Geographic Distribution of Desert Locusts in Africa, Asia and Europe Using Multiple Sources of Remote-Sensing Data
Abstract
:1. Introduction
2. Materials and Methods
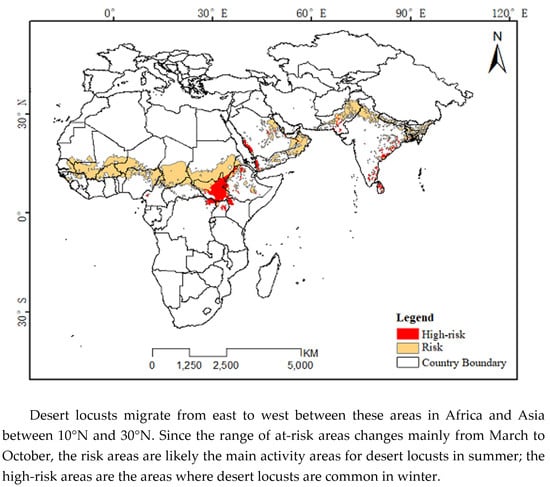
2.1. Study Area
2.2. Data Collection and Pre-Processing
2.3. Methods
2.3.1. Model Establishment
2.3.2. Assessment of Potential Desert Locust Habitat
3. Results
3.1. Model Validation and Variable Contribution
3.2. Potential Distribution of Desert Locusts
3.3. Changes in the Potential Distribution Area
4. Discussion
4.1. The Important Influence of Temperature Change on Desert Locusts Distribution
4.2. Prediction and Analysis of the Migration Path of Desert Locust
4.3. Uncertainties and Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A


References
- Latchininsky, A.V. Locusts and remote sensing: A review. J. Appl. Remote Sens. 2013, 7, 075099. [Google Scholar] [CrossRef] [Green Version]
- Skaf, R.; Popov, G.B.; Roffey, J. The Desert Locust: An international challenge. Philos. Trans. R. Soc. B Biol. Sci. 1990, 328, 525–538. [Google Scholar] [CrossRef]
- Gómez, D.; Salvador, P.; Sanz, J.; Casanova, C.; Taratiel, D.; Casanova, J. Desert locust detection using Earth observation satellite data in Mauritania. J. Arid Environ. 2019, 164, 29–37. [Google Scholar] [CrossRef]
- Hielkema, J.U.; Roffey, J.; Tucker, C.J. Assessment of ecological conditions associated with the 1980/81 desert locust plague upsurge in West Africa using environmental satellite data. Int. J. Remote Sens. 1986, 7, 1609–1622. [Google Scholar] [CrossRef]
- Ceccato, P.; Cressman, K.; Giannini, A.; Trzaska, S. The desert locust upsurge in West Africa (2003–2005): Information on the desert locust early warning system and the prospects for seasonal climate forecasting. Int. J. Pest Manag. 2007, 53, 7–13. [Google Scholar] [CrossRef]
- Buhl, J.; Sumpter, D.J.T.; Couzin, I.D.; Hale, J.J.; Despland, E.; Miller, E.R.; Simpson, S.J. From Disorder to Order in Marching Locusts. Science 2006, 312, 1402–1406. [Google Scholar] [CrossRef] [Green Version]
- Al-Ajlan, A.M. Relationship between desert locust, Schistocerca gregaria (Forskål), infestation, environmental factors and control measures in Gazan and Makkah Regions, Saudi Arabia. Pak. J. Biol. Sci. 2007, 10, 3507. [Google Scholar]
- Cressman, K. Role of remote sensing in desert locust early warning. J. Appl. Remote Sens. 2013, 7, 075098. [Google Scholar] [CrossRef]
- Navratil, P.; Wilps, H. Object-based locust habitat mapping using high-resolution multispectral satellite data in the southern Aral Sea basin. J. Appl. Remote Sens. 2013, 7, 075097. [Google Scholar] [CrossRef]
- Nishide, Y.; Tanaka, S.; Saeki, S. Adaptive difference in daily timing of hatch in two locust species, Schistocerca gregaria and Locusta migratoria: The effects of thermocycles and phase polyphenism. J. Insect Physiol. 2015, 72, 79–87. [Google Scholar] [CrossRef]
- Tucker, C.J.; Hielkema, J.U.; Roffey, J. The potential of satellite remote sensing of ecological conditions for survey and forecasting desert-locust activity. Int. J. Remote Sens. 1985, 6, 127–138. [Google Scholar] [CrossRef]
- Powell, L.; Berg, A.A.; Johnson, D.; Warland, J. Relationships of pest grasshopper populations in Alberta, Canada to soil moisture and climate variables. Agric. For. Meteorol. 2007, 144, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Waldner, F.; Ebbe, M.A.O.B.; Cressman, K.; Defourny, P. Operational Monitoring of the Desert Locust Habitat with Earth Observation: An Assessment. ISPRS Int. J. Geo-Infor. 2015, 4, 2379–2400. [Google Scholar] [CrossRef] [Green Version]
- Lazar, M.; Piou, C.; Doumandji-Mitiche, B.; Lecoq, M. Importance of solitarious desert locust population dynamics: Lessons from historical survey data in Algeria. Ѐntomol. Exp. Appl. 2016, 161, 168–180. [Google Scholar] [CrossRef] [Green Version]
- Bryceson, K.P.; Wright, D. An analysis of the 1984 locust plague in Australia using multitemporal landsat multispectral data and a simulation model of locust development. Agric. Ecosyst. Environ. 1986, 16, 87–102. [Google Scholar] [CrossRef]
- Deveson, E.D. Satellite normalized difference vegetation index data used in managing Australian plague locusts. J. Appl. Remote Sens. 2013, 7, 075096. [Google Scholar] [CrossRef] [Green Version]
- Gómez, D.; Salvador, P.; Sanz, J.; Casanova, C.; Taratiel, D.; Casanova, J.L. Machine learning approach to locate desert locust breeding areas based on ESA CCI soil moisture. J. Appl. Remote Sens. 2018, 12, 036011. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Deveson, E.D.; Waters, C.; Spessa, A.; Lawton, D.; Feng, P.; Liu, D.L. Future climate change likely to reduce the Australian plague locust (Chortoicetes terminifera) seasonal outbreaks. Sci. Total Environ. 2019, 668, 947–957. [Google Scholar] [CrossRef]
- Hunter, D.; Walker, P.W.; Elder, R.J. Adaptations of locusts and grasshoppers to the low and variable rainfall of Australia. J. Orthoptera Res. 2001, 10, 347–351. [Google Scholar] [CrossRef] [Green Version]
- Beck, H.E.; Zimmermann, N.E.; McVicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data 2018, 5, 180214. [Google Scholar] [CrossRef] [Green Version]
- Wan, Z.; Li, Z. A physics-based algorithm for retrieving land-surface emissivity and temperature from EOS/MODIS data. IEEE Trans. Geosci. Remote Sens. 1997, 35, 980–996. [Google Scholar] [CrossRef]
- Wan, Z.; Zhang, Y.; Zhang, Q.; Li, Z.-L. Validation of the land-surface temperature products retrieved from Terra Moderate Resolution Imaging Spectroradiometer data. Remote Sens. Environ. 2002, 83, 163–180. [Google Scholar] [CrossRef]
- Rodell, M.; Houser, P.R.; Jambor, U.; Gottschalck, J.; Mitchell, K.; Meng, C.-J.; Arsenault, K.; Cosgrove, B.; Radakovich, J.; Bosilovich, M.; et al. The Global Land Data Assimilation System. Bull. Am. Meteorol. Soc. 2004, 85, 381–394. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Yang, K.; Qin, J.; Zhao, L.; Tang, W.; Han, M. Evaluation of AMSR-E retrievals and GLDAS simulations against observations of a soil moisture network on the central Tibetan Plateau. J. Geophys. Res. Atmos. 2013, 118, 4466–4475. [Google Scholar] [CrossRef]
- Hijmans, R.J.; Cameron, S.E.; Parra, J.L.; Jones, P.G.; Jarvis, A. Very high resolution interpolated climate surfaces for global land areas. Int. J. Clim. 2005, 25, 1965–1978. [Google Scholar] [CrossRef]
- Zeng, Y.; Low, B.W.; Yeo, D.C. Novel methods to select environmental variables in MaxEnt: A case study using invasive crayfish. Ecol. Model. 2016, 341, 5–13. [Google Scholar] [CrossRef]
- Sun, P.; Zhang, Q.; Wen, Q.; Singh, V.P.; Shi, P. Multisource Data-Based Integrated Agricultural Drought Monitoring in the Huai River Basin, China. J. Geophys. Res. Atmos. 2017, 122, 10751–10772. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Monserud, R.A.; Leemans, R. Comparing global vegetation maps with the Kappa statistic. Ecol. Model. 1992, 62, 275–293. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H.; Anderson, R.P.; Dudík, M.; Ferrier, S.; Guisan, A.; Hijmans, R.J.; Huettmann, F.; Leathwick, J.R.; Lehmann, A.; et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography 2006, 29, 129–151. [Google Scholar] [CrossRef] [Green Version]
- Phillips, S.J. Transferability, sample selection bias and background data in presence-only modelling: A response to Peterson et al. (2007). Ecography 2008, 31, 272–278. [Google Scholar] [CrossRef]
- Johnson, J.W.; Lebreton, J.M. History and Use of Relative Importance Indices in Organizational Research. Organ. Res. Methods 2004, 7, 238–257. [Google Scholar] [CrossRef]
- Jiménez-Valverde, A.; Lobo, J.M. Threshold criteria for conversion of probability of species presence to either–or presence–absence. Acta Oecologica 2007, 31, 361–369. [Google Scholar] [CrossRef]
- Manel, S.; Williams, H.C.; Ormerod, S.J. Evaluating presence-absence models in ecology: The need to account for prevalence. J. Appl. Ecol. 2001, 38, 921–931. [Google Scholar] [CrossRef]
- Zhang, K.; Yao, L.; Meng, J.; Tao, J. Maxent modeling for predicting the potential geographical distribution of two peony species under climate change. Sci. Total Environ. 2018, 634, 1326–1334. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chang, H.; Liu, T.; Zhang, C. The potential geographical distribution of Haloxylon across Central Asia under climate change in the 21st century. Agric. For. Meteorol. 2019, 275, 243–254. [Google Scholar] [CrossRef]
- Chitale, V.S.; Behera, M.D.; Roy, P.S. Future of Endemic Flora of Biodiversity Hotspots in India. PLoS ONE 2014, 9, e115264. [Google Scholar] [CrossRef]
- MacFadyen, S.; McDonald, G.; Hill, M.P. From species distributions to climate change adaptation: Knowledge gaps in managing invertebrate pests in broad-acre grain crops. Agric. Ecosyst. Environ. 2018, 253, 208–219. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-Cabral, N.Y.Z.; Kumar, L.; Shabani, F. Suitable areas of Phakopsora pachyrhizi, Spodoptera exigua, and their host plant Phaseolus vulgaris are projected to reduce and shift due to climate change. Theor. Appl. Clim. 2018, 135, 409–424. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, C.; Hu, Q.; Tian, H. Temperature Changes in Central Asia from 1979 to 2011 Based on Multiple Datasets. J. Clim. 2014, 27, 1143–1167. [Google Scholar] [CrossRef]





| Variables | Data Source/Producer | Unit | Period |
|---|---|---|---|
| NDVI | https://modis.gsfc.nasa.gov/ | —— | 2005–2020 |
| LAI | https://modis.gsfc.nasa.gov/ | —— | 2005–2020 |
| SM | http://disc.gsfc.nasa.gov/ | kg/m2 | 2005–2020 |
| RF | http://disc.gsfc.nasa.gov/ | mm | 2005–2020 |
| LST | http://www.worldclim.org/ | °C | 2005–2020 |
| Elevation | http://www.worldclim.org/ | m | 2005–2020 |
| Month | Desert Locust Area (×107 km2) | ||
|---|---|---|---|
| Increased | Decreased | Unchanged | |
| January | 0.18 | 0.16 | 0.52 |
| February | 0.45 | 0.12 | 0.59 |
| March | 0.54 | 0.33 | 0.70 |
| April | 0.91 | 0.28 | 0.96 |
| May | 0.64 | 0.13 | 1.74 |
| June | 0.53 | 0.26 | 2.12 |
| July | 0.30 | 0.18 | 2.48 |
| August | 0.07 | 0.24 | 2.53 |
| September | 0.23 | 0.49 | 2.11 |
| October | 0.37 | 1.19 | 1.15 |
| November | 0.26 | 0.99 | 0.67 |
| December | 0.21 | 0.39 | 0.47 |
| Code | Environmental Variables | Factor Contribution (%) | VIF |
|---|---|---|---|
| NDVI | Average Monthly Normalized Difference Vegetation Index | 12.59 | 2.29 |
| LAI | Average Monthly Leaf Area Index | 25.63 | 3.92 |
| LST | Average Monthly Land Surface Temperature | 27.02 | 2.75 |
| RF | Average Monthly Precipitation | 22.25 | 1.21 |
| SM | Average Monthly Soil Moisture | 2.7 | 3.72 |
| Elevation | Elevation | 9.81 | 2.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Qian, J.; Chen, X.; Hu, Z.; Sun, J.; Wei, S.; Xu, K. Geographic Distribution of Desert Locusts in Africa, Asia and Europe Using Multiple Sources of Remote-Sensing Data. Remote Sens. 2020, 12, 3593. https://doi.org/10.3390/rs12213593
Chen C, Qian J, Chen X, Hu Z, Sun J, Wei S, Xu K. Geographic Distribution of Desert Locusts in Africa, Asia and Europe Using Multiple Sources of Remote-Sensing Data. Remote Sensing. 2020; 12(21):3593. https://doi.org/10.3390/rs12213593
Chicago/Turabian StyleChen, Chaoliang, Jing Qian, Xi Chen, Zengyun Hu, Jiayu Sun, Shujie Wei, and Kaibin Xu. 2020. "Geographic Distribution of Desert Locusts in Africa, Asia and Europe Using Multiple Sources of Remote-Sensing Data" Remote Sensing 12, no. 21: 3593. https://doi.org/10.3390/rs12213593