
Physiology News Magazine
How does the visual brain code contours?
Neurones in the primary visual cortex of mammals show a remarkable selectivity for the orientation of contours in the visual scene. The neuronal basis of this property has been the subject of intense debate for nearly half a century. Our recent work throws new light on this problem.
Features
How does the visual brain code contours?
Neurones in the primary visual cortex of mammals show a remarkable selectivity for the orientation of contours in the visual scene. The neuronal basis of this property has been the subject of intense debate for nearly half a century. Our recent work throws new light on this problem.
Features
Trichur R. Vidyasagar, Jaikishan Jayakumar and Sivaram Viswanathan
Department of Optometry & Vision Sciences, University of Melbourne, Melbourne, Victoria, Australia
https://doi.org/10.36866/pn.84.27

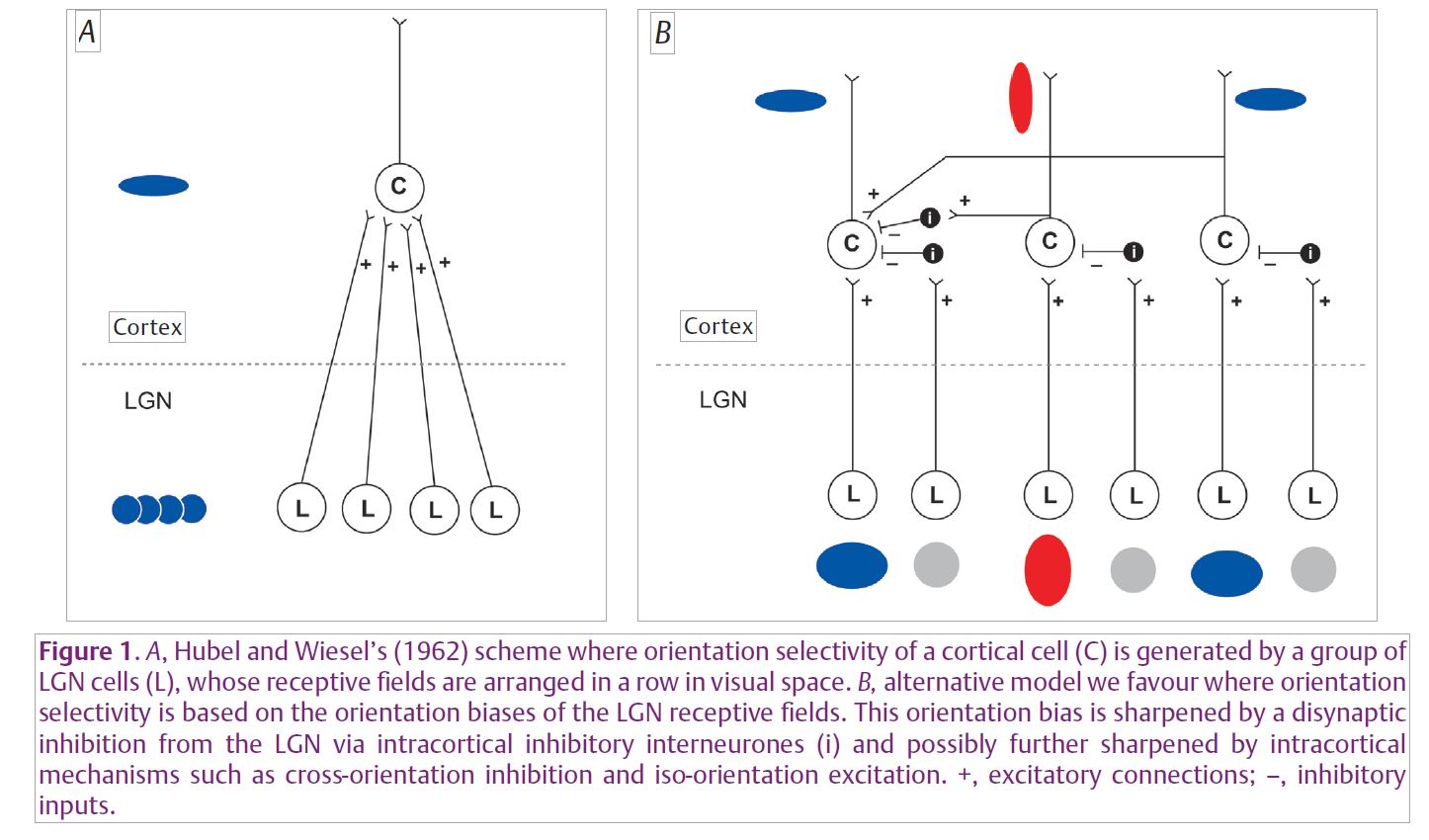
Various parts of the cerebral cortex, whether they receive visual, auditory or other sensory inputs or signals from other parts of the cortex, do not fundamentally differ in structure, barring minor variations. Thus, the distinctive function of a cortical region may be determined largely by its inputs and not so much by any intrinsic structure specific to each cortical area. This suggests that there exists a canonical microcircuitry of the cortex that enables the impressive transformations in the coding of the sensory signals arriving at the primary sensory areas. The major breakthrough in our understanding of such a fundamental circuitry came with the studies on the mammalian primary visual cortex by Hubel and Wiesel (1962). Their discovery of the remarkable selectivity for the orientation of an edge or a line shown by primary visual cortical cells and an elegant model they put forward to account for this has inspired a whole host of experiments over the last 50 years. It has also remained as one of the most intensely debated schemes in all sensory neuroscience. Many laboratories, including ours, are now bringing us closer to a comprehensive understanding of a basic circuitry of the way sensory signals are coded by primary sensory areas.
Hubel and Wiesel suggested that the orientation selectivity of single cortical cells arises from an excitatory convergence they receive from neurones in the dorsal lateral geniculate nucleus (LGN) of the thalamus, that themselves have circular receptive fields and lack any orientation selectivity. In this scheme, the receptive fields (RFs) of these LGN cells were assumed to be along a row in visual space, so that a long contour of the right orientation that stimulates this row of LGN RFs would best excite the cortical cell that they all project to (Fig. 1A). There have been two other schools of thought that have consistently questioned this scheme: (1) Intracortical mechanisms, especially excitation from cells tuned to the optimal orientation and inhibition from cells tuned to orthogonal orientations, act on an excitatory input from the LGN that is not tuned to stimulus orientation (Creutzfeldt et al. 1974; Sillito et al. 1980; Douglas et al. 1991); (2) The mild degrees of orientation sensitivity that are already present in single thalamic neurones, when acted upon by intracortical inhibition, can lead to sharp orientation selectivity at the cortical level (Vidyasagar & Heide, 1984; Vidyasagar et al. 1996) (Fig. 1B).
Though the debate has been dragging on without a general consensus, recent experiments may be taking us towards a resolution. Kara et al. (2002) invented a clever protocol to ‘isolate’ the raw geniculate input to the cortex without any cortical influences by applying a particular train of electrical stimulation to the LGN. Such stimulation excites, among other cells, cortical inhibitory interneurones and leads to a profound suppression of all intracortical interactions. Kara et al. (2002) found that the orientation selectivity of cells receiving direct geniculate inputs was not significantly reduced by the electrical stimulation in the LGN. They interpreted this as support for Hubel & Wiesel’s scheme of excitatory convergence, since the stimulation had effectively ‘silenced’ the cortex. However, an alternative interpretation that we favour is that the electrical stimulation in the LGN also activates intrageniculate inhibition which would sharpen the orientation bias that the geniculate cells inherit from the retina. In that case, during the electrical stimulation, the signal reaching cortical cells in single geniculate afferents could already be sharply tuned for orientation. To test this suggestion, we constructed special electrodes that had a recording electrode glued to the LGN stimulating electrode and we recorded the orientation selectivity of the LGN cells themselves before and during electrical stimulation in the close vicinity (Viswanathan et al. 2011). Figure 2 (left panel) shows our variation on Kara et al.’s design. We found that LGN cells showed a significant increase in their orientation selectivity during local electrical stimulation (Fig. 2, right panel). Thus, the sharp tuning seen in the raw geniculocortical input (Kara et al. 2002) could reflect to a large extent the sharpening that happens in the LGN itself and need not be due to the pattern of geniculocortical convergence originally suggested by Hubel and Wiesel.

Our results imply that any non-specific inhibition, such as that happening within the cortex during normal visual stimulation and acting on an orientationally biased input to a cortical cell, can lead to sharp orientation selectivity in the cortex. Consistent with this suggestion, we also found that iontophoretic application of the inhibitory transmitter, GABA, near an LGN cell could also markedly sharpen the orientation selectivity of the cell. The sharper, band-pass spatial frequency selectivity of cortical cells compared to the low-pass LGN and retinal cells indicates that non-specific inhibition from a larger surround does occur at the cortex, as indeed it has been shown in experiments using an antagonist of GABA-mediated inhibition (Vidyasagar & Mueller, 1994). It is also now well established that the orientation bias seen in the LGN (Vidyasagar & Heide, 1984; Soodak et al. 1987) and retina (Levick & Thibos, 1980) are apparent mainly towards the higher spatial frequency end of the broad spectrum of spatial frequencies that these cells respond to. Thus, non-specific intracortical inhibition simultaneously bestows on the cell selectivities for both spatial frequency and orientation (Vidyasagar & Heide, 1984).
Other recent experiments (Jin et al. 2011) have suggested that the receptive field scatter of thalamic afferents in the cortex is less than what was believed based upon the classical scheme of Hubel and Wiesel, and hint at a substantial role for subcortical mechanisms in generating the cortical selectivity for stimulus orientation. If orientation is indeed coded in the retina and sharpened further at thalamic and cortical levels, there are some constraints if the system is to preserve resolution and sensitivity and still have a broad range of orientations represented in the cortex. Orientation can be coded by retinal ganglion cells only in a limited number of broadly tuned channels, as is done for processing of colour by the three cone types. Consistent with this, we find that retinal and LGN cells are not only broadly tuned for orientation, but their preferred orientations seem to be close to the radial orientation (i.e. pointing towards the centre of the retina) or its tangential orientation (Levick & Thibos, 1980; Vidyasagar & Urbas, 1982; Shou & Leventhal, 1989). Thus, the remarkable orientation selectivity of visual cortical cells first described by Hubel and Wiesel in a pioneering article in The Journal of Physiology almost 50 years ago may turn out to be the outcome of a number of mechanisms that build upon the receptive field asymmetry present in retinal ganglion cells.
References
Creutzfeldt OD, Kuhnt U & Benevento LA (1974). An intracellular analysis of visual cortical neurones to moving stimuli: response in a co-operative neuronal network. Exp Brain Res 21, 251–274.
Douglas RJ, Martin KA & Whitteridge D (1991). An intracellular analysis of the visual responses of neurones in cat visual cortex. J Physiol 440, 659–696.
Hubel DH & Wiesel TN (1962). Receptive fields, binocular interaction and functional architecture in the cat’s visual cortex. J Physiol 160, 106–154.
Jin J, Wang Y, Swadlow HA & Alonso JM (2011). Population receptive fields of ON and OFF thalamic inputs to an orientation column in visual cortex. Nat Neurosci 14, 232–240.
Kara P, Pezaris JS, Yurgenson S & Reid RC (2002). The spatial receptive field of thalamic inputs to single cortical simple cells revealed by the interaction of visual and electrical stimulation. Proc Natl Acad Sci U S A 99, 16261–16266.
Levick WR & Thibos LN (1980). Orientation bias of cat retinal ganglion cells. Nature 286, 389–390.
Shou TD & Leventhal AG (1989). Organized arrangement of orientation-sensitive relay cells in the cat’s dorsal lateral geniculate nucleus. J Neurosci 9, 4287–4302.
Sillito AM, Kemp JA, Milson JA & Berardi N (1980). A re-evaluation of the mechanisms underlying simple cell orientation selectivity. Brain Res 194, 517–520.
Soodak RE, Shapley RM & Kaplan E (1987). Linear mechanism of orientation tuning in the retina and lateral geniculate nucleus of the cat. J Neurophysiol 58, 267–275.
Vidyasagar TR & Heide W (1984). Geniculate orientation biases seen with moving sine wave gratings: implications for a model of simple cell afferent connectivity. Exp Brain Res 57, 176–200.
Vidyasagar TR & Mueller A (1994). Function of GABAA inhibition in specifying spatial frequency and orientation selectivities in cat striate cortex. Exp Brain Res 98, 31–38.
Vidyasagar TR, Pei X & Volgushev M (1996). Multiple mechanisms underlying the orientation selectivity of visual cortical neurones. Trends Neurosci 19, 272–277
Vidyasagar TR & Urbas JV (1982). Orientation sensitivity of cat LGN neurones with and without inputs from visual cortical areas 17 and 18. Exp Brain Res 46, 157–169.
Viswanathan S, Jayakumar J & Vidyasagar TR (2011). Role of feedforward geniculate inputs in the generation of orientation selectivity in the cat’s primary visual cortex. J Physiol 589, 2349–2361. http://jp.physoc.org/content/589/9/2349.long
