
Physiology News Magazine
Legs pay out for cost of breathing!
Lee Romer and Jerome Dempsey propose that the demand placed on the respiratory muscles during high-intensity endurance exercise plays a pivotal role in determining exercise performance through its effect on limb blood flow and muscle fatigue
Features
Legs pay out for cost of breathing!
Lee Romer and Jerome Dempsey propose that the demand placed on the respiratory muscles during high-intensity endurance exercise plays a pivotal role in determining exercise performance through its effect on limb blood flow and muscle fatigue
Features
Lee M Romer (1) & Jerome A Dempsey (2)
1: Centre for Sports Medicine and Human Performance, Brunel University, Uxbridge, UK
2: John Rankin Laboratory of Pulmonary Medicine, Department of Population Health Sciences, University of Wisconsin, Madison, USA
https://doi.org/10.36866/pn.65.25

The respiratory muscles are, with few exceptions, structurally suited to the high ventilatory demands of endurance exercise. The diaphragm, for example, has a high oxidative capacity, multiple sources of blood supply and a unique resistance to vasoconstrictor influences, making it the most fatigue resistant of all skeletal muscles. Such structural characteristics, in combination with precise neural regulation of breathing pattern and respiratory muscle recruitment, mean that the capacity of these muscles for pressure generation is usually well in excess of the demands placed upon them. Thus, in most untrained healthy individuals the pressures produced by the inspiratory muscles during progressively increasing exercise are only about 50% of maximum dynamic capacity, the
oxygen cost of breathing approximates 10% of maximum oxygen uptake and diaphragm fatigue does not occur.
Despite the considerable aerobic capacity of the diaphragm, this muscle will fatigue in response to exercise sustained to exhaustion at intensities greater than 80-85% of maximum oxygen uptake. For example, the pressure measured across the diaphragm in response to bilateral stimulation of the phrenic nerves (1-20 Hz) was reduced 15-50% below preexercise baseline and did not return to control values until 1-2 hours after exercise (Johnson et al. 1993). The reason for this exercise-induced diaphragmatic fatigue is due in part to the high levels of diaphragmatic work that must be sustained throughout highintensity exercise, as demonstrated by the finding that diaphragmatic fatigue was prevented when diaphragmatic work during exercise was reduced by over 50% using a mechanical ventilator. However, other factors besides diaphragmatic work must also be responsible for the exercise-induced diaphragmatic fatigue, because fatigue does not occur when the resting subject mimics the magnitude and duration of diaphragmatic work incurred during exercise. Indeed, fatigue does not occur until diaphragmatic work is voluntarily increased two-fold greater than that required during maximal exercise. The probable explanation of this substantial effect of whole body exercise on diaphragm fatigability is that, at rest, the volitional increases in diaphragmatic work mean large shares of the total cardiac output are devoted to the diaphragm, whereas during exercise the diaphragm must compete with locomotor muscles for their share of the available cardiac output. Less blood flow to the diaphragm promotes inadequate oxygen transport and increases the likelihood of fatigue.
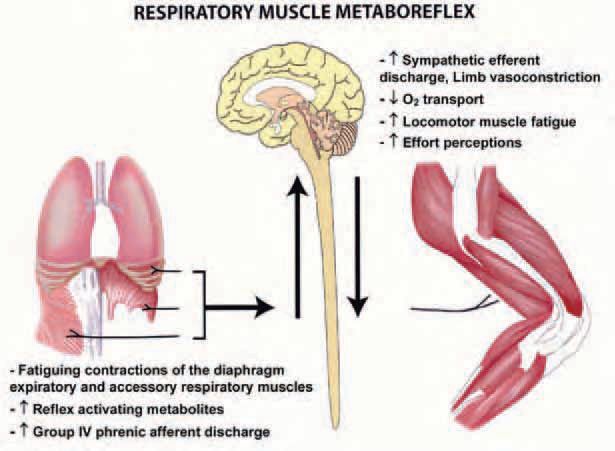
The aforementioned physiological effects of the work of breathing have important functional consequences, as demonstrated by an approximately 15% improvement in performance during high-intensity endurance exercise with partial unloading of the respiratory muscles via mechanical ventilation (Harms et al. 2000). One aspect of respiratory muscle work that might limit exercise performance is a reflex effect from fatiguing respiratory muscles, which increases sympathetic vasoconstrictor outflow and compromises perfusion of limb muscle thereby limiting its ability to perform work. Fatiguing contractions and accumulation of metabolites in the respiratory muscles activate the unmylenated Type IV phrenic afferents which, via a supraspinal reflex, evokes an increase in muscle sympathetic nerve activity (St Croix et al. 2000) and a reduction in blood flow and vascular conductance in the resting leg (Sheel et al. 2001) (see Fig. 1). During maximal exercise, concomitant increases in vascular conductance and blood flow to working limb muscles have been observed when the normally occurring work of breathing is reduced using a mechanical ventilator (Harms et al. 1997). Furthermore, in resting and exercising dogs, a bolus injection of lactic acid into the diaphragm by way of the phrenic artery elicits a transient reduction in hind limb blood flow and vascular conductance – these changes appear to be mediated sympathetically because they are prevented by pharmacological blockade of the adrenergic receptors (Rodman et al. 2003).
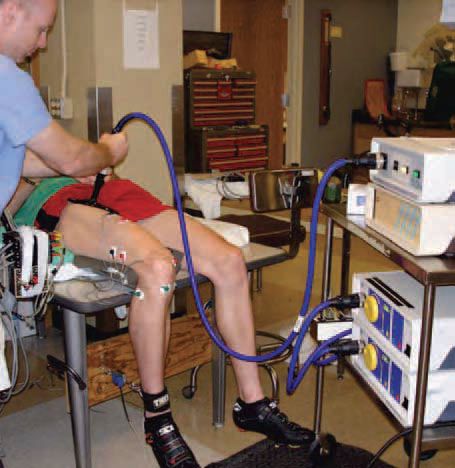
In our most recent experiments we attempted to determine whether the aforementioned changes in vascular conductance and limb blood flow with manipulation of the work of breathing impact upon the fatigability of the working limb muscles (Romer et al. 2006). We assessed limb muscle fatigue by magnetically stimulating the femoral nerve using a paired twitch technique (see Fig. 2). Force:frequency curves for the quadriceps were constructed by altering the duration between paired twitches. These measurements were performed before and after high-intensity cycle exercise under conditions of control, inspiratory muscle unloading (via mechanical ventilation) and inspiratory muscle loading (via inspiratory resistors). When exercise at 90% of peak work rate was continued to exhaustion with breathing unimpeded, quadriceps twitch force averaged across a wide range of stimulation frequencies (1-100 Hz) was reduced by almost 30% in the period immediately after exercise. When the exercise was repeated on a different day at equal workload and duration as the control condition, but with the normally occurring work of breathing reduced by 50-60%, the decrease in quadriceps force output was attenuated by about one-third. Increasing the work of breathing by 80% above control exacerbated the severity of fatigue by about 40% compared with identical exercise with breathing unimpeded.
The effect of inspiratory muscle unloading on limb muscle fatigue was consistent among subjects but relatively small compared with the much larger reductions in limb fatigue we recently found by preventing the mild arterial oxygen desaturation that can accompany high-intensity exercise. Nevertheless, it is likely that this effect of unloading on fatigue represents an underestimation of what might be attributed to the total work of breathing. For example, the normal work of breathing was reduced by only slightly more than one half. Furthermore, the loading and unloading arms of the study were confined to inspiration. Recent evidence in healthy individuals suggests that the expiratory abdominal muscles also fatigue in response to high-intensity endurance exercise and that expiratory loading to the point of task failure elicits an increase in leg muscle sympathetic nerve activity. Furthermore, the high expiratory pressures often encountered during high-intensity exercise in response to active expiration and expiratory flow limitation have been shown to reduce stroke volume, which may be expected to exacerbate limb fatigue via concomitant reductions in limb blood flow.
In health, it is likely that exerciseinduced respiratory muscle work contributes to limb fatigue only during sustained high-intensity exercise that is sufficient to elicit significant levels of fatigue in the diaphragm or accessory respiratory muscles. Environmental hypoxia, and particularly chronic hypoxic exposure which potentiates the hyperventilatory response to exercise and consequently the work of breathing, might also be expected to exacerbate the effect of respiratory muscle work on limb fatigue. From a clinical perspective, changes in the work of breathing may play a particularly important role in determining limb fatigue in patients with chronic obstructive pulmonary disease (COPD) and chronic heart failure (CHF). In patients with COPD the limb muscles are more fatigable than in healthy subjects and the inspiratory and expiratory pressures developed during exercise are substantial. Many such patients exhibit a plateau in leg blood flow early during progressively increasing exercise and these patients also tend to have the highest levels of ventilation and dyspnea during exercise, suggesting that blood flow may be redirected to the respiratory muscles. Patients with CHF also have an elevated work of breathing, in addition to blunted cardiac output and sympathetic vasoconstrictor responses to exercise. In combination, these factors could compromise limb blood flow and fatigue, even during submaximal exercise.
In summary, unloading the respiratory muscles in normal healthy individuals improves performance during highintensity exercise coincident with increases in limb vascular conductance and blood flow and a reduction in leg muscle fatigue. We propose that exercise-induced respiratory muscle work contributes to both ‘peripheral’ and ‘central’ fatigue influences on exercise performance. In other words, quadriceps muscle fatigue plays a pivotal role in determining exercise performance both through its direct effect on muscle force output (i.e., peripheral fatigue) and also through its feedback effect on effort perceptions, causing reduced motor output to the working limb muscles (i.e., central fatigue).
Acknowledgements
The authors are grateful to the NHLBI for supporting their work.
References
Harms CA, Babcock MA, McClaran SR, Pegelow DR, Nickele GA, Nelson WB & Dempsey JA (1997). Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol 82, 1573-1583.
Harms CA, Wetter TJ, St Croix CM, Pegelow DF & Dempsey JA (2000). Effects of respiratory muscle work on exercise performance. J Appl Physiol 89, 131-138.
Johnson BD, Babcock MA, Suman OE & Dempsey JA (1993). Exercise-induced diaphragmatic fatigue in healthy humans. J Physiol 460, 385-405.
Rodman JR, Henderson KS, Smith CA & Dempsey JA (2003). Cardiovascular effects of the respiratory muscle metaboreflexes in dogs: rest and exercise. J Appl Physiol 95, 1159-1169.
Romer LM, Lovering AT, Haverkamp HC, Pegelow DF & Dempsey JA (2006). Effect of inspiratory muscle work on peripheral fatigue of locomotor muscles in healthy humans. J Physiol 571, 425-439.
Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ & Dempsey JA (2001). Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol 537, 277289.
St Croix CM, Morgan BJ, Wetter TJ & Dempsey JA (2000). Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol 529, 493-504.
