Published online May 14, 2015. doi: 10.3748/wjg.v21.i18.5739
Peer-review started: November 19, 2014
First decision: January 8, 2015
Revised: January 28, 2015
Accepted: February 11, 2015
Article in press: February 11, 2015
Published online: May 14, 2015
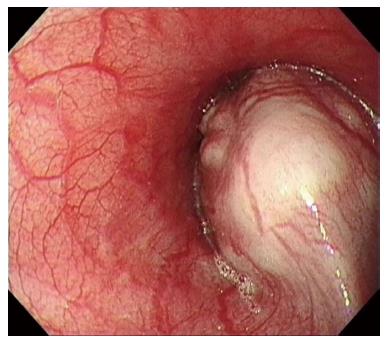
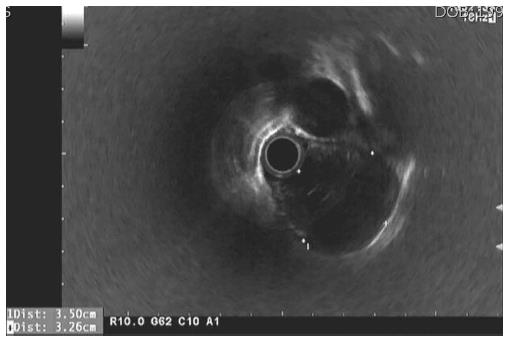
A 21-year-old male visited our hospital with a complaint of aggravating dysphagia and odynophagia for a few days. Esophagogastroduodenoscopy showed huge bulging mucosa with an intact surface causing luminal narrowing at 35 cm from the incisor teeth. Endoscopic ultrasonography showed an about 35 mm sized irregular margined in-homogenous hypoechoic lesion with an obscure layer of origin. Endoscopic ultrasonography fine needle aspiration revealed spindle cell proliferation without immunoreactivity for CD117, SMA, and cytokeratin. The patient underwent excision of the subepithelial lesion at the distal esophagus. On pathologic examination of the specimen, the tumor was composed of short fascicles of oval to spindle cells with eosinophilic and clear cytoplasm and vesicular nuclei. The tumor cells were positive for S-100 and SOX10 and negative for CD117, SMA, HMB-45, melan-A, cytokeratin, and CD99. The split-apart signal was detected in EWSR1 on FISH, suggesting a malignant gastrointestinal neuroectodermal tumor. At the time of writing, the patient is on radiation therapy at the operated site of esophagus and doing well, with no recurrence for three months. Malignant gastrointestinal neuroectodermal tumor is a rare gastrointestinal tumor with features of clear cell sarcoma, without melanocytic differentiation, and shows a poor prognosis. This is the first reported case of malignant gastrointestinal neuroectodermal tumor arising as subepithelial lesion in the esophagus.
Core tip: This is the first reported case of malignant gastrointestinal neuroectodermal tumor arising in the esophagus. Malignant gastrointestinal neuroectodermal tumor is a tumor with a similar morphology, immunophenotype, and molecular genetic features to clear cell sarcoma of tendons and aponeurosis lacking melanocytic differentiation arising in the gastrointestinal tract. Malignant gastrointestinal neuroectodermal tumors show aggressive disease behavior with a poor prognosis.
- Citation: Kim SB, Lee SH, Gu MJ. Esophageal subepithelial lesion diagnosed as malignant gastrointestinal neuroectodermal tumor. World J Gastroenterol 2015; 21(18): 5739-5743
- URL: https://www.wjgnet.com/1007-9327/full/v21/i18/5739.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i18.5739
Subepithelial lesion (SEL) of the gastrointestinal tract is defined as any bulging covered with intact mucosa, and represents either intraluminal lesions arising from any layers of the gastrointestinal wall or external compression caused by neighboring organs[1]. Most SELs are found incidentally during esophagogastroduodenoscopy and, in some cases, may cause symptoms. Endoscopic ultrasonography (EUS) and/or fine needle aspiration is useful in making differential diagnoses of SEL, with the average accuracy of fine needle aspiration reaching 80%[2]. Differential diagnosis of esophageal SEL includes leiomyoma, granular cell tumor, glomus tumor, gastrointestinal stromal tumor, lipoma, cyst, varices, submucosal cancer/metastasis, or external compression by adjacent structures. The majority of mesenchymal tumors of the gastrointestinal tract are gastrointestinal stromal tumor or leiomyoma, with the diagnosis of malignant gastrointestinal neuroectodermal tumor (MGINET) being reported in rare cases[3].
We report on a case of a SEL of the esophagus diagnosed as MGINET, which is the first to be reported in the esophagus. We also provide a short review of the literature on MGINET.
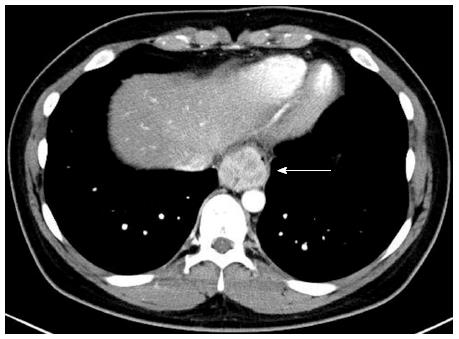
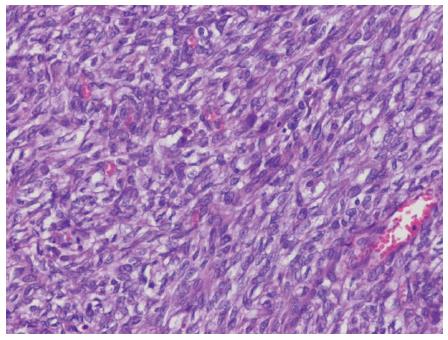
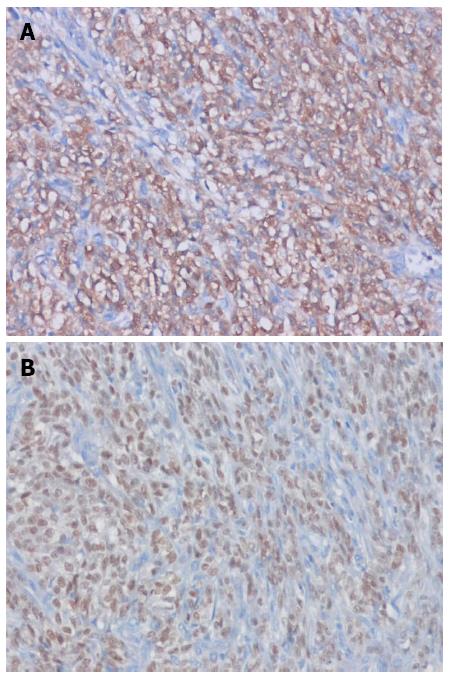
A 21-year-old male visited our hospital with a complaint of aggravating dysphagia for a few days. The patient had experienced intermittent dysphagia after over-eating for a year. He had no significant past medical or familial history, and was a non-smoker and a social alcohol drinker. On physical examination, he had the appearance of relative well-being, with an alert mentality and a soft, non-tender abdomen with no palpable mass. Initial vital signs included 140/90 mmHg blood pressure, 97 beats/min heart rate, 20 breaths/min respiration rate, and 36.9 °C body temperature. The initial laboratory findings were as follows: 9820 cells/μL white blood cells, 16.5 g/dL hemoglobin, 2.09 × 105 cells/μL platelets, 0.67 mg/dL total bilirubin, 7.18 g/dL total protein, 4.18 g/dL albumin, 13 IU/L aspartate aminotransferase, 24 IU/L alanine aminotransferase, 263 IU/L alkaline phosphatase, 166 IU/L γ-glutamyl transpeptidase, 301 IU/L lactate dehydrogenase, 12.0 s prothrombin time, 7.5 mg/dL blood urea nitrogen, 1.01 mg/dL creatinine, 134 mEq/L Na+, 4 mEq/L K+, and 97 mEq/L Cl-. Esophagogastroduodenoscopy showed a huge bulging mucosa with an intact surface causing luminal narrowing at 35 cm from the incisor teeth (Figure 1). On EUS, an irregular margined in-homogenous hypoechoic lesion measuring approximately 35 mm in size with an obscure layer of origin was observed (Figure 2). EUS guided fine needle aspiration was performed and, on microscopic view, spindle cell proliferation without immunoreactivity for CD117 and CD34. DOG-1, smooth muscle actin, and cytokeratin were observed. An abdominal computed tomography scan showed a well-defined round mass measuring approximately 3.5 cm in size in the distal esophagus (Figure 3). The patient underwent excision of the subepithelial lesion at the distal esophagus. Gross finding showed a mass measuring 3.5 cm × 3.5 cm in size with the cut surface showing a heterogeneous white grayish appearance with focal hemorrhage. On microscopic examination of the specimen, the tumor was composed of short fascicles of oval to spindle cells with eosinophilic and clear cytoplasm and vesicular nuclei (Figure 4). Mitosis was seen frequently, with a mitotic count of 55/50 under a high power field. The tumor cells were positive for S-100, SOX10 (Figure 5), and vimentin, and negative for CD117, CD34, Dog-1, smooth muscle actin, desmin, HMB-45, melan-A, cytokeratin (AE1/AE3), and CD99. The split-apart signal was detected in the Ewing sarcoma break point region 1 gene (EWSR1) on fluorescence in situ hybridization. Further evaluation with positron emission tomography showed no evidence of regional or distant metastasis. At the time of writing, the patient is on radiation therapy at the operated site of the esophagus and is doing well, with no recurrence for 5 mo.
Clear cell sarcoma of tendons and aponeurosis is a malignant melanoma arising in soft tissue, with characteristic features of melanocytic differentiation and chromosomal translocation of EWSR1-ATF1 t(11;22)(q13;q12). Tumors with similar morphology, immunophenotype, molecular genetic features to clear cell sarcoma of tendons and aponeurosis lacking melanocytic differentiation arising in the gastrointestinal tract have been previously reported. Zambrano first designated these tumors as clear cell sarcoma-like tumor of the gastrointestinal tract in 2003[4]. Further studies have demonstrated that clear cell sarcoma-like tumor of the gastrointestinal tract arises from the autonomic nervous system, and Stockman et al[3] re-designated clear cell sarcoma-like tumor of the gastrointestinal tract as MGINET in 2012. Immunohistochemical staining of MGINET characteristically shows positive results for vimentin, S100, and SOX10, and negative results for human melanoma black (HMB) 45, melan-A, tyrosinase, CD117, CD34, DOG-1, CD99, α-smooth muscle actin, desmin, and glial fibrillary acidic protein. Conflicting immunohistochemical staining results for CD56, synaptophysin, NB 84, non-specific enolase, and neurofilament protein have been reported. Consistent with previous reports[3,5-11], our case showed positive immunohistochemical staining results for S-100 protein, SOX 10, and vimentin, and negative results for HMB 45 and melan-A. Differential diagnosis with gastrointestinal stromal tumor was made by negative immunohistochemical staining results for CD117, CD34, and DOG-1. In our case, final diagnosis of MGINET was confirmed by split-apart signal detected in the EWSR1 on fluorescence in situ hybridization.
For our review of English language case reports on the subject, we included 40 cases of MGINET (39 from a search of PubMed and Google Scholar® in addition to our own case)[3-16]. Median age of the 40 patients diagnosed as MGINET was 35 years (range: 10-81), with 25 (62.5%) patients being diagnosed at younger than 40 years. Male to female ratio was equal. The most commonly affected site of MGINET was the small intestine with 28 patients (70%), followed by the stomach in 8 (20%) cases, the colon in 3 (7.5%) cases, and the esophagus in 1 (2.5%) case. Mean size of the primary tumor was 4.86 cm. Among 33 patients with information on lymph node status, 23 (69.7%) patients had lymph node metastasis at initial diagnosis and among 36 patients with information on local or distant metastasis, 14 (38.9%) patients had local or distant metastasis at initial diagnosis. The most common site of metastasis in MGINET was the liver with 16 cases (40%), followed by the peritoneum, mesentery, omentum, pelvis, and lung. Among 13 patients with information on tumor invasion depth, 11 (84.6%) had transluminal involvement. Stockman et al[3] reported clinical follow-up data of 12 patients with MGINET; six patients died with tumor and four patients were alive with regional or distant metastasis, leaving only two patients alive without recurrence. MGINET showed aggressive clinical feature with a high rate of recurrence even after complete resection and a high mortality rate. Among 12 patients with both initial and follow-up clinical information, even patients with a small tumor size of 3.5 cm developed liver metastasis at 12 mo. Among two patients with intact serosa, one patient developed liver metastasis at 24 mo and one had no metastasis at 24 mo. Among five patients with positive lymph node metastasis, three patients developed or died from metastasis and, among four patients without lymph node metastasis, two patients developed or died from metastasis. Four patients were free of disease at last follow-up, although long-term data were not available. As one patient developed metastasis at 60 mo from initial diagnosis, further follow-up may increase the incidence of metastasis or death from tumor in these patients. Involved site, tumor size, presence of trans-mural involvement, and lymph node involvement at initial diagnosis do not appear to affect prognosis of MGINET. More cases and further follow-up data are needed in order to understand MGINET behavior. In our case, the patient had localized disease resected with negative margin and had no evidence of regional or distant metastasis on imaging studies, including computed tomography and PET. Early detection of the esophageal lesion due to symptoms caused by narrowing of the esophageal lumen might have led to a better prognosis in our patient, however further clinical follow-up data are needed. We report on a case of subepithelial lesion diagnosed as malignant gastrointestinal neuroectodermal tumor, which is the first report case found in the esophagus.
A 21-year-old male presented with aggravating dysphagia for a few days.
Physical examination on the abdomen revealed unremarkable findings.
Leiomyoma, granular cell tumor, glomus tumor, gastrointestinal stromal tumor, lipoma, cyst, varices, submucosal cancer or metastasis, or external compression by adjacent structures.
Initial laboratory findings were unremarkable.
Esophagogastroduodenoscopy revealed a huge bulging mucosa with an intact surface causing luminal narrowing at 35 cm from the incisor teeth. Endoscopic ultrasonography showed an irregular margined inhomogeneous hypoechoic lesion measuring approximately 35 mm x 32 mm in size with an obscure layer of origin. An abdominal computed tomography scan showed a well-defined mass measuring approximately 35 mm in size in the distal esophagus.
Histological examination showed spindle cells with eosinophilic and clear cytoplasm and vesicular nuclei. Immunohistochemical staining results were positive for S-100 protein, SOX 10, and vimentin and negative for HMB 45 and melan. The final pathological result was confirmed as a malignant gastrointestinal neuroectodermal tumor.
The patient received excision of the subepithelial lesion in the distal esophagus and was subsequently treated with radiation therapy.
Malignant gastrointestinal neuroectodermal tumor most commonly involves the small intestine, with the stomach and colon being the next most common sites, respectively.
Malignant gastrointestinal neuroectodermal tumor: a tumor with a similar morphology, immunophenotype, and molecular genetic features to clear cell sarcoma of tendons and aponeurosis lacking melanocytic differentiation arising in the gastrointestinal tract.
This case report presents a case of malignant gastrointestinal neuroectodermal tumor arising in the esophagus. The most malignant gastrointestinal neuroectodermal tumors show aggressive behavior and a poor prognosis.
The case report on an esophageal subepithelial lesion diagnosed as malignant gastrointestinal neuroectodermal tumor is well written. The topic of the paper is interesting and important.
P- Reviewer: Guo YM, Saha L S- Editor: Ma YJ L- Editor: Rutherford A E- Editor: Ma S
| 1. | Hawes RH, Fockens P, Varadarajulu S. Endosonography: Expert Consult - Online and Print. 3e. : Saunders Pub 2014; 400. [Cited in This Article: ] |
| 2. | Hoda KM, Rodriguez SA, Faigel DO. EUS-guided sampling of suspected GI stromal tumors. Gastrointest Endosc. 2009;69:1218-1223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 156] [Cited by in F6Publishing: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 3. | Stockman DL, Miettinen M, Suster S, Spagnolo D, Dominguez-Malagon H, Hornick JL, Adsay V, Chou PM, Amanuel B, Vantuinen P. Malignant gastrointestinal neuroectodermal tumor: clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of 16 cases with a reappraisal of clear cell sarcoma-like tumors of the gastrointestinal tract. Am J Surg Pathol. 2012;36:857-868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 4. | Zambrano E, Reyes-Mugica M, Franchi A, Rosai J. An osteoclast-rich tumor of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts: reports of 6 cases of a GIST simulator. Int J Surg Pathol. 2003;11:75-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 119] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 5. | Antonescu CR, Nafa K, Segal NH, Dal Cin P, Ladanyi M. EWS-CREB1: a recurrent variant fusion in clear cell sarcoma--association with gastrointestinal location and absence of melanocytic differentiation. Clin Cancer Res. 2006;12:5356-5362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 262] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 6. | Friedrichs N, Testi MA, Moiraghi L, Modena P, Paggen E, Plötner A, Wiechmann V, Mantovani-Löffler L, Merkelbach-Bruse S, Buettner R. Clear cell sarcoma-like tumor with osteoclast-like giant cells in the small bowel: further evidence for a new tumor entity. Int J Surg Pathol. 2005;13:313-318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Shenjere P, Salman WD, Singh M, Mangham DC, Williams A, Eyden BP, Howard N, Knight B, Banerjee SS. Intra-abdominal clear-cell sarcoma: a report of 3 cases, including 1 case with unusual morphological features, and review of the literature. Int J Surg Pathol. 2012;20:378-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Donner LR, Trompler RA, Dobin S. Clear cell sarcoma of the ileum: the crucial role of cytogenetics for the diagnosis. Am J Surg Pathol. 1998;22:121-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Huang W, Zhang X, Li D, Chen J, Meng K, Wang Y, Lu Z, Zhou X. Osteoclast-rich tumor of the gastrointestinal tract with features resembling those of clear cell sarcoma of soft parts. Virchows Arch. 2006;448:200-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Joo M, Chang SH, Kim H, Gardner JM, Ro JY. Primary gastrointestinal clear cell sarcoma: report of 2 cases, one case associated with IgG4-related sclerosing disease, and review of literature. Ann Diagn Pathol. 2009;13:30-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Terazawa K, Otsuka H, Morita N, Yamashita K, Nishitani H. Clear-cell sarcoma of the small intestine detected by FDG-PET/CT during comprehensive examination of an inflammatory reaction. J Med Invest. 2009;56:70-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Lagmay JP, Ranalli M, Arcila M, Baker P. Clear cell sarcoma of the stomach. Pediatr Blood Cancer. 2009;53:214-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 13. | Venkataraman G, Quinn AM, Williams J, Hammadeh R. Clear cell sarcoma of the small bowel: a potential pitfall. Case report. APMIS. 2005;113:716-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Pauwels P, Debiec-Rychter M, Sciot R, Vlasveld T, den Butter B, Hagemeijer A, Hogendoorn PC. Clear cell sarcoma of the stomach. Histopathology. 2002;41:526-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 56] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Granville L, Hicks J, Popek E, Dishop M, Tatevian N, Lopez-Terrada D. Visceral clear cell sarcoma of soft tissue with confirmation by EWS-ATF1 fusion detection. Ultrastruct Pathol. 2006;30:111-118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Comin CE, Novelli L, Tornaboni D, Messerini L. Clear cell sarcoma of the ileum: report of a case and review of literature. Virchows Arch. 2007;451:839-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |