Published online Jun 21, 2015. doi: 10.3748/wjg.v21.i23.7074
Peer-review started: January 27, 2015
First decision: March 10, 2015
Revised: March 23, 2015
Accepted: May 4, 2015
Article in press: May 4, 2015
Published online: June 21, 2015
Hepatitis B virus (HBV) infection is still a serious worldwide problem, and vaccination is the most effective strategy for primary prevention of the infection. Although universal vaccination may be required for total eradication, several countries, including Japan, have not yet adopted universal vaccination programs. Some individuals are non-responders to HBV vaccine and several mechanisms responsible for their poor response have been proposed. To overcome non-response, third generation vaccines with pre-S proteins have been developed. These vaccines have shown better anti-HBs responses and may also be effective in preventing infection by HBV with S mutant. Improvement of vaccine efficacy by intradermal administration, or co-administration with cytokines or adjuvants, may also be effective in non-responders. The necessity, timing and method of booster vaccination in responders with decreased anti-HBs responses, and effective vaccination against S-mutant HBV, are issues requiring resolution in the global prevention of HBV infection.
Core tip: Hepatitis B virus (HBV) infection is still a serious worldwide problem, and vaccination is most effective for primary prevention of infection. This review summarizes current unsolved issues and future perspectives on vaccination required for global prevention of HBV infection.
- Citation: Tajiri K, Shimizu Y. Unsolved problems and future perspectives of hepatitis B virus vaccination. World J Gastroenterol 2015; 21(23): 7074-7083
- URL: https://www.wjgnet.com/1007-9327/full/v21/i23/7074.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i23.7074
About 400 million people worldwide are chronically infected with hepatitis B virus (HBV), with about half being infected perinatally or during early childhood via vertical and/or horizontal routes[1]. HBV infection can induce acute hepatitis, which may result in fulminant hepatitis, and chronic hepatitis, which may eventually lead to liver cirrhosis and/or hepatocellular carcinoma (HCC)[1]. HBV infection is responsible for 500000 to 1.2 million deaths per year due to chronic hepatitis, cirrhosis, and HCC[2], as well as being responsible for 60% to 80% of HCCs worldwide[3]. Treatment with antiviral agents, including nucleotide analogues and interferon, has been effective in some, but not all, patients with chronic HBV. Therefore, prophylactic approaches are essential to prevent viral infection. Many countries have introduced universal prophylactic vaccination programs, and some individuals, especially those who are immunocompromised, are unable to develop anti-HBs antibody following conventional vaccination. Moreover, HBV in vaccinated subjects may develop mutants, allowing them to escape the effects of vaccination. Attempts have been made to improve the immunogenicity of HBV vaccines. This review describes the current status and unresolved issues of HBV vaccination.
Methods of HBV transmission at an early age include perinatal, vertical from mother to infant, and horizontal from an infected household member to the child[4]. Perinatal infection occurs in 70% to 90% of babies born to HBeAg-positive mothers, and in less than 15% of babies born to HBeAg-negative mothers[5,6]. The risk of vertical transmission was significantly increased by high serum levels of maternal HBV-DNA[7], and the rate of infection increased from 0% in mothers with serum HBV-DNA < 5 log10 copies/mL to 50% in those with HBV-DNA ≥ 9 log10 copies/mL[8]. Moreover, some reports from China have shown that the reductions of serum HBV DNA in the 3rd trimester of HBsAg-positive pregnant women by the administration of hepatitis B immunoglobulin (HBIG)[9] or nucleoside analogue[10,11] significantly decreased the neonatal intrauterine HBV infection. These data suggest that the serum level of HBV DNA in pregnant women is the major determinant in the occurrence of mother-to-infant transmission.
In infected persons, HBV is found not only in blood but in body fluids, including saliva, semen and vaginal secretions, all of which are capable of transmitting the virus. Furthermore, HBV remains viable for 7 d or longer on environmental surfaces at room temperature.
The World Health Organization (WHO) has classified countries into three categories according to the prevalence of chronic HBV carrier: high (8% or greater); intermediate (2%-8%); and low (less than 2%) (Table 1)[12]. In high endemic areas, the life risk of acquiring HBV infection is greater than 60% and most infections are transmitted vertically/perinatally or horizontally during early childhood. In intermediate endemic areas, the life risk of HBV ranges from 20% to 60%, and infections are found in all age groups. In low endemic areas, the life risk is low, especially in normal living environments, and infections occur primarily in adults through sexual or parenteral transmission[12]. Regardless, vaccination should be considered to prevent HBV infection.
| Prevalence | High | Intermediate | Low |
| Carrier rate | ≥ 8% | 2%-7% | ≤ 1% |
| Region | Southeast Asia | Mediterranean basin | United States |
| China | Eastern Europe | Canada | |
| Pacific islands | Central Asia | Central Asia | |
| Sub-Saharan Africa | Japan | Western Europe | |
| Alaska (Eskimos) | Latin and South America | Australia | |
| Middle East | New Zealand | ||
| Predominant age at infection | Perinatal | Early childhood | Adult |
| Early childhood | |||
| Predominant mode of infection | Maternal to infant | Percutaneous | Sexual contact |
| Percutaneous | Sexual contact | Percutaneous |
The first plasma-derived HB vaccine, containing highly purified 22 nm HBsAg inactivated by urea, pepsin, formaldehyde, and heat, was introduced in 1982[13]. Subsequently, yeast-derived recombinant HB vaccines were introduced in the mid-1980s because of the potential risk of blood-borne infection in plasma-derived vaccines[14]. Yeast-derived vaccines were manufactured by cloning the HBV S gene in yeast cells, yielding nonglycosylated HBV small S protein but not the pre-S region[15]. Due to the potential neurotoxicity of the preservative thimerosal, two yeast-derived recombinant thimerosal-free vaccines (Recombivax HB® and Engerix-B®) were developed and have been widely available[16]. The third generation HB vaccines are mammalian cell-derived recombinant vaccines containing the pre-S region. These vaccines may be more immunogenic in a controlled trial[17], but are not widely available at present.
HB vaccines used for primary prophylaxis have been shown to reduce the risk of infection in most populations[18]. A combination vaccine (Twinrix®), containing Engerix-B and HAVRIX (hepatitis A vaccine), is also available[19,20]. The HepB3 hepatitis B vaccination program, consisting of a series of three doses, the second administered one month and the third six months after the first dose, resulted in the production of anti-HBs in about 95% of recipients[21]. No significant side effects have been observed, except for pain at the injection site and mild to moderate fever[22].
Although HBV is transmitted at a high rate by parenteral, percutaneous and sexual contact, a study from the United States showed that vaccination of high-risk groups had little impact on the incidence of HBV infection[23]. In contrast, since perinatal or early postnatal transmission is the primary cause of chronic infections worldwide, the first dose of HB vaccine should be given as soon as possible after birth (< 24 h) even in low-endemic countries. Primary protection regardless of maternal HBsAg status has been recommended[24].
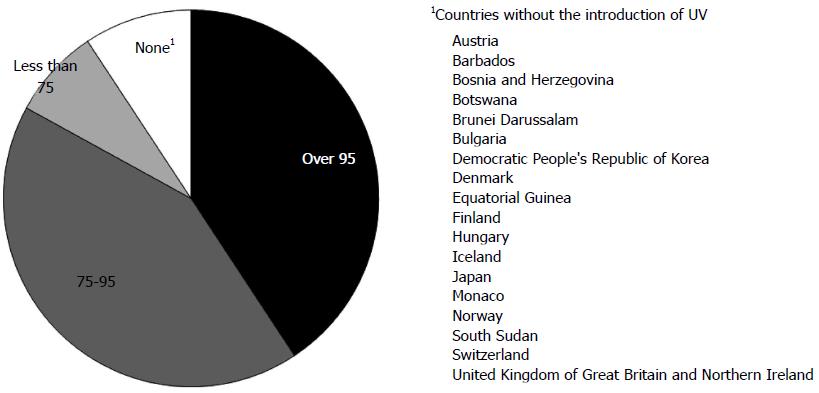
To prevent HBV infection, the WHO advocated HBV vaccination of all infants in 1992, or universal vaccination (UV). By the end of 2013, UV had been introduced nationwide in 183 countries. In Japan, UV has not yet been introduced, but will be introduced in the near future. Global coverage with HepB3 is estimated at 81% and is as high as 92% in the Western Pacific[25] (Figure 1). A meta-analysis of randomized controlled trials of HB vaccination of newborns showed that vaccinated infants born to HBV-positive mothers were 3.5-fold less likely to become infected with HBV (RR = 0.28, 95%CI: 0.20-0.40)[26].
In Taiwan, a high endemic area, UV of all newborns was implemented in 1986, reducing the HBsAg prevalence rate from 9.8% in 1984 to 0.6% in 2004[27]. Furthermore, the prevalence of HCC in childhood decreased from 0.7 per 100000 in 1981-1986 to 0.36 per 100000 in 1990-1994[28]. After 20 years of this program, the risk of HCC has been decreased by 70% in young adults, indicating that HB vaccine was the first vaccine to successfully prevent a major human cancer[27,29]. Furthermore, almost 30 years later, the prevalence of HBV infection was markedly lower, with HBsAg positivity decreasing from 10% to 0.9% and anti-HBc positivity from 28% to 7%[30]. In the Gambia, HBsAg prevalence in childhood decreased from 10% to 0.6% 10 years after UV introduction[31,32]. In Alaska, the incidence of acute hepatitis B was decreased to almost zero and the prevalence of chronic hepatitis among children and young adults was also decreased[33,34]. Furthermore, the incidence of HCC in patients under 20 years of age decreased from 3 per 100000 in 1984-1988 to zero in 1995-1999[33,34]. These findings showed that UV can prevent vertical and horizontal transmission of HBV infection, reducing rates of chronic HBV infection, especially in high endemic areas.
In the United States, a low endemic area, the incidence of acute hepatitis B has declined 82%, from 8.5 per 100000 in 1990 to 1.5 per 100000 in 2007[35]. In Malaysia, HBsAg prevalence in children decreased from 1.6% in 1997 to 0.3% in 2003[36]. In Italy, the morbidity of acute hepatitis B in patients aged 15 to 24 years old decreased from 17 per 100000 in 1990 to less than 0.5 per 100000 in 2005[37,38].
Despite vaccination at birth, however, 5% of babies become HBV carriers[39-41]. The precise mechanism is unclear, but vaccinated individuals, especially in HBV endemic areas, should be carefully monitored for HBV infection.
Mechanisms of nonresponse to HB vaccine: Three conditions have been shown associated with nonresponse to HB vaccine (Table 2). In addition, around 10% of the general population shows poor responses. Antibody response rates decline gradually after age 40 years, with age being a factor determining response to HB vaccine[42]. The mechanism of nonresponse is not fully understood, but several hypotheses have been proposed.
| Factors |
| Underlying medical conditions |
| Chronic HBV infection |
| Hemodialysis |
| Immature neonates |
| HIV infection |
| Immunosuppressor administration |
| Genetic factors |
| HLA haplotypes |
| Celiac disease |
| SNPs (cytokine, chemokine) |
| Technical errors |
| Intra-gluteal injection |
| Inappropriate storage conditions |
Because human leukocyte antigen (HLA) alleles are one of the determinants of the repertoire of peptides presented to T cells, HLA alleles are associated with response to HBV vaccine.
A report from eastern Turkey demonstrated that high frequencies of HLA-A11 and HLA-A24 and a low frequency of HLA-CW6 were associated with nonresponse to HB vaccine[43]. Another report from Turkey showed an association between HLA-DR (HLA-DRB1*04X, DRB1*0401X, DRB1*11/13, and DRB1*0401X0201) haplotypes and non-response, whereas Class-I (HLA-B13) was associated with a good response to HB vaccine[44]. A report from Italy showed that the haplotypes HLA-B8, DR3, and DQ2 were associated with nonresponse to HB vaccine, both in patients with celiac disease and healthy subjects[45]. A recent report from Korea showed that B62, DRB1*07 and DRB1*08(-) were significantly associated with poor responses to vaccine[46]. A meta-analysis of the association between HLA alleles and response to HB vaccine in 2308 subjects, including responders, non-responders, and healthy subjects, found that the DRB1 alleles DRB1*01, DRB1*1301, and DRB1*15 were associated with good responses whereas DRB1 *03 (DRB1*0301), DRB1*04, DRB1*07, and DRB1*1302 were associated with poor responses[47]. Furthermore, evaluations of DQB1 alleles showed that DQB1*05 (DQB1*0501), DQB1*06, and DQB1*0602 were associated with good responses, whereas DQB1*02 was associated with poor response[47].
Associations between single nucleotide polymorphisms (SNPs) at the interleukin (IL)-2 and IL4 loci, along with insertion/deletion variants at the IL12B locus, have been associated with responses to HB vaccines[48]. Moreover, three SNPs (rs497916, rs3922, rs676925) in CXCR5 and one SNP (rs355687) in CXCL13 were associated with response to HB vaccine. These findings indicate that cytokines and chemokines are actively involved in responses to HB vaccine[49].
The poor responses of HIV-infected patients to HB vaccine were thought to be due to low numbers of CD4+ T cells[50]. However, the percent of regulatory T-cells was found to be negatively associated with response to HB vaccine in HIV-infected patients, suggesting another mechanism for low anti-HBs production in response to HB vaccine[51].
Hepatitis C virus (HCV)-infected patients have been reported to show a poorer response to HB vaccine than healthy subjects[52]. This may be due to the differential regulation of IL-12/IL-23 production secondary to overexpression of T cell immunoglobulin mucin-domain-3 on monocytes[53].
Attempts to improve the efficacy of HB vaccine: Because the existence of non-responders to HB vaccine is a serious social problem, several attempts have been made to improve the efficacy of vaccine. Persons unresponsive to a first series of three injections (i.e., anti-HBs < 10 mIU/mL) are recommended to complete a second 3-dose vaccine series, with about 50% of these individuals showing an anti-HBs response[16,54]. Non-responders to the second course should be evaluated for underlying chronic HBV infection. Response in patients on hemodialysis may be improved using double-dose vaccine[55]. However, some individuals are non-responsive to multiple series of vaccinations, suggesting the need for other strategies to induce anti-HBs production in these subjects.
New HB vaccines have been developed to improve anti-HBs response in non-responders. HepageneTM, a third generation vaccine containing pre-S1 and pre-S2 proteins in addition to S protein, has been shown to induce anti-HBs in 76% of previously non-responsive individuals[56]. Another third generation HB vaccine, Sci-B-Vac™, was recently shown to induce anti-HBs in 20 of 21 non- or low-responders, with 12 non-responders and all 6 low responders showing a high anti-HBs response (> 100 mIU/mL)[57]. Moreover, the preventive effect of Sci-B-Vac™ has been assessed in newborns[58,59].
Granulocyte-colony stimulating factor: Recently, HB vaccine was administered together with granulocyte-colony stimulating factor (G-CSF) to patients with liver cirrhosis[60]. However, adjuvant G-CSF did not significantly enhance anti-HBs production.
Granulocyte-macrophage colony stimulating factor: The effect of granulocyte-macrophage colony stimulating factor (GM-CSF) on the response to HBV revaccination in non-responders has been evaluated[61]. Although the combination of GM-CSF (150 μg) and HB vaccine (20 μg) induced a higher anti-HBs response than HB vaccine (20 μg) alone, a higher dose (40 μg) of HB vaccine induced a higher response than the combination. GM-CSF was effective in patients with end-stage renal disease[62], but was ineffective during booster vaccination of HIV-infected patients with low anti-HBs antibody titer[63]. Thus, the stimulatory effect of GM-CSF has not yet been determined.
Interleukin (IL)-2 was not effective as an adjuvant[64,65]. In contrast, levamisole used as an adjuvant in HB vaccination has shown promising results[66,67]. New chemical adjuvants [AS02(v) and AS04] have also been found to increase response to HB vaccine[68-70].
HB vaccine is usually given intramuscularly, but intradermal vaccination was shown to be superior[71]. Intradermal vaccination induced higher anti-HBs positivity rates or a similar rate as intramuscular vaccine with smaller doses of vaccine[72]. The ability of intradermal administration to improve the effectiveness of vaccination has been tested, but intradermal vaccination is technically difficult[73].
Intradermal administration every two weeks into non-responders to conventional intramuscular vaccination induced anti-HBs responses in 94% of the subjects[74]. Intradermal vaccination has induced better responses to HB vaccine than conventional intramuscular vaccination in healthcare workers[75,76], dialysis patients[77-79], HIV infected patients[80], and patients with celiac disease[81]. A comprehensive review of the efficacy of intradermal HB vaccination in non-responders found that this method induced a superior response compared with intramuscular vaccination under various conditions[82].
Several studies have shown that the protective effects of HB vaccination may continue for at least 10 to 15 years[83,84] or even beyond 15 years, especially in subjects with a high anti-HBs titer after the initial course of vaccination[85]. Routine booster vaccinations have been regarded as unnecessary, due to the immunological memory acquired after the initial round of HB vaccination[86,87], and booster vaccination of previously vaccinated individuals has shown to result in a rapid increase in anti-HBs antibody[88]. However, the long-term protective effects of HB vaccination have not been fully investigated. Of 6156 high school students vaccinated during infancy, approximately 10% to 25% lost immune responses to HBsAg[86,87]. A recent report showed that 17.8% of vaccinated students born to highly infectious mothers positive for HBeAg became HBsAg-positive, compared with 11.1% of those born to maternal HBeAg-negative (P = 0.014), suggesting that booster vaccination may be needed for complete prevention of HBV infection[41].
Policies regarding booster vaccination vary among countries. In patients on hemodialysis, vaccine-induced antibody protection may persist only at anti-HBs titers above 10 mIU/mL, suggesting that a booster be administered if the antibody level declines to below 10 mIU/mL[89]. A European consensus statement also recommended booster vaccination if antibody concentration declines to below 10 mIU/mL, depending on the risk of exposure to HBV (Table 3)[90].
| Individuals | Booster recommendation |
| Health-care workers | Single dose if anti-HBs < 10 mIU/mL |
| Men who have sex with men | Ensure primary vaccination, boosters are unnecessary |
| Persons with multiple sexual partners | Ensure primary vaccination, boosters are unnecessary |
| Injection drug users | No evidence to support booster vaccinations |
| Patients with hemodialysis | Additional boosters to maintain anti-HBs > 10 mIU/mL |
| Institutionalized patients | Not recommended |
| Public safety workers | Single dose if anti-HBs < 10 mIU/mL |
| Spouse, sexual partners and household members of HBV carriers | Receive primary vaccination, boosters are unnecessary |
| Recipients of liver transplantation | Additional boosters to maintain anti-HBs > 10 mIU/mL |
In liver transplantation, it was shown that 18 out of 23 recipients from isolated anti-HBc-positive donors developed HBV infection after liver transplantation[91], suggesting a recommendation of booster vaccination to acquire anti-HBs levels above 10 mIU/mL in all HBsAg-negative recipients before transplantation even if the donors are negative for HBsAg.
Mutations in the small-S protein, most frequently a glycine to arginine substitution at codon 145 (G145R), have been found in some children born to HBV-infected mothers who were found to be infected despite previous vaccination[92,93]. Viruses containing the G145R mutant have been shown to be infectious in chimpanzees[94]. Other S-gene mutations have been identified in codons 120-147, and these mutations may evade neutralizing anti-HBs and infect vaccinated people[95-97]. In Italy, S-gene mutations, such as G145R, P120S and P127S, have been identified in liver transplant patients and in children born to HBsAg carriers and treated with HB vaccine. Although these mutants have been found in many regions around the world, including Taiwan[95], their prevalence appears to be low and constant, and reductions in the efficacy of HB vaccine have not been observed[97,98]. However, a recent report showed that HBV containing G145R had been transmitted by sexual contact to a subject who had received universal HB vaccination[39]. Recently, a vaccinated individual was found to have developed acute hepatitis B, caused by a vaccine-escape mutant[99]. Mathematical modeling predicted that over 50 years were required before any vaccine-escape mutants become predominant[100]. Continued monitoring is necessary to determine if the prevalence of these mutants is increasing and if the protective efficacy of conventional vaccines is maintained. Strategies for practical application of HB vaccine containing pre-S protein should be investigated.
HBV strains have been classified into eight genotypes[101,102], with the prevalence of different genotypes varying geographically[103]. However, infection with an HBV genotype different from the native strain has increased in various regions, resulting in the progressive globalization of HBV infection. For example, infection with HBV genotype A strains from foreign countries and subsequent acute hepatitis are increasing in Japan, in which HBV genotype C is the major strain[104]. Infections of Japanese individuals with non-native strains have mainly occurred in adults through sexual contact[104], and UV has not yet been introduced in Japan (Figure 1). Recombinant vaccines generated from HBsAg of HBV genotype A2 have been used worldwide. Although these A2-type vaccines were suggested to be effective in preventing non-A2 HBV infection[105], this cross-genotype preventive effect has not been fully investigated. Using an animal model, we recently showed that monoclonal antibodies derived from vaccines with genotype C could prevent infection by HBV genotype A[106]. Further investigations and careful observation are required for cross-genotype prevention of HB vaccine.
First, 5% to 10% of vaccinated healthy subjects are non-responders, and dialysis patients, HIV-infected patients and patients with celiac disease show poorer response to HB vaccine than healthy subjects. Booster vaccination overcomes the non-response in some of these subjects, but a third generation HB vaccine, intradermal vaccination or vaccination with adjuvants may be more effective. Methods are therefore needed to develop anti-HBs responses in non-responders.
Anti-HBs response gradually decreases after a single course of vaccination. However, there are no standard guidelines for the necessity, timing, or method of booster vaccination under various situations.
S-gene mutant HBV may escape from HB vaccination with S-protein alone. To prevent infection and spread of HBV with S-mutation, the effectiveness of third generation HB vaccines containing pre-S proteins in addition to S-protein should be determined.
Several countries have not yet introduced UV. Promotion of UV in these countries is mandatory for global eradication of HBV infection.
The significance of co-administration of HBIG with HB vaccine for prevention of mother-to-infant transmission of HBV needs to be fully evaluated. Although the administration of HBIG was shown to prevent intrauterine transmission of HBV and reduce overt infantile hepatitis[107,108], appropriate randomized control trials should be performed in the future.
HBV infection remains a serious problem worldwide, and vaccination is the most effective strategy for primary prevention of the infection. Although UV is needed for global eradication of HBV infection, it has not yet been introduced in many countries, including Japan. Third generation vaccines with pre-S proteins have shown better anti-HBs responses even in non-responders, but data on their efficacy are limited. Third generation HB vaccines may also be effective in preventing infection with HBV containing an S-mutation. Strategies are needed to improve vaccine efficacy in non-responders, whether with new vaccines or adjuvants, or by intradermal vaccination. Moreover, guidelines are needed regarding the necessity, timing and method of booster injection of responders with decreased anti-HBs response.
P- Reviewer: Chiu KW, Frider B S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | McMahon BJ. Epidemiology and natural history of hepatitis B. Semin Liver Dis. 2005;25 Suppl 1:3-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 313] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 2. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1734] [Cited by in F6Publishing: 1694] [Article Influence: 84.7] [Reference Citation Analysis (0)] |
| 3. | Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2651] [Cited by in F6Publishing: 2570] [Article Influence: 111.7] [Reference Citation Analysis (0)] |
| 4. | Leung N. Chronic hepatitis B in Asian women of childbearing age. Hepatol Int. 2009;3 Suppl 1:24-31. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Xu DZ, Yan YP, Choi BC, Xu JQ, Men K, Zhang JX, Liu ZH, Wang FS. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol. 2002;67:20-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 205] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 6. | Bai H, Zhang L, Ma L, Dou XG, Feng GH, Zhao GZ. Relationship of hepatitis B virus infection of placental barrier and hepatitis B virus intra-uterine transmission mechanism. World J Gastroenterol. 2007;13:3625-3630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 66] [Cited by in F6Publishing: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 7. | Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis. 1994;170:1418-1423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 153] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 8. | Li XM, Shi MF, Yang YB, Shi ZJ, Hou HY, Shen HM, Teng BQ. Effect of hepatitis B immunoglobulin on interruption of HBV intrauterine infection. World J Gastroenterol. 2004;10:3215-3217. [PubMed] [Cited in This Article: ] |
| 9. | Li XM, Yang YB, Hou HY, Shi ZJ, Shen HM, Teng BQ, Li AM, Shi MF, Zou L. Interruption of HBV intrauterine transmission: a clinical study. World J Gastroenterol. 2003;9:1501-1503. [PubMed] [Cited in This Article: ] |
| 10. | Deng M, Zhou X, Gao S, Yang SG, Wang B, Chen HZ, Ruan B. The effects of telbivudine in late pregnancy to prevent intrauterine transmission of the hepatitis B virus: a systematic review and meta-analysis. Virol J. 2012;9:185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 11. | Pan CQ, Han GR, Jiang HX, Zhao W, Cao MK, Wang CM, Yue X, Wang GJ. Telbivudine prevents vertical transmission from HBeAg-positive women with chronic hepatitis B. Clin Gastroenterol Hepatol. 2012;10:520-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 129] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 12. | Romano' L, Paladini S, Van Damme P, Zanetti AR. The worldwide impact of vaccination on the control and protection of viral hepatitis B. Dig Liver Dis. 2011;43 Suppl 1:S2-S7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Szmuness W, Stevens CE, Zang EA, Harley EJ, Kellner A. A controlled clinical trial of the efficacy of the hepatitis B vaccine (Heptavax B): a final report. Hepatology. 1981;1:377-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 334] [Cited by in F6Publishing: 293] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | McAleer WJ, Buynak EB, Maigetter RZ, Wampler DE, Miller WJ, Hilleman MR. Human hepatitis B vaccine from recombinant yeast. Nature. 1984;307:178-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 541] [Cited by in F6Publishing: 471] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 15. | Stephenne J. Development and production aspects of a recombinant yeast-derived hepatitis B vaccine. Vaccine. 1990;8 Suppl:S69-S73; discussion S79-S80. [PubMed] [Cited in This Article: ] |
| 16. | Availability of hepatitis B vaccine that does not contain thimerosal as a preservative. MMWR Morb Mortal Wkly Rep. 1999;48:780-782. [Cited in This Article: ] |
| 17. | Young MD, Schneider DL, Zuckerman AJ, Du W, Dickson B, Maddrey WC. Adult hepatitis B vaccination using a novel triple antigen recombinant vaccine. Hepatology. 2001;34:372-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Mahoney FJ, Woodruff BA, Erben JJ, Coleman PJ, Reid EC, Schatz GC, Kane MA. Effect of a hepatitis B vaccination program on the prevalence of hepatitis B virus infection. J Infect Dis. 1993;167:203-207. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Rendi-Wagner P, Kundi M, Stemberger H, Wiedermann G, Holzmann H, Hofer M, Wiesinger K, Kollaritsch H. Antibody-response to three recombinant hepatitis B vaccines: comparative evaluation of multicenter travel-clinic based experience. Vaccine. 2001;19:2055-2060. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Abraham B, Parenti D. Antibody production in response to hepatitis B surface antigen in a combination hepatitis A/hepatitis B vaccine. J Infect Dis. 2000;182:1005-1006. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Jackson Y, Chappuis F, Mezger N, Kanappa K, Loutan L. High immunogenicity of delayed third dose of hepatitis B vaccine in travellers. Vaccine. 2007;25:3482-3484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Greenberg DP. Pediatric experience with recombinant hepatitis B vaccines and relevant safety and immunogenicity studies. Pediatr Infect Dis J. 1993;12:438-445. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 71] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 23. | Hoofnagle JH. Toward universal vaccination against hepatitis B virus. N Engl J Med. 1989;321:1333-1334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Hepatitis B. Available from: http://www.who.int/mediacentre/factsheets/fs204/en/. [Cited in This Article: ] |
| 25. | Immunization Coverage. Available from: http://www.who.int/mediacentre/factsheets/fs378/en/. [Cited in This Article: ] |
| 26. | Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Hepatitis B immunisation for newborn infants of hepatitis B surface antigen-positive mothers. Cochrane Database Syst Rev. 2006;CD004790. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 74] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Ni YH, Huang LM, Chang MH, Yen CJ, Lu CY, You SL, Kao JH, Lin YC, Chen HL, Hsu HY. Two decades of universal hepatitis B vaccination in taiwan: impact and implication for future strategies. Gastroenterology. 2007;132:1287-1293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 224] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 28. | Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med. 1997;336:1855-1859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1328] [Cited by in F6Publishing: 1350] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 29. | Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, Chu HC, Wu TC, Yang SS, Kuo HS. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: a 20-year follow-up study. J Natl Cancer Inst. 2009;101:1348-1355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 433] [Cited by in F6Publishing: 393] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 30. | Ni YH, Chang MH, Wu JF, Hsu HY, Chen HL, Chen DS. Minimization of hepatitis B infection by a 25-year universal vaccination program. J Hepatol. 2012;57:730-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 156] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 31. | Viviani S, Jack A, Hall AJ, Maine N, Mendy M, Montesano R, Whittle HC. Hepatitis B vaccination in infancy in The Gambia: protection against carriage at 9 years of age. Vaccine. 1999;17:2946-2950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 110] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | van der Sande MA, Waight P, Mendy M, Rayco-Solon P, Hutt P, Fulford T, Doherty C, McConkey SJ, Jeffries D, Hall AJ. Long-term protection against carriage of hepatitis B virus after infant vaccination. J Infect Dis. 2006;193:1528-1535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 103] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 33. | McMahon BJ, Bulkow LR, Singleton RJ, Williams J, Snowball M, Homan C, Parkinson AJ. Elimination of hepatocellular carcinoma and acute hepatitis B in children 25 years after a hepatitis B newborn and catch-up immunization program. Hepatology. 2011;54:801-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 140] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 34. | McMahon BJ, Dentinger CM, Bruden D, Zanis C, Peters H, Hurlburt D, Bulkow L, Fiore AE, Bell BP, Hennessy TW. Antibody levels and protection after hepatitis B vaccine: results of a 22-year follow-up study and response to a booster dose. J Infect Dis. 2009;200:1390-1396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 35. | Daniels D, Grytdal S, Wasley A. Surveillance for acute viral hepatitis - United States, 2007. MMWR Surveill Summ. 2009;58:1-27. [PubMed] [Cited in This Article: ] |
| 36. | Ng KP, Saw TL, Baki A, Rozainah K, Pang KW, Ramanathan M. Impact of the Expanded Program of Immunization against hepatitis B infection in school children in Malaysia. Med Microbiol Immunol. 2005;194:163-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Bonanni P, Pesavento G, Bechini A, Tiscione E, Mannelli F, Benucci C, Nostro AL. Impact of universal vaccination programmes on the epidemiology of hepatitis B: 10 years of experience in Italy. Vaccine. 2003;21:685-691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 38. | Mele A, Tosti ME, Mariano A, Pizzuti R, Ferro A, Borrini B, Zotti C, Lopalco P, Curtale F, Balocchini E. Acute hepatitis B 14 years after the implementation of universal vaccination in Italy: areas of improvement and emerging challenges. Clin Infect Dis. 2008;46:868-875. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 39. | Stramer SL, Wend U, Candotti D, Foster GA, Hollinger FB, Dodd RY, Allain JP, Gerlich W. Nucleic acid testing to detect HBV infection in blood donors. N Engl J Med. 2011;364:236-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 227] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 40. | Lai MW, Lin TY, Tsao KC, Huang CG, Hsiao MJ, Liang KH, Yeh CT. Increased seroprevalence of HBV DNA with mutations in the s gene among individuals greater than 18 years old after complete vaccination. Gastroenterology. 2012;143:400-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Wu TW, Lin HH, Wang LY. Chronic hepatitis B infection in adolescents who received primary infantile vaccination. Hepatology. 2013;57:37-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 42. | Averhoff F, Mahoney F, Coleman P, Schatz G, Hurwitz E, Margolis H. Immunogenicity of hepatitis B Vaccines. Implications for persons at occupational risk of hepatitis B virus infection. Am J Prev Med. 1998;15:1-8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 183] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Albayrak A, Ertek M, Tasyaran MA, Pirim I. Role of HLA allele polymorphism in chronic hepatitis B virus infection and HBV vaccine sensitivity in patients from eastern Turkey. Biochem Genet. 2011;49:258-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 44. | Mert G, Sengul A, Gul HC, Karakas A, Eyigun CP. The role of human leukocyte antigen tissue groups in hepatitis B virus vaccination in Turkey. J Microbiol Immunol Infect. 2014;47:9-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Vitaliti G, Praticò AD, Cimino C, Di Dio G, Lionetti E, La Rosa M, Leonardi S. Hepatitis B vaccine in celiac disease: yesterday, today and tomorrow. World J Gastroenterol. 2013;19:838-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 28] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Yoon JH, Shin S, In Jw, Chang JY, Song EY, Roh EY. Association of HLA alleles with the responsiveness to hepatitis B virus vaccination in Korean infants. Vaccine. 2014;32:5638-5644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 47. | Li ZK, Nie JJ, Li J, Zhuang H. The effect of HLA on immunological response to hepatitis B vaccine in healthy people: a meta-analysis. Vaccine. 2013;31:4355-4361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 48. | Wang C, Tang J, Song W, Lobashevsky E, Wilson CM, Kaslow RA. HLA and cytokine gene polymorphisms are independently associated with responses to hepatitis B vaccination. Hepatology. 2004;39:978-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 147] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 49. | Duan Z, Chen X, Liang Z, Zeng Y, Zhu F, Long L, McCrae MA, Zhuang H, Shen T, Lu F. Genetic polymorphisms of CXCR5 and CXCL13 are associated with non-responsiveness to the hepatitis B vaccine. Vaccine. 2014;32:5316-5322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 50. | Kim HN, Harrington RD, Van Rompaey SE, Kitahata MM. Independent clinical predictors of impaired response to hepatitis B vaccination in HIV-infected persons. Int J STD AIDS. 2008;19:600-604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | del Pozo Balado Mdel M, Leal M, Méndez Lagares G, Mata RC, López-Cortés LF, Viciana P, Pacheco YM. Increased regulatory T cell counts in HIV-infected nonresponders to hepatitis B virus vaccine. J Infect Dis. 2010;202:362-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Shi L, Wang JM, Ren JP, Cheng YQ, Ying RS, Wu XY, Lin SM, Griffin JW, Li GY, Moorman JP. KLRG1 impairs CD4+ T cell responses via p16ink4a and p27kip1 pathways: role in hepatitis B vaccine failure in individuals with hepatitis C virus infection. J Immunol. 2014;192:649-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Wang JM, Ma CJ, Li GY, Wu XY, Thayer P, Greer P, Smith AM, High KP, Moorman JP, Yao ZQ. Tim-3 alters the balance of IL-12/IL-23 and drives TH17 cells: role in hepatitis B vaccine failure during hepatitis C infection. Vaccine. 2013;31:2238-2245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Tan KL, Goh KT, Oon CJ, Chan SH. Immunogenicity of recombinant yeast-derived hepatitis B vaccine in nonresponders to perinatal immunization. JAMA. 1994;271:859-861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 55. | Rangel MC, Coronado VG, Euler GL, Strikas RA. Vaccine recommendations for patients on chronic dialysis. The Advisory Committee on Immunization Practices and the American Academy of Pediatrics. Semin Dial. 2000;13:101-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 56. | McDermott AB, Cohen SB, Zuckerman JN, Madrigal JA. Hepatitis B third-generation vaccines: improved response and conventional vaccine non-response--evidence for genetic basis in humans. J Viral Hepat. 1998;5 Suppl 2:9-11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Krawczyk A, Ludwig C, Jochum C, Fiedler M, Heinemann FM, Shouval D, Roggendorf M, Roggendorf H, Lindemann M. Induction of a robust T- and B-cell immune response in non- and low-responders to conventional vaccination against hepatitis B by using a third generation PreS/S vaccine. Vaccine. 2014;32:5077-5082. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 58. | Hellström UB, Madalinski K, Sylvan SP. PreS1 epitope recognition in newborns after vaccination with the third-generation Sci-B-Vac vaccine and their relation to the antibody response to hepatitis B surface antigen. Virol J. 2009;6:7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Sylvan SP, Madalinski K, Hellström UB. Anti-preS responses influence the anti-HBs response in newborns after vaccination with the third generation Sci-B-Vac vaccine. Vaccine. 2009;28:446-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Lankarani KB, Talebzadeh M, Eshraghian A, Malek-Hosseini SA. Granulocyte colony stimulating factor adjuvant role on the immunological response to hepatitis B vaccine in patients with cirrhosis: a double blind randomized placebo controlled trial. Hepat Mon. 2014;14:e15447. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Lin C, Zhu J, Zheng Y, Chen Y, Wu Z, Chong Y, Gao Z. Effect of GM-CSF in combination with hepatitis B vaccine on revacination of healthy adult non-responders. J Infect. 2010;60:264-270. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 62. | Fabrizi F, Ganeshan SV, Dixit V, Martin P. Meta-analysis: the adjuvant role of granulocyte macrophage-colony stimulating factor on immunological response to hepatitis B virus vaccine in end-stage renal disease. Aliment Pharmacol Ther. 2006;24:789-796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 63. | Overton ET, Sungkanuparph S, Klebert M, Royal M, Demarco-Shaw D, Powderly WG, Aberg JA. GM-CSF Fails to Improve Immune Responses to Booster Hepatitis B Vaccination in HIV-Infected Individuals. Open Virol J. 2011;5:109-113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 64. | Jungers P, Devillier P, Salomon H, Cerisier JE, Courouce AM. Randomised placebo-controlled trial of recombinant interleukin-2 in chronic uraemic patients who are non-responders to hepatitis B vaccine. Lancet. 1994;344:856-857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 65. | Mauri JM, Vallès M. Effects of recombinant interleukin-2 and revaccination for hepatitis B in previously vaccinated, non-responder, chronic uraemic patients. Collaborative Group of Girona. Nephrol Dial Transplant. 1997;12:729-732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 30] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 66. | Fabrizi F, Dixit V, Martin P, Messa P. Hepatitis C virus and the immunological response to hepatitis B virus vaccine in dialysis patients: meta-analysis of clinical studies. J Viral Hepat. 2011;18:871-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 67. | Alavian SM, Tabatabaei SV. Effects of oral levamisole as an adjuvant to hepatitis B vaccine in adults with end-stage renal disease: a meta-analysis of controlled clinical trials. Clin Ther. 2010;32:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | Tong NK, Beran J, Kee SA, Miguel JL, Sánchez C, Bayas JM, Vilella A, de Juanes JR, Arrazola P, Calbo-Torrecillas F. Immunogenicity and safety of an adjuvanted hepatitis B vaccine in pre-hemodialysis and hemodialysis patients. Kidney Int. 2005;68:2298-2303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 145] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 69. | Kong NC, Beran J, Kee SA, Miguel JL, Sánchez C, Bayas JM, Vilella A, Calbo-Torrecillas F, López de Novales E, Srinivasa K. A new adjuvant improves the immune response to hepatitis B vaccine in hemodialysis patients. Kidney Int. 2008;73:856-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 70. | Tielemans CL, Vlasak J, Kosa D, Billiouw JM, Verpooten GA, Mezei I, Ryba M, Peeters PC, Mat O, Jadoul MY. Immunogenicity and safety of an investigational AS02(v)-adjuvanted hepatitis B vaccine in patients with renal insufficiency who failed to respond or to maintain antibody levels after prior vaccination: results of two open, randomized, comparative trials. Vaccine. 2011;29:1159-1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Yasumura S, Shimizu Y, Yasuyama T, Hiroki O, Okada K, Tsukishiro T, Tsuchida T, Higuchi K, Watanabe A. Intradermal hepatitis B virus vaccination for low- or non-responded health-care workers. Acta Med Okayama. 1991;45:457-459. [PubMed] [Cited in This Article: ] |
| 72. | Das HS, Sawant P, Shirhatti RG, Vyas K, Vispute S, Dhadphale S, Patrawalla V, Desai N. Efficacy of low dose intradermal hepatitis B vaccine: results of a randomized trial among health care workers. Trop Gastroenterol. 2002;23:120-121. [PubMed] [Cited in This Article: ] |
| 73. | Fabrizi F, Andrulli S, Bacchini G, Corti M, Locatelli F. Intradermal versus intramuscular hepatitis b re-vaccination in non-responsive chronic dialysis patients: a prospective randomized study with cost-effectiveness evaluation. Nephrol Dial Transplant. 1997;12:1204-1211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 72] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 74. | Playford EG, Hogan PG, Bansal AS, Harrison K, Drummond D, Looke DF, Whitby M. Intradermal recombinant hepatitis B vaccine for healthcare workers who fail to respond to intramuscular vaccine. Infect Control Hosp Epidemiol. 2002;23:87-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 75. | Levitz RE, Cooper BW, Regan HC. Immunization with high-dose intradermal recombinant hepatitis B vaccine in healthcare workers who failed to respond to intramuscular vaccination. Infect Control Hosp Epidemiol. 1995;16:88-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 76. | Ghebrehewet S, Baxter D, Falconer M, Paver K. Intradermal recombinant hepatitis B vaccination (IDRV) for non-responsive healthcare workers (HCWs). Hum Vaccin. 2008;4:280-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 77. | Fabrizi F, Dixit V, Messa P, Martin P. Intradermal vs intramuscular vaccine against hepatitis B infection in dialysis patients: a meta-analysis of randomized trials. J Viral Hepat. 2011;18:730-737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 78. | Barraclough KA, Wiggins KJ, Hawley CM, van Eps CL, Mudge DW, Johnson DW, Whitby M, Carpenter S, Playford EG. Intradermal versus intramuscular hepatitis B vaccination in hemodialysis patients: a prospective open-label randomized controlled trial in nonresponders to primary vaccination. Am J Kidney Dis. 2009;54:95-103. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Chanchairujira T, Chantaphakul N, Thanwandee T, Ong-Ajyooth L. Efficacy of intradermal hepatitis B vaccination compared to intramuscular vaccination in hemodialysis patients. J Med Assoc Thai. 2006;89 Suppl 2:S33-S40. [PubMed] [Cited in This Article: ] |
| 80. | Whitaker JA, Rouphael NG, Edupuganti S, Lai L, Mulligan MJ. Strategies to increase responsiveness to hepatitis B vaccination in adults with HIV-1. Lancet Infect Dis. 2012;12:966-976. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 81. | Leonardi S, Praticò AD, Lionetti E, Spina M, Vitaliti G, La Rosa M. Intramuscular vs intradermal route for hepatitis B booster vaccine in celiac children. World J Gastroenterol. 2012;18:5729-5733. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 82. | Filippelli M, Lionetti E, Gennaro A, Lanzafame A, Arrigo T, Salpietro C, La Rosa M, Leonardi S. Hepatitis B vaccine by intradermal route in non responder patients: an update. World J Gastroenterol. 2014;20:10383-10394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 57] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 83. | Wu JS, Hwang LY, Goodman KJ, Beasley RP. Hepatitis B vaccination in high-risk infants: 10-year follow-up. J Infect Dis. 1999;179:1319-1325. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 81] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 84. | Zanetti AR, Mariano A, Romanò L, D’Amelio R, Chironna M, Coppola RC, Cuccia M, Mangione R, Marrone F, Negrone FS. Long-term immunogenicity of hepatitis B vaccination and policy for booster: an Italian multicentre study. Lancet. 2005;366:1379-1384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 256] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 85. | Chaves SS, Fischer G, Groeger J, Patel PR, Thompson ND, Teshale EH, Stevenson K, Yano VM, Armstrong GL, Samandari T. Persistence of long-term immunity to hepatitis B among adolescents immunized at birth. Vaccine. 2012;30:1644-1649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 86. | Lu CY, Ni YH, Chiang BL, Chen PJ, Chang MH, Chang LY, Su IJ, Kuo HS, Huang LM, Chen DS. Humoral and cellular immune responses to a hepatitis B vaccine booster 15-18 years after neonatal immunization. J Infect Dis. 2008;197:1419-1426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 87. | Jan CF, Huang KC, Chien YC, Greydanus DE, Davies HD, Chiu TY, Huang LM, Chen CJ, Chen DS. Determination of immune memory to hepatitis B vaccination through early booster response in college students. Hepatology. 2010;51:1547-1554. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 88. | Wiström J, Ahlm C, Lundberg S, Settergren B, Tärnvik A. Booster vaccination with recombinant hepatitis B vaccine four years after priming with one single dose. Vaccine. 1999;17:2162-2165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 89. | Propst T, Propst A, Lhotta K, Vogel W, König P. Reinforced intradermal hepatitis B vaccination in hemodialysis patients is superior in antibody response to intramuscular or subcutaneous vaccination. Am J Kidney Dis. 1998;32:1041-1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 90. | Are booster immunisations needed for lifelong hepatitis B immunity? European Consensus Group on Hepatitis B Immunity. Lancet. 2000;355:561-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 347] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 91. | Wachs ME, Amend WJ, Ascher NL, Bretan PN, Emond J, Lake JR, Melzer JS, Roberts JP, Tomlanovich SJ, Vincenti F. The risk of transmission of hepatitis B from HBsAg(-), HBcAb(+), HBIgM(-) organ donors. Transplantation. 1995;59:230-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 306] [Cited by in F6Publishing: 307] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 92. | Zanetti AR, Tanzi E, Manzillo G, Maio G, Sbreglia C, Caporaso N, Thomas H, Zuckerman AJ. Hepatitis B variant in Europe. Lancet. 1988;2:1132-1133. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 83] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 93. | Carman WF, Zanetti AR, Karayiannis P, Waters J, Manzillo G, Tanzi E, Zuckerman AJ, Thomas HC. Vaccine-induced escape mutant of hepatitis B virus. Lancet. 1990;336:325-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 817] [Cited by in F6Publishing: 749] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 94. | Ogata N, Zanetti AR, Yu M, Miller RH, Purcell RH. Infectivity and pathogenicity in chimpanzees of a surface gene mutant of hepatitis B virus that emerged in a vaccinated infant. J Infect Dis. 1997;175:511-523. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 69] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 95. | Hsu HY, Chang MH, Ni YH, Lin HH, Wang SM, Chen DS. Surface gene mutants of hepatitis B virus in infants who develop acute or chronic infections despite immunoprophylaxis. Hepatology. 1997;26:786-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 141] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 96. | Zuckerman AJ. Effect of hepatitis B virus mutants on efficacy of vaccination. Lancet. 2000;355:1382-1384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 124] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 97. | Hsu HY, Chang MH, Ni YH, Chen HL. Survey of hepatitis B surface variant infection in children 15 years after a nationwide vaccination programme in Taiwan. Gut. 2004;53:1499-1503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 173] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 98. | Hsu HY, Chang MH, Ni YH, Chiang CL, Chen HL, Wu JF, Chen PJ. No increase in prevalence of hepatitis B surface antigen mutant in a population of children and adolescents who were fully covered by universal infant immunization. J Infect Dis. 2010;201:1192-1200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 99. | Luongo M, Critelli R, Grottola A, Gitto S, Bernabucci V, Bevini M, Vecchi C, Montagnani G, Villa E. Acute hepatitis B caused by a vaccine-escape HBV strain in vaccinated subject: sequence analysis and therapeutic strategy. J Clin Virol. 2015;62:89-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 100. | Wilson JN, Nokes DJ, Carman WF. The predicted pattern of emergence of vaccine-resistant hepatitis B: a cause for concern? Vaccine. 1999;17:973-978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 68] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 101. | Kramvis A, Kew M, François G. Hepatitis B virus genotypes. Vaccine. 2005;23:2409-2423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 255] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 102. | Miyakawa Y, Mizokami M. Classifying hepatitis B virus genotypes. Intervirology. 2003;46:329-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 237] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 103. | Kurbanov F, Tanaka Y, Mizokami M. Geographical and genetic diversity of the human hepatitis B virus. Hepatol Res. 2010;40:14-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 104. | Matsuura K, Tanaka Y, Hige S, Yamada G, Murawaki Y, Komatsu M, Kuramitsu T, Kawata S, Tanaka E, Izumi N. Distribution of hepatitis B virus genotypes among patients with chronic infection in Japan shifting toward an increase of genotype A. J Clin Microbiol. 2009;47:1476-1483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 105. | Cassidy A, Mossman S, Olivieri A, De Ridder M, Leroux-Roels G. Hepatitis B vaccine effectiveness in the face of global HBV genotype diversity. Expert Rev Vaccines. 2011;10:1709-1715. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 106. | Hamada-Tsutsumi S, Iio E, Watanabe T, Murakami S, Isogawa M, Iijima S, Inoue T, Matsunami K, Tajiri K, Ozawa T. Validation of cross-genotype neutralization by hepatitis B virus-specific monoclonal antibodies by in vitro and in vivo infection. PLoS One. 2015;10:e0118062. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 107. | Chen HL, Lin LH, Hu FC, Lee JT, Lin WT, Yang YJ, Huang FC, Wu SF, Chen SC, Wen WH. Effects of maternal screening and universal immunization to prevent mother-to-infant transmission of HBV. Gastroenterology. 2012;142:773-781.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 108. | Shi Z, Li X, Ma L, Yang Y. Hepatitis B immunoglobulin injection in pregnancy to interrupt hepatitis B virus mother-to-child transmission-a meta-analysis. Int J Infect Dis. 2010;14:e622-e634. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |