, F.; Wei, Z.; Wu, M.; Lin, X.; Du, X.; Chen, G.; Zhang, D.; Zhang, Z.; Liu, X.; et al. Photodynamic Therapy Combined with Antihypoxic Signaling and CpG Adjuvant as an In Situ Tumor Vaccine Based on Metal–Organic Framework Nanoparticles to Boost Cancer Immunotherapy. Adv. Healthc. Mater. 2020, 9, 1900996. [Google Scholar] [CrossRef]
- Ni, K.; Luo, T.; Lan, G.; Culbert, A.; Song, Y.; Wu, T.; Jiang, X.; Lin, W. A Nanoscale Metal–Organic Framework to Mediate Photodynamic Therapy and Deliver CpG Oligodeoxynucleotides to Enhance Antigen Presentation and Cancer Immunotherapy. Angew. Chem.–Int. Ed. 2020, 59, 1108–1112. [Google Scholar] [CrossRef]
- Lan, G.; Ni, K.; Xu, Z.; Veroneau, S.S.; Song, Y.; Lin, W. Nanoscale Metal-Organic Framework Overcomes Hypoxia for Photodynamic Therapy Primed Cancer Immunotherapy. J. Am. Chem. Soc. 2018, 140, 5670–5673. [Google Scholar] [CrossRef]
- Xie, B.R.; Yu, Y.; Liu, X.H.; Zeng, J.Y.; Zou, M.Z.; Li, C.X.; Zeng, X.; Zhang, X.Z. A near Infrared Ratiometric Platform Based π-Extended Porphyrin Metal-Organic Framework for O2 Imaging and Cancer Therapy. Biomaterials 2021, 272, 120782. [Google Scholar] [CrossRef]
- Ni, K.; Aung, T.; Li, S.; Fatuzzo, N.; Liang, X.; Lin, W. Nanoscale Metal-Organic Framework Mediates Radical Therapy to Enhance Cancer Immunotherapy. Chem 2019, 5, 1892–1913. [Google Scholar] [CrossRef]
- Fan, Z.; Liu, H.; Xue, Y.; Lin, J.; Fu, Y.; Xia, Z.; Pan, D.; Zhang, J.; Qiao, K.; Zhang, Z.; et al. Reversing Cold Tumors to Hot: An Immunoadjuvant-Functionalized Metal-Organic Framework for Multimodal Imaging-Guided Synergistic Photo-Immunotherapy. Bioact. Mater. 2021, 6, 312–325. [Google Scholar] [CrossRef]
- Ni, W.; Wu, J.; Fang, H.; Feng, Y.; Hu, Y.; Lin, L.; Chen, J.; Chen, F.; Tian, H. Photothermal-Chemotherapy Enhancing Tumor Immunotherapy by Multifunctional Metal-Organic Framework Based Drug Delivery System. Nano Lett. 2021, 21, 7796–7805. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, C.; Meng, M.; Li, S.; Sheng, S.; Zhang, S.; Ni, W.; Tian, H.; Wang, Q. Metal-Organic Framework-Mediated Multifunctional Nanoparticles for Combined Chemo-Photothermal Therapy and Enhanced Immunotherapy against Colorectal Cancer. Acta Biomater. 2022, 144, 132–141. [Google Scholar] [CrossRef]
- Shao, Y.; Liu, B.; Di, Z.; Zhang, G.; Sun, L.D.; Li, L.; Yan, C.H. Engineering of Upconverted Metal-Organic Frameworks for Near-Infrared Light-Triggered Combinational Photodynamic/Chemo-/Immunotherapy against Hypoxic Tumors. J. Am. Chem. Soc. 2020, 142, 3939–3946. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; He, C.; Guo, N.; Chan, C.; Ni, K.; Weichselbaum, R.R.; Lin, W. Chlorin-Based Nanoscale Metal-Organic Framework Systemically Rejects Colorectal Cancers via Synergistic Photodynamic Therapy and Checkpoint Blockade Immunotherapy. J. Am. Chem. Soc. 2016, 138, 12502–12510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; He, Y.; Wang, Y.; Li, C.; Zhang, Y.; Guo, Q.; Zhang, Y.; Chu, Y.; Liu, P.; Chen, H.; et al. Penetrable Nanoplatform for “Cold” Tumor Immune Microenvironment Reeducation. Adv. Sci. 2020, 7, 2000411. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Y.; Du, L.; Deng, Z.; Jiang, M.; Zeng, S. Soft X-Ray Stimulated Lanthanide@MOF Nanoprobe for Amplifying Deep Tissue Synergistic Photodynamic and Antitumor Immunotherapy. Adv. Healthc. Mater. 2021, 10, 2101174. [Google Scholar] [CrossRef]
- Wang, Q.X.; Yang, Y.F.; Yang, X.F.; Pan, Y.; Sun, L.D.; Zhang, W.Y.; Shao, Y.; Shen, J.; Lin, J.; Li, L.; et al. Upconverted/Downshifted NaLnF4 and Metal-Organic Framework Heterostructures Boosting NIR-II Imaging-Guided Photodynamic Immunotherapy toward Tumors. Nano Today 2022, 43, 101439. [Google Scholar] [CrossRef]
- Cano-Mejia, J.; Burga, R.A.; Sweeney, E.E.; Fisher, J.P.; Bollard, C.M.; Sandler, A.D.; Cruz, C.R.Y.; Fernandes, R. Prussian Blue Nanoparticle-Based Photothermal Therapy Combined with Checkpoint Inhibition for Photothermal Immunotherapy of Neuroblastoma. Nanomedicine 2017, 13, 771–781. [Google Scholar] [CrossRef]
- Shukla, A.; Cano-Mejia, J.; Andricovich, J.; Burga, R.A.; Sweeney, E.E.; Fernandes, R. An Engineered Prussian Blue Nanoparticles-Based Nanoimmunotherapy Elicits Robust and Persistent Immunological Memory in a TH-MYCN Neuroblastoma Model. Adv. Nanobiomed. Res. 2021, 1, 2100021. [Google Scholar] [CrossRef] [PubMed]
- Cano-Mejia, J.; Shukla, A.; Ledezma, D.K.; Palmer, E.; Villagra, A.; Fernandes, R. CpG-Coated Prussian Blue Nanoparticles-Based Photothermal Therapy Combined with Anti-CTLA-4 Immune Checkpoint Blockade Triggers a Robust Abscopal Effect against Neuroblastoma. Transl. Oncol. 2020, 13, 100823. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Fang, C.; Tian, J.; Zhou, T.; Liang, X.; Wang, P.; Hu, Y.; Qi, Y.; Jin, Y. A Hepatocellular Carcinoma Targeting Nanostrategy with Hypoxia-Ameliorating and Photothermal Abilities That, Combined with Immunotherapy, Inhibits Metastasis and Recurrence. ACS Nano 2020, 14, 12679–12696. [Google Scholar] [CrossRef]
- Balakrishnan, P.B.; Ledezma, D.K.; Cano-Mejia, J.; Andricovich, J.; Palmer, E.; Patel, V.A.; Latham, P.S.; Yvon, E.S.; Villagra, A.; Fernandes, R.; et al. CD137 Agonist Potentiates the Abscopal Efficacy of Nanoparticle-Based Photothermal Therapy for Melanoma. Nano Res. 2022, 15, 2300–2314. [Google Scholar] [CrossRef]
- Ji, B.; Wei, M.; Yang, B. Recent Advances in Nanomedicines for Photodynamic Therapy (PDT)-Driven Cancer Immunotherapy. Theranostics 2022, 12, 434–458. [Google Scholar] [CrossRef] [PubMed]
- Dolmans, D.E.J.G.J.; Fukumura, D.; Jain, R.K. Photodynamic Therapy for Cancer. Nat. Rev. Cancer 2003, 3, 380–387. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive Oxygen Species Generating Systems Meeting Challenges of Photodynamic Cancer Therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [Green Version]
- Kwon, N.; Kim, H.; Li, X.; Yoon, J. Supramolecular Agents for Combination of Photodynamic Therapy and Other Treatments. Chem. Sci. 2021, 12, 7248–7268. [Google Scholar] [CrossRef]
- Shen, L.; Zhou, T.; Fan, Y.; Chang, X.; Wang, Y.; Sun, J.; Xing, L.; Jiang, H. Recent Progress in Tumor Photodynamic Immunotherapy. Chin. Chem. Lett. 2020, 31, 1709–1716. [Google Scholar] [CrossRef]
- Pardoll, D.M. The Blockade of Immune Checkpoints in Cancer Immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Marin-Acevedo, J.A.; Dholaria, B.; Soyano, A.E.; Knutson, K.L.; Chumsri, S.; Lou, Y. Next Generation of Immune Checkpoint Therapy in Cancer: New Developments and Challenges. J. Hematol. Oncol. 2018, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Hargadon, K.M.; Johnson, C.E.; Williams, C.J. Immune Checkpoint Blockade Therapy for Cancer: An Overview of FDA-Approved Immune Checkpoint Inhibitors. Int. Immunopharmacol. 2018, 62, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Zappasodi, R.; Merghoub, T.; Wolchok, J.D. Emerging Concepts for Immune Checkpoint Blockade-Based Combination Therapies. Cancer Cell 2018, 33, 581–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalbasi, A.; Ribas, A. Tumour-Intrinsic Resistance to Immune Checkpoint Blockade. Nat. Rev. Immunol. 2020, 20, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Hui, E.; Cheung, J.; Zhu, J.; Su, X.; Taylor, M.J.; Wallweber, H.A.; Sasmal, D.K.; Huang, J.; Kim, J.M.; Mellman, I.; et al. T Cell Costimulatory Receptor CD28 Is a Primary Target for PD-1-Mediated Inhibition. Science 2017, 355, 1428–1433. [Google Scholar] [CrossRef]
- Zou, W.; Wolchok, J.D.; Chen, L. PD-L1 (B7-H1) and PD-1 Pathway Blockade for Cancer Therapy: Mechanisms, Response Biomarkers, and Combinations. Sci. Transl. Med. 2016, 8, 328rv4. [Google Scholar] [CrossRef] [Green Version]
- Dobosz, P.; Stępień, M.; Golke, A.; Dzieciątkowski, T. Challenges of the Immunotherapy: Perspectives and Limitations of the Immune Checkpoint Inhibitor Treatment. Int. J. Mol. Sci. 2022, 23, 2847. [Google Scholar] [CrossRef]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A Transmembrane Intracellular Estrogen Receptor Mediates Rapid Cell Signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef] [Green Version]
- Seacat, A.M.; Kuppusamy, P.; Zweier, J.L.; Yager, J.D. ESR Identification of Free Radicals Formed from the Oxidation of Catechol Estrogens by Cu2+. Arch. Biochem. Biophys. 1997, 347, 45–52. [Google Scholar] [CrossRef] [Green Version]
- Fang, R.H.; Hu, C.M.J.; Luk, B.T.; Gao, W.; Copp, J.A.; Tai, Y.; O’Connor, D.E.; Zhang, L. Cancer Cell Membrane-Coated Nanoparticles for Anticancer Vaccination and Drug Delivery. Nano Lett. 2014, 14, 2181–2188. [Google Scholar] [CrossRef]
- Zhu, J.; Sevencan, C.; Zhang, M.; McCoy, R.S.A.; Ding, X.; Ye, J.; Xie, J.; Ariga, K.; Feng, J.; Bay, B.H.; et al. Increasing the Potential Interacting Area of Nanomedicine Enhances Its Homotypic Cancer Targeting Efficacy. ACS Nano 2020, 14, 3259–3271. [Google Scholar] [CrossRef]
- Hornyák, L.; Dobos, N.; Koncz, G.; Karányi, Z.; Páll, D.; Szabó, Z.; Halmos, G.; Székvölgyi, L. The Role of Indoleamine-2,3-Dioxygenase in Cancer Development, Diagnostics, and Therapy. Front. Immunol. 2018, 9, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Li, H.J.; Luo, Y.L.; Chen, Y.F.; Fan, Y.N.; Du, J.Z.; Wang, J. Programmable Delivery of Immune Adjuvant to Tumor-Infiltrating Dendritic Cells for Cancer Immunotherapy. Nano Lett. 2020, 20, 4882–4889. [Google Scholar] [CrossRef] [PubMed]
- Krieg, A.M. Antiinfective Applications of Toll-like Receptor 9 Agonists. Proc. Am. Thorac. Soc. 2007, 4, 289–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Jiang, M.; Yu, W.; Xu, Z.; Liu, X.; Jia, Q.; Guan, X.; Zhang, W. CpG-Based Nanovaccines for Cancer Immunotherapy. Int. J. Nanomed. 2021, 16, 5281–5299. [Google Scholar] [CrossRef] [PubMed]
- Jahrsdörfer, B.; Weiner, G.J. CpG Oligodeoxynucleotides as Immunotherapy in Cancer. Update Cancer 2008, 3, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Qin, L.; Zhang, H.; Zhou, Y.; Umeshappa, C.S.; Gao, H. Nanovaccine-Based Strategies to Overcome Challenges in the Whole Vaccination Cascade for Tumor Immunotherapy. Small 2021, 17, 2006000. [Google Scholar] [CrossRef]
- Emami Nejad, A.; Najafgholian, S.; Rostami, A.; Sistani, A.; Shojaeifar, S.; Esparvarinha, M.; Nedaeinia, R.; Haghjooy Javanmard, S.; Taherian, M.; Ahmadlou, M.; et al. The Role of Hypoxia in the Tumor Microenvironment and Development of Cancer Stem Cell: A Novel Approach to Developing Treatment. Cancer Cell Int. 2021, 21, 62. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The Role of Hypoxia in Cancer Progression, Angiogenesis, Metastasis, and Resistance to Therapy. Hypoxia 2015, 3, 83. [Google Scholar] [CrossRef] [Green Version]
- You, L.; Wu, W.; Wang, X.; Fang, L.; Adam, V.; Nepovimova, E.; Wu, Q.; Kuca, K. The Role of Hypoxia-Inducible Factor 1 in Tumor Immune Evasion. Med. Res. Rev. 2021, 41, 1622–1643. [Google Scholar] [CrossRef]
- Masoud, G.N.; Li, W. HIF-1α Pathway: Role, Regulation and Intervention for Cancer Therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef] [Green Version]
- Ziello, J.E.; Jovin, I.S.; Huang, Y. Hypoxia-Inducible Factor (HIF)-1 Regulatory Pathway and Its Potential for Therapeutic Intervention in Malignancy and Ischemia. Yale J. Biol. Med. 2007, 80, 51–60. [Google Scholar]
- Semenza, G.L. Hypoxia-Inducible Factor 1 (HIF-1) Pathway. Sci. STKE 2007, 2007, cm8. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Zhang, H.; Qian, D.Z.; Rey, S.; Liu, J.O.; Semenza, G.L. Acriflavine Inhibits HIF-1 Dimerization, Tumor Growth, and Vascularization. Proc. Natl. Acad. Sci. USA 2009, 106, 17910–17915. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J. Autophagy and Mitophagy in Cellular Damage Control. Redox Biol. 2013, 1, 19–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youle, R.J.; Narendra, D.P. Mechanisms of Mitophagy. Nat. Rev. Mol. Cell Biol. 2011, 12, 9–14. [Google Scholar] [CrossRef]
- Wanderoy, S.; Tabitha Hees, J.; Klesse, R.; Edlich, F.; Harbauer, A.B. Kill One or Kill the Many: Interplay between Mitophagy and Apoptosis. Biol. Chem. 2020, 402, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Tang, Y.; Elmenoufy, A.H.; Xu, H.; Cheng, Z.; Yang, X. Nanocomposite-Based Photodynamic Therapy Strategies for Deep Tumor Treatment. Small 2015, 11, 5860–5887. [Google Scholar] [CrossRef]
- Chen, X.; Song, J.; Chen, X.; Yang, H. X-Ray-Activated Nanosystems for Theranostic Applications. Chem. Soc. Rev. 2019, 48, 3073–3101. [Google Scholar] [CrossRef]
- Eriksson, E.; Wenthe, J.; Irenaeus, S.; Loskog, A.; Ullenhag, G. Gemcitabine Reduces MDSCs, Tregs and TGFβ-1 While Restoring the Teff/Treg Ratio in Patients with Pancreatic Cancer. J. Transl. Med. 2016, 14, 282. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Bush, X.; Yan, B.; Chen, J.A. Gemcitabine Nanoparticles Promote Antitumor Immunity against Melanoma. Biomaterials 2019, 189, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Lu, Y.; Guo, M.; Du, S.; Han, N. Recent Strategies for Nano-Based PTT Combined with Immunotherapy: From a Biomaterial Point of View. Theranostics 2021, 11, 7546–7569. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Han, H.S.; Lee, E.S.; Shin, J.M.; Almquist, B.D.; Lee, D.S.; Park, J.H. Hyaluronic Acid–Based Activatable Nanomaterials for Stimuli-Responsive Imaging and Therapeutics: Beyond CD44-Mediated Drug Delivery. Adv. Mater. 2019, 31, 1803549. [Google Scholar] [CrossRef]
- Otte, J.; Dyberg, C.; Pepich, A.; Johnsen, J.I. MYCN Function in Neuroblastoma Development. Front. Oncol. 2021, 10, 624079. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.-F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.-L.; Forner, A.; et al. Sorafenib in Advanced Hepatocellular Carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [Green Version]
- Zhu, W.; Liu, Y.; Yang, Z.; Zhang, L.; Xiao, L.; Liu, P.; Wang, J.; Yi, C.; Xu, Z.; Ren, J. Albumin/Sulfonamide Stabilized Iron Porphyrin Metal Organic Framework Nanocomposites: Targeting Tumor Hypoxia by Carbonic Anhydrase IX Inhibition and: T 1- T 2 Dual Mode MRI Guided Photodynamic/Photothermal Therapy. J. Mater. Chem. B 2018, 6, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Chen, W.C.; Yan, L.; Tian, S.; Liu, B.; Chen, X.; Lee, C.S.; Zhang, W. Harnessing Combinational Phototherapy: Via Post-Synthetic PpIX Conjugation on Nanoscale Metal-Organic Frameworks. J. Mater. Chem. B 2019, 7, 4763–4770. [Google Scholar] [CrossRef]
- Zhang, K.; Meng, X.; Cao, Y.; Yang, Z.; Dong, H.; Zhang, Y.; Lu, H.; Shi, Z.; Zhang, X. Metal-Organic Framework Nanoshuttle for Synergistic Photodynamic and Low-Temperature Photothermal Therapy. Adv. Funct. Mater. 2018, 28, 1804634. [Google Scholar] [CrossRef]
- Milling, L.; Zhang, Y.; Irvine, D.J. Delivering Safer Immunotherapies for Cancer. Adv. Drug Deliv. Rev. 2017, 114, 79–101. [Google Scholar] [CrossRef]
- Ibrahim, M.; Abuwatfa, W.H.; Awad, N.S.; Sabouni, R.; Husseini, G.A. Encapsulation, Release, and Cytotoxicity of Doxorubicin Loaded in Liposomes, Micelles, and Metal-Organic Frameworks: A Review. Pharmaceutics 2022, 14, 254. [Google Scholar] [CrossRef]
- Bieniek, A.; Terzyk, A.P.; Wiśniewski, M.; Roszek, K.; Kowalczyk, P.; Sarkisov, L.; Keskin, S.; Kaneko, K. MOF Materials as Therapeutic Agents, Drug Carriers, Imaging Agents and Biosensors in Cancer Biomedicine: Recent Advances and Perspectives. Prog. Mater. Sci. 2021, 117, 100743. [Google Scholar] [CrossRef]
- Nirosha Yalamandala, B.; Shen, W.; Min, S.; Chiang, W.; Chang, S.; Hu, S. Advances in Functional Metal-Organic Frameworks Based On-Demand Drug Delivery Systems for Tumor Therapeutics. Adv. Nanobiomed. Res. 2021, 1, 2100014. [Google Scholar] [CrossRef]
- Freund, R.; Lächelt, U.; Gruber, T.; Rühle, B.; Wuttke, S. Multifunctional Efficiency: Extending the Concept of Atom Economy to Functional Nanomaterials. ACS Nano 2018, 12, 2094–2105. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhao, Y.; Guan, Z.J.; Fang, Y. Porous Framework Materials for Bioimaging and Cancer Therapy. Molecules 2023, 28, 1360. [Google Scholar] [CrossRef]
- Iranpour, S.; Bahrami, A.R.; Saljooghi, A.S.; Matin, M.M. Application of Smart Nanoparticles as a Potential Platform for Effective Colorectal Cancer Therapy. Coord. Chem. Rev. 2021, 442, 213949. [Google Scholar] [CrossRef]
- Salehipour, M.; Rezaei, S.; Rezaei, M.; Yazdani, M.; Mogharabi-Manzari, M. Opportunities and Challenges in Biomedical Applications of Metal–Organic Frameworks. J. Inorg. Organomet. Polym. Mater. 2021, 31, 4443–4462. [Google Scholar] [CrossRef]
- Souri, M.; Soltani, M.; Moradi Kashkooli, F.; Kiani Shahvandi, M.; Chiani, M.; Shariati, F.S.; Mehrabi, M.R.; Munn, L.L. Towards Principled Design of Cancer Nanomedicine to Accelerate Clinical Translation. Mater. Today Bio 2022, 13, 100208. [Google Scholar] [CrossRef]
- Azevedo, S.; Costa-Almeida, R.; Santos, S.G.; Magalhães, F.D.; Pinto, A.M. Advances in Carbon Nanomaterials for Immunotherapy. Appl. Mater. Today 2022, 27, 101397. [Google Scholar] [CrossRef]
- Hendon, C.H.; Rieth, A.J.; Korzyński, M.D.; Dincǎ, M. Grand Challenges and Future Opportunities for Metal-Organic Frameworks. ACS Cent. Sci. 2017, 3, 554–563. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, L.; Yang, Y.; Qian, X.; Fu, T.; Li, X.; Yang, Z.; Yan, H.; Cui, C.; Tan, W. Metal–Organic Framework Nanocarriers for Drug Delivery in Biomedical Applications. Nanomicro. Lett. 2020, 12, 103. [Google Scholar] [CrossRef]










| Original MOFs/Metal Node | MOFs Composites | Further Modifications | Particle Dimensions | Irradiation In Vivo | Tumor Cell Models | Photo/Chemo/Immunotherapy | Ref | |
|---|---|---|---|---|---|---|---|---|
| Mechanism | Efficacy | |||||||
| ZIF-8/ Zn2+ | HA/IR820@ZIF-8 | IR820 adsorption to ZIF-8 surface + HA coating | 120 nm | Laser PTT: 808 nm, 1 W/cm2, 5 min | B16F10 | HA tumor targeting and ICD↑DAMPs MAN targeted DC delivery↑DC maturation and antitumoral response | ↓97.7% tumor growth inhibition Systemic anti- metastatic response Immunologic memory | [120] |
| MAN/(R837+1MT)@ZIF-8 | Immune adjuvant R837 and immunomodulator 1-MT adsorption to ZIF-8 surface + MAN coating | 221 nm | ||||||
| ZIF-8/ Zn2+ | CuZPMn@PpIX/DOX/CpG | CuS nanoparticles + PpIX + DOX encapsulation into ZIF-8 + CpG adsorption + PDA and MnO2 nanosheets coating | 120–150 nm | Laser PTT: 808 nm, 2 W/cm2, 10 min Laser PDT: 655 nm, 0.2 W/cm2, 10 min | 4T1 | CuS and PDA coating↑PTT MnO2 O2 generation + PpIX↑PDT PTT, PDT, DOX chemotherapy + CpG immunotherapy synergistic effect | Primary tumors eradication No recurrence or metastasis | [121] |
| ZIF-8/ Zn2+ | HA/ZIF- 8@ICG@IMQ | ICG and IMQ encapsulation + HA coating | 134 nm | Laser PTT: 808 nm, 0.1 W/cm2, 5 min | CT26 | ICG↑PTT TAAs release + IMQ ↑DC maturation and antitumoral response | Elimination of primary tumors Distant tumor growth inhibition Tumor recurrence prevention | [122] |
| PCN-224/Zr4+ | M@O-A | OXA encapsulation + aptPD-L1 adsorption | 139.1 nm | LED PDT: 640 nm; 0.1 W/cm2, 30 min | MC38 | aptPD-L1 specific targeting of PD-L1-positive tumor cells PDT + chemotherapy ↑ICD and antitumor immune response Synergy with ICB | Tumor growth inhibition Longer survival rates Complete distant tumor inhibition | [123] |
| PCN-224/Zr4+ | msiPCN | sicdk4 -protamine encapsulation + CT26 cell membrane coating | ≈150 nm | He−Ne laser PDT: 660 nm, 0.1 W/cm2,2 min | CT26 | Tumor cells homotypic targeting Cdk4 inhibition + PDT↑ICD, antigens release and PD-L1 expression Synergy with anti-PD-L1 anti-bodies | Tumor cell cycle arrest Tumor proliferation inhibition 100% survival rate after 30 days | [124] |
| PCN-224/Zr4+ | MnO2@CPCN | CCCP encapsulation + MnO2 shell and PAH coating | 117.6 nm | Laser PDT: 660 nm, 0.2 W/cm2, 10 min | 4T1 | MnO2 shell↓tumor hypoxia and↑ PDT efficiency MnO2 shell GSH scavenge releases CCCP↑pro death Mitophagy PDT + CCCP synergy ↑ICD , autophagy and antitumor immune response | 100% survival rate Tumor growth inhibition Tumor tissue eradication in 20% of mice Prevention of tumor metastasis and recurrence | [125] |
| PCN-224/Zr4+ | PCN@FM | FMs (DC cells + 4T1 cells) coating. | ≈175 nm | Laser PDT: 660 nm, 0.4 W/cm2, 5min | 4T1 | FM coating tumor homotypic targeting PDT↑ICD and antigen production FM coating + PDT synergistic effect | Primary tumors rebound proliferation inhibition Distant tumors proliferation suppression 70 days survival in 40% of the mice | [126] |
| PCN-224/Zr4+ | PCN-ACF-CpG@HA | ACF and CpG adsorption + HA coating | 105.4 -117.5 nm | Laser PDT: 670 nm, 0.25 W/cm2, 10 min | H22 | HA tumor cells specific targeting PDT↑ICD and antigen release PDT+ CpG↑DCs maturation ACF HIF-1α inhibition ↓immunosuppression | Tumor growth inhibition and cell destruction Metastasis inhibition | [127] |
| W-TBP/W6+ | W-TBP/CpG | CpG adsorption | Diameter: 114.0 ± 6.7 nm Width: 100 nm Length: 200 nm | Light PDT: 650 nm, 0.1 W/cm2, 7.5 min | TUBO | PDT↑ICD and TAAs PDT+ CpG↑DCs maturation Synergy with ICB | 96.6% tumor regression Abscopal effects when synergizing with ICB | [128] |
| Fe-TBP/Fe3+ | Fe-TBP | ________ | 100 nm in length | LED PDT: 650 nm, 0.1 W/cm2, 7.5 min | CT26 | Fe-TBP Fenton-like reaction ↑O2 and ↑ PDT PDT↑ICD Synergy with ICB | >90% regression of primary and distant tumors | [129] |
| Pd-TBP/Pd2+ | PTP@M | 4T1 cell membrane coating | 165 nm | Laser PDT: 630 nm, 0.3 W/cm2, 5 min | 4T1 | 4T1 cell membrane coating tumor cell homotypic targeting π-extended Pd-TBP in PTP ↑ PDT and ↑ ICD Synergy with ICB ↑ antitumor immune response | Tumor inhibition Anti-metastasis effect | [130] |
| Cu-TBP/Cu2+ | Cu-TBP | ________ | 164.1 ± 48.5 nm | LED PDT: 650 nm, 0.1 W/cm2, 30 min | B16F10 and SKOV-3 | pH dependent release of Cu2+ and H4TBP Cu2+ E2 metabolism catalysation ↑ ROS E2 metabolism-ROS + PDT ↑ ICD Synergy with ICB↑ systemic antitumoral immune response | 96.6% tumor growth inhibition 98.3% primary tumor regression and 94.9% in distal tumors inhibition with α-PD-L1 synergy Metastasis regression and long term antitumoral memory effects | [131] |
| MIL-101/Fe3+ | ICG-CpG@MOF | ICG and CpG adsorption | >150 nm | Laser PDT and PTT: 808 nm, 1.5 W/cm2, 5 min | 4T1 | HA mediated tumor cells targeting GSH dependent delivery of CpG PDT + PTT + CpG synergy | Tumor disappearance 18 days after treatment Metastasis inhibition | [132] |
| MIL-101/Fe3+ | MMH-NPs | MTO encapsulation + HA coating | 173.9 ± 3.7 nm | Laser PTT: 671 nm, 1.0 W/cm2, 5 min | CT26 | HA mediated tumor cells targeting PTT + chemotherapy ↑ICD and tumor antigen presentation αOX40 administration ↓ immunosuppressive cells | Tumor growth inhibition Abscopal effects and metastasis inhibition | [133] |
| MIL-100/Fe3+ | OIMH NPs | ICG and OXA encapsulation + HA coating | 127 nm | Laser PTT: 808 nm, 0.8 W/cm2, 10 min | CT26 | PTT + chemotherapy ↑ICD and antitumor response -Synergy with ICB | Inhibition of primary and distant tumors | [134] |
| UCS/ Zr4+ | TPZ/UCS | UCNPs core + CA coating + porphyrin MOF shell + TPZ encapsulation | 38–65 nm | Laser PTT: 980 nm, 1.2 W/cm2, 20 min (5 min interval every 1 min of irradiation) | CT26 | UCNP energy transference to the MOF porphyrin shell↑ ROS production pH dependent release and hypoxia activation of TPZ ↑ ROS production PDT+ TPZ ↑ICD and antitumor immunity Synergy with ICB | Complete tumor suppression Abscopal effects in synergy with α-PD-L1 | [135] |
| TBC-Hf/Hf4+ | IDOi@TBC-Hf | IDOi encapsulation | 83.2 nm | LED PDT: 650 nm, 0.1 W/cm2, 15 min | CT26 and MC38 | PDT ↑ICD in primary tumors IDO inhibition by IDOi ↓ immunosuppressive TME | Near elimination of the primary tumors Abscopal effect | [136] |
| pMOF/Zr4+ | Apt/PDGs-s@pMOF | PDG adsorption + ROS-sensitive crosslinking + Periostin- targeting Apt coating. | 96.96 nm | Laser PDT: 660 nm, 0.3 W/cm2, 5 min | 4T1 | Periotin-targeting Apt targeting of tumor cells Deeper penetration of the PDG by crosslinking destruction ↓ intratumoral MDSCs PDT↑ICD a systemic immune response | Primary tumor proliferation inhibition Abscopal effect in distant tumors | [137] |
| Zr-MOF/Zr4+ | SNPs@Zr-MOF@RB | PAA coating of SNP core-shell + Zr ions and TCCP adsorption for in situ growth + RB incorporation | ≈30 nm | Soft X-ray light for 5 min | 4T1 | Energy transfer from SNP to the MOF + RB ↑ROS production and ↑deep tissue PDT ↑ICD + deep tissue anti tumor immune response ↓ immunosuppressive TME | Tumor growth inhibition | [138] |
| Zr-MOF/Zr4+ | NaLnF4@MOF | NaLnF4 NPs DHCA modification + growth of the Zr-MOF around NaLnF4 | 36.6 ± 2.2 nm | Light PDT: 980 nm, 0.61 W/cm2, 10 min (3 min break for each minute) | CT26 | UCL from NaLnF4 to MOF ↑ PDT PDT-induced ICD synergises with ICB | Complete eradication of primary tumors 95% tumor inhibition Distant tumor suppression in synergy with α-PD-L1 | [139] |
| PBNP/Fe3+ and Fe2+ | PBNP | ________ | ≈60–90 nm | Laser PTT: 808 nm, 1.875 W/cm2, 10 min | Neuro2a | PTT↑ICD and antigen presentation Synergize with ICB | Primary tumor shrinkage Suppression and elimination of primary tumors in synergy with aCTLA-4 Rechallenging tumors eradication | [140] |
| PBNP/Fe3+ and Fe2+ | CpG- PBNP | CpG adsorption | 100–1000 nm | Laser PTT: 808 nm, 1.5 W/cm2, 10 min | 9464D | PTT↑ICD and antigen presentation PDT+ CpG↑DCs maturation | Complete tumor regression 100% survival rate after 80 days Slower distant tumor growth Rechallenged tumor regression 80% mice survival rate after 125 days | [141] |
| PBNP/Fe3+ and Fe2+ | CpG- PBNP | CpG adsorption | 100–1000 nm | Laser PTT: 808 nm, 0.75 W/cm2, 10 min | Neuro2a | PTT↑ICD and antigen presentation PDT+ CpG↑DCs maturation and ↑ synergy with ICB | Primary and distant tumor regression Fast elimination of rechallenged tumors | [142] |
| PBNP/Fe3+ and Fe2+ | SP94-PB-SF- Cy5.5 | SP94 adsorption +SF encapsulation + Cy5.5 adsorption | 90–110 nm | Laser PTT: 808 nm, 1.5 W/cm2, 10 min | HepG2 and Hepa1-6 | SP94 selectively targets HCC cells PTT + SF ↑ICD Synergy with ICB ↑ immune response | 100% tumor inhibition 80% mice survival rate Primary, distant and rechallenged tumors suppression in synergy with ICB | [143] |
| PBNP/Fe3+ and Fe2+ | PBNP | ________ | 51 nm | Laser PTT: 808 nm, 2 W/cm2, 10 min | SM1 | PTT- induced ICD + aCD137 ICB ↑ antitumoral response | Primary tumor elimination 60% distant tumor growth inhibition 66% rechallenged tumor rejection | [144] |
| Therapeutic Modality | Most Common MOFs | Most Common Modifications | Overall Immunotherapeutic Approaches | Best Therapeutic Outcomes In Vivo |
|---|---|---|---|---|
| PDT + immunotherapy | Porphyrin-based MOFs | Tumor cell membrane coating CpG adsorption | ICD through ROS production Immune adjuvants ↑DC maturation ↓TME immunosupression + immune checkpoints inhibition | Erradication of primary tumors 95% distant tumor inhibition |
| PTT + immunotherapy | ZIF-8, MIL-100 and PBNPs | HA coating CpG adsorption ICG encapsulation | Robust ICD induction ↑DC maturation ↓TME immunosupression + immune checkpoints inhibition | 97.7% tumor inhibition Primary tumor erradication in 40% of the mice Nearly no recurrence or metastasis |
| PTT + PDT + immunotherapy | ZIF-8 and MIL-101 | CpG adsorption | Robust ICD induction Immune adjuvants ↑DC maturation | 100% primary tumor inhibition and regression No recurrence or metastasis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, P.D.; Magalhães, F.D.; Pereira, R.F.; Pinto, A.M. Metal-Organic Frameworks Applications in Synergistic Cancer Photo-Immunotherapy. Polymers 2023, 15, 1490. https://doi.org/10.3390/polym15061490
Fernandes PD, Magalhães FD, Pereira RF, Pinto AM. Metal-Organic Frameworks Applications in Synergistic Cancer Photo-Immunotherapy. Polymers. 2023; 15(6):1490. https://doi.org/10.3390/polym15061490
Chicago/Turabian StyleFernandes, Pedro D., Fernão D. Magalhães, Rúben F. Pereira, and Artur M. Pinto. 2023. "Metal-Organic Frameworks Applications in Synergistic Cancer Photo-Immunotherapy" Polymers 15, no. 6: 1490. https://doi.org/10.3390/polym15061490