4.1. Differences among N Nutrition Parameters: N Concentration versus N Accumulation
The estimation accuracies for LNC and PNC were low when using VIs and the best-performing VI could explain no more than 30% of the variability in N concentration. However, the estimation accuracies for LNA and PNA were significantly higher than those for LNC and PNC. The optimal VI (NDRE) could explain 77% and 73% of the variability in LNA and PNA, respectively. The LNC (or PNC) varied in a narrow range across the growing season of rice crops. It decreased from the beginning, leveled off in the middle and then decreased until harvesting [
6]. This trend could be characterized by NDRE with a linear but weak relationship. However, LNA (or PNA) varied in a relatively wider range and kept increasing across the entire season. Particularly, NDRE tended to saturate when LNA (PNA) increased to a certain level. A linear regression would not be able to capture this decreasing rate of nitrogen accumulation. Therefore, LNC or PNC exhibited weak relationships with VIs in linear regressions and LNA or PNA in exponential regression [
3,
5]. Besides, the N absorption features are located in the shortwave infrared (SWIR) region rather than the visible and NIR region [
30]. Li et al. [
31] improved crop LNC estimation from the SWIR reflectance spectra of fresh leaves through enhancing the absorption features of nitrogen in the SWIR region. However, those VIs derived from UAV multispectral imagery were all based on visible and NIR bands, which could be easily affected by chlorophyll content and canopy structure [
32]. Furthermore, the N dilution effect might be another reason for the low estimation accuracy of LNC and PNC with VIs due to the decrease of N concentration along with the increase of biomass [
33]. The good relationships between NA and VIs might be attributed to the strong capabilities of VIs in retrieving biomass [
34] and the close correlation between N accumulation and biomass (
Table 3).
In texture analysis, the optimal NDTI for LNC and PNC estimation were all composed of NIR and RE bands with similar texture metrics (e.g., CON, DIS, COR). Although both LNC and PNC could be estimated with a common index NDTI (DIS
B4_D3, COR
B5_D3), the relationships between texture indices and NC were not strong in both linear and exponential regressions. That might be because leaf color turned light to dark repeatedly in response to N fertilization at the vegetative stages and kept yellowing at reproductive stages [
6]. This reduced the sensitivity of texture metrics to the heterogeneity of tonal variation caused by N status in crops across the whole growing season. Specifically, NDTI(MEA
B5, MEA
B4) and NDTI(MEA
B5, MEA
B2) were the optimal texture indices across the whole growing season in exponential regression for LNA and PNA estimation, respectively (
Table 5). Because allometric variation of LNA or PNA in the season was largely attributed to biomass (
Table 3), texture indices could characterize the biomass related variation with an exponential regression [
7,
8].
Furthermore, the variables in the multivariate model for LNA included one N-sensitive variable (NDTI(DIS
B4_D3, COR
B5_D3)) and two biomass-sensitive variables (NDRE and NGRDI). Therefore, LNA could reflect part of the variation in LNC [
35,
36], but the exact proportion needs to be investigated in future work. However, the optimal multivariate PNA estimation model consisted of entirely AGB-sensitive indices such as NDTI(MEA
B5_D3, MEA
B2_D4), MTVI2 [
29], and NGRDI [
20]. This also suggests that the variation in PNA might be dominated by biomass and contain weak information on N concentration which corresponds well with the findings in Reference [
5].
4.2. Directional Effect of Texture Analysis on Row-Planted Crops
The directional effect of texture analysis was rarely investigated in the existing literature, since the majority of previous studies executed texture analysis with the default direction (45°) [
7,
15]. Some studies calculated texture metrics with different directions but did not explicitly explain the reason [
10,
16]. That might be because most of them studied naturally grown forests, and the trees were distributed disorderly. In contrast, we found that texture metrics had a significant directional effect (
Figure 2). That is because rice plants are grown in rows, and the local window sliding along the row orientation contains more homogeneous vegetation than from other directions. Furthermore, the rice plants in the same row grow more homogenously than those in different rows, resulting in lower contrast and higher correlation in the along-row direction than in other directions (
Figure 5). Texture indices calculated with texture metrics from different directions had different performances on the N nutrition parameter estimation (
Table 6). However, the texture feature MEA represents the average values within the moving window and the textural information extracted from the UAV images is the average value from an ROI in the non-sampling area. As a result, MEA was not affected by directions, and the same
R2 value was obtained with the optimal NDTIs composed of MEA features calculated in different directions for LNA and PNA estimation (
Table 6).
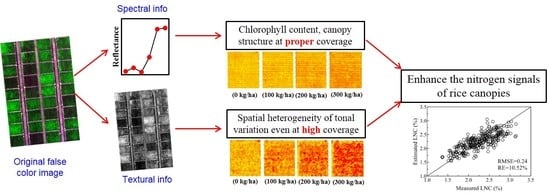
Texture metrics calculated in directions D3 and D4 constructed the optimal NDTIs for LNC and PNC estimation which might be explained by the strong capability of across-row texture metrics in differentiating the tonal variations caused by N status. Moreover, the estimation accuracy (RMSE = 0.24, RRMSE = 10.52%) of LNC decreased marginally if the texture indices in the multivariate model of LNC were derived from the direction D3 alone. Additionally, the same estimation accuracies were obtained for other N parameters (LNA, PNC, and PNA) through the multivariate models with all the texture indices calculated in the direction D3 when compared to the original multivariate models (data not shown). Therefore, all N nutrition parameters could be estimated at nearly the highest accuracies with the combination of VIs and texture indices calculated with texture metrics in the direction D3 which could simplify the use of texture analysis significantly.
Texture analysis also involved the optimal selection of texture calculation algorithm, spectral band, and window size [
14]. Although window size has a considerable effect on the estimation accuracy of forest biomass due to the mismatch of spatial scale between remotely sensed pixel size and tree canopy [
14,
16], it showed no significant influence in the present study. This was because most window sizes contained a large proportion of crops due to the large canopy coverage since the jointing stage. Rice crops are often planted in the row distance of 24–30 cm, and multispectral images collected at 100 m usually possess a spatial resolution of 5–7 cm. Hence, a larger window size might be a good choice for texture analysis at early growth stages. Yue et al. [
7] also found the optimal image resolution for using image textures to estimate AGB in winter wheat depended on the crop canopy size and row spacing. Therefore, the optimal window size has to be taken into consideration in terms of row space and image spatial resolution for other row-planted crops (e.g., soybean, corn).
4.3. The Benefits of Fused Information for Enhancing N Signals
Numerous studies were dedicated to improving N concentration estimation with different approaches due to the importance of N nutrition status in crop management. The most commonly used way was to propose new VIs for N concentration estimation [
37,
38]. Although those VIs yielded high estimation accuracies as shown in the literature, the unstable performance was reported in other studies [
39]. In this study, VIs had poor performance in N concentration estimation, which is in line with References [
3,
5]. Texture indices showed comparable performance when compared to VIs which is in contrast to the performance of texture ratios in forest AGB estimation [
10,
16]. However, a significant improvement in LNC estimation was obtained when using the combination of texture indices and VIs as compared to using VIs or texture indices alone, with an increase of more than 35% in RMSE (
Table 7). This finding agrees well with the results of References [
7] and [
8] which reported that the combination of texture indices and VIs improved the estimation of crop AGB significantly. In contrast to relevant studies [
6,
40], a universal model could be used to estimate N concentration across the entire season, which was a substantial improvement in crop N status monitoring with remotely sensed data. Compared with the new spectral index proposed by Stroppiana et al. [
38] with hyperspectral data (
R2 = 0.65), our multivariate model derived from multispectral data could even yield a similar
R2 value (
R2 = 0.68) for PNC. A universal model suitable for the entire season could not only be used to guide N fertilization applications at the early growth stages [
1,
41] but also to predict crop yield and quality before harvest [
42,
43].
The improvement in LNC and PNC estimation induced by the addition of texture indices stemmed from the enhancement of N signals for the early stages. VIs could not be used to estimate N concentration at the early growth stages (from tillering to booting) with a universal model, because N signals were hampered by the rapid increase of biomass and the large proportion of background materials [
5,
6]. However, leaf color changed remarkably due to the N fertilizer applications conducted at jointing stage [
11,
12], and texture metrics could characterize the spatial distribution of tonal variations caused by N status. The values of CON feature had a greater variation between jointing and booting stages than reflectance (
Figure 6). Furthermore, VIs had weak capability in N status monitoring at reproductive stages due to the high canopy coverage [
2]. However, leaf tone had a visual color change from the vegetative period to the reproductive period, and the values of CON and DIS were significantly different at reproductive stages (
Figure 6). NDTI has a wider variation than NDRE at high N levels at late growth stages (
Figure 7). Therefore, the N signal could also be detected by those texture metrics at the late growth stages.
Moreover, texture metrics have the capability of smoothing the spatial heterogeneity between vegetation and background materials with a sliding window [
9,
16]. Therefore, the complementary information between texture and VIs might be useful for solving the saturation problem and reducing background interference. Although a similar conclusion has been drawn by Yue et al. [
7] and Zheng et al. [
8] for AGB estimation, this study represents the first solid evidence on improving the estimation of N nutrition parameters, especially leaf and plant N concentrations.
Multi-source data fusion was commonly used in previous studies to improve the estimation of crop biomass [
44,
45,
46]. For example, Yue et al. [
45] improved wheat biomass estimation by combining VIs and plant height derived from crop surface models (CSMs), but the construction of CSMs was tedious and time consuming. Although the fusion of data from different platforms could also acquire higher estimation accuracy [
44], the data (e.g., LiDAR data) processing needs more professional skill. However, UAV-based image texture and spectral data are more convenient to acquire over small fields. Furthermore, the fusion of texture and spectral data could improve the accuracy of N status estimation of multiple growth stages, which solves the problem that N status was often estimated at only one growth stage or a short time window [
6,
40]. Therefore, monitoring N status across the whole season could be realized through the integration of texture and spectral information into the crop growth monitoring systems.
There might be some concerns that it is challenging to build a universal model for predicting the N status of rice plants across the entire growing season [
3]. With spectral information alone, VIs often exhibit weak capabilities of detecting N status at late growth stages due to the fact of crop senescence. Textural information could capture the variation of leaf color within and between plots, especially at the late stages when leaf color changes dramatically. However, our results demonstrated that the fusion of spectral and textural information could improve N status monitoring efficiently. Furthermore, the multivariate models for LNC and PNC were tested on different dataset groups with satisfactory validation performance (
Table 8). The applicability of those models still needs to be improved through further testing with more datasets from different geographic sites.
4.4. Potentials for Other Platforms
In this study, we found the combination of spectral and texture information in NIR and RE bands was superior to that in other bands in rice N status monitoring. As for monitoring crop N status in large areas, satellite imagery has been widely used but with low estimation accuracy. That is because satellite imagery has low spatial resolution and low spectral resolution normally with blue, green, red and NIR wavebands. RapidEye is the first launched satellite with a RE band and successfully applied to many aspects of precision agriculture [
47,
48]. Other satellites with RE bands (e.g., Sentinel-2, WorldView-2, and Gaofen-6) have been launched and have yielded significant improvement in the estimation accuracy on agronomic variables [
49,
50]. These findings from satellite observations were consistent with our results that the RE-based VIs were superior to other indices.
To date, texture analysis on satellite imagery was mostly used for forest AGB [
14,
15,
16]. Our findings provide a strong reference on crop N status with texture analysis of satellite images equipped with the RE band. However, in terms of spatial resolution and crop row spacing, the spatial resolution of satellite imagery (highest spatial resolution is 0.3 m by WorldView 3) is insufficient for distinguishing the narrow row spacing in cereal crops (e.g., rice, wheat). Furthermore, crops could be planted in multiple directions over large areas and the direction effect of texture metrics may not be significant in satellite images with lower spatial resolutions. Given the suitability of UAV platforms for small-scale applications for the moment, the direction of texture analysis could still exist in UAV imagery. When using texture analysis on row-planted vegetation (e.g., staple crops, vegetable crops, and fruit trees) at the farm level with high resolution images, it is still beneficial to take consideration of the direction effect for improved nitrogen nutrition monitoring.