Published online May 14, 2021. doi: 10.3748/wjg.v27.i18.2219
Peer-review started: January 25, 2021
First decision: February 27, 2021
Revised: March 13, 2021
Accepted: April 22, 2021
Article in press: April 22, 2021
Published online: May 14, 2021
Faecal microbiota transplantation (FMT) seems to be a promising treatment for irritable bowel syndrome (IBS) patients. In Western countries (United States and Europe), there is a female predominance in IBS. A sex difference in the response to FMT has been reported recently in IBS patients.
To investigate whether there was a sex difference in the response to FMT in the IBS patients who were included in our previous randomized controlled trial of the efficacy of FMT.
The study included 164 IBS patients who participated in our previous randomized controlled trial. These patients had moderate-to-severe IBS symptoms belonging to the IBS-D (diarrhoea-predominant), IBS-C (constipation-predominant) and IBS-M (mixed) subtypes, and had not responded to the National Institute for Health and Care Excellence (NICE)-modified diet. They belonged in three groups: placebo (own faeces), and active treated group (30-g or 60-g superdonor faeces). The patients completed the IBS severity scoring system (IBS-SSS), Fatigue Assessment Scale (FAS) and the IBS quality of life scale (IBS-QoL) questionnaires at the baseline and 2 wk, 1 mo and 3 mo after FMT. They also provided faecal samples at the baseline and 1 mo after FMT. The faecal bacteria profile and dysbiosis were determined using the 16S rRNA gene polymerase chain reaction DNA amplification covering V3-V9; probe labelling by single nucleotide extension and signal detection. The levels of short-chain fatty acids (SCFAs) were determined by gas chromatography and flame ionization.
There was no sex difference in the response to FMT either in the placebo group or active treated group. There was no difference between females and males in either the placebo group or actively treated groups in the total score on the IBS-SSS, FAS or IBS-QoL, in dysbiosis, or in the faecal bacteria or SCFA level. However, the response rate was significantly higher in females with diarrhoea-predominant (IBS-D) than that of males at 1 mo, and 3 mo after FMT. Moreover, IBS-SSS total score was significantly lower in female patients with IBS-D than that of male patients both 1 mo and 3 mo after FMT.
There was no sex difference in the response to FMT among IBS patients with moderate-to-severe symptoms who had previously not responded to NICE-modified diet. However, female patients with IBS-D respond better and have higher reduction of symptoms than males after FMT.
Core Tip: A sex difference in the response to faecal microbiota transplantation (FMT) was previous reported for a subgroup of refractory irritable bowel syndrome (IBS) patients with severe bloating who had not responded to at least three conventional therapies for IBS. This subgroup only contained patients with diarrhoea-predominant (IBS-D) or mixed (IBS-M) IBS. The present study found no sex difference in the response to FMT among IBS patients with moderate-to-severe symptoms of IBS-D, constipation-predominant (IBS-C) and IBS-M. However, female patients with IBS-D respond better and have higher reduction of symptoms than males after FMT.
- Citation: El-Salhy M, Casen C, Valeur J, Hausken T, Hatlebakk JG. Responses to faecal microbiota transplantation in female and male patients with irritable bowel syndrome. World J Gastroenterol 2021; 27(18): 2219-2237
- URL: https://www.wjgnet.com/1007-9327/full/v27/i18/2219.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i18.2219
The gut microbiota plays an important role in the pathophysiology of irritable bowel syndrome (IBS)[1,2]. The composition of the gut bacteria in IBS patients differs from that of healthy subjects[2-6]. IBS patients have lower abundances of the butyrate-producing bacteria, Erysipelotrichaceae and Ruminococcaceae compared with healthy controls[7,8]. Methane-producing bacteria, Methanobacteriales were found to be more abundant in IBS patients with constipation as a predominant symptom (IBS-C) and less abundant in IBS patients with diarrhoea as a predominant symptom (IBS-D) compared with healthy individuals[7,8]. Moreover, IBS patients have been found to have increased abundances of Veillonella, Lactobacillus and Ruminococcus bacteria and decreased abundances of Bifidobacterium, Faecalibacterium and Erysipelotrichaceae methanogens[7,8]. IBS patients also have a lower diversity of gut bacteria (dysbiosis) than healthy subjects[4-6,9].
Faecal microbiota transplantation (FMT) has previously been performed in IBS patients in seven randomized controlled trials (RCTs)[10-16]. Four of these RCTs showed that FMT had good effects on symptoms and the quality of life[10,12,15,16]. while the other three RCTs found no effects[11,13,14]. It soon became clear that carefully selecting the donor based on clinical and microbial criteria as well as the dose of the transplant are important for a successful outcome of FMT[17].
In Western countries (United States and Europe) there is a sex difference in IBS, with a female:male ratio of 2:1[18-20]. However, in Asia there is no such female predominance[21-24]. A recently published RCT on FMT in IBS found that females responded better to FMT than did males[16]. A recent RCT of IBS patients performed by our group found that FMT led to marked reductions in IBS symptoms and fatigue and an improvement in the quality of life[12]. These improvements were accompanied by marked changes in the faecal bacteria profile and the profile of short-chain fatty acids (SCFAs) of the patients[12,25].
The present study investigated whether there is a sex difference in the response to FMT in terms of symptoms, dysbiosis, and bacteria and SCFA profiles in the same cohort of patients that we had investigated in our previous study[12].
The design of this study has been described in detail previously[12]. In brief, patients completed three questionnaires to assess their symptoms and quality of life at the baseline and 2 wk, 1 mo and 3 mo after FMT. They also provided faecal samples at the baseline and 1 mo after FMT. Polyethylene glycol and loperamide were allowed as rescue medication during the study. The patients were randomized 1:1:1 to placebo (own faeces), 30-g (superdonor faeces) or 60-g (superdonor faeces) FMT[12]. The 30- and 60-g superdonor-faeces groups were pooled together and called the active treated group in order to increase the sample size and reduce the probability of type-II statistical errors.
This study included 164 patients who had participated in our previous study[12]. The characteristics of these patients are given in Table 1. The patients enrolled in this study have been described in detail previously[12]. In brief, patients attending the outpatient clinic at Stord Hospital who fulfilled the Rome IV criteria for a diagnosis of IBS were recruited. All of the recruited patients had previously not responded to consuming the National Institute for Health and Care Excellence (NICE)-modified diet for at least 3 mo[12]. They also received a course of IBS treatment that slightly improved their symptoms.
| Placebo | P value | Active treated | P value | |||||
| Total | Females | Males | Total | Females | Males | |||
| n | 55 | 47 | 8 | 109 | 85 | 24 | ||
| Age, yr (median, range) | 38.5 (18-75) | 38.0 (18-73) | 47.0 (20-75) | 0.3 | 39.0 (18-73) | 40.0 (18-73) | 32.0 (21-65) | 0.07 |
| IBS-D | 21 | 19 | 2 | 0.7 | 42 | 30 | 12 | 0.4 |
| IBS-C | 22 | 18 | 4 | 40 | 32 | 8 | ||
| IBS-M | 12 | 10 | 2 | 27 | 23 | 4 | ||
| IBS duration, yr | 15.5 ± 7.9 | 16.2 ± 8.0 | 15.0 ± 9.0 | 0.9 | 17.3 ± 8.9 | 16.8 ± 8.2 | 18.0 ± 9.2 | 0.9 |
| Age at IBS onset, yr (median, range) | 20.0 (15-35) | 20.5 (16-35) | 19.0 (15-30) | 0.4 | 20.0 (15-36) | 20.0 (16-35) | 20 (15-33) | 0.6 |
| IBS-SSS total score | 315.2 ± 77.1 | 320.1 ± 77.8 | 286.9 ± 69.3 | 0.5 | 312.9 ± 82.0 | 319.1 ± 77.3 | 297.7 ± 82.0 | 0.4 |
| Moderate symptoms1 (%) | 23 (42) | 17 (36) | 6 (75) | 0.06 | 45 (41) | 30 (35) | 13 (54) | 0.1 |
| Severe symptoms2 (%) | 32 (58) | 30 (64) | 2 (25) | 64 (59) | 55 (65) | 11 (46) | ||
The inclusion criteria were being aged between 18 and 75 years and having moderate-to-severe IBS symptoms, as indicated by a score of 175 on the IBS severity scoring system (IBS-SSS). The exclusion criteria were being pregnant or planning pregnancy, lactating, the presence of systemic disease, having immune deficiency or being treated by immune-modulating medication, or having a psychiatric illness, excessive alcohol consumption or drug abuse. Patients who took probiotics, antibiotics or IBS medications within 8 wk prior to study inclusion were also excluded[12].
The single superdonor used in this study has been described in detail previously[12]. Briefly, he was screened according to the European guidelines for FMT donors[26]. He was a healthy 36-year-old male, non-smoker, not taking any medication regularly and had a normal body mass index. He had been born via a vaginal delivery, breastfed and had taken only a few courses of antibiotics during his life. He exercised regularly and took sport-specific dietary supplements, which made his diet richer than average in protein, fibre, minerals and vitamins. He was normobiotic, but his faecal bacteria profile deviated from the healthy subjects abundance in 14 of the 39 bacteria markers[12].
Faecal samples were frozen immediately and kept at -20 °C until they were delivered frozen to the laboratory, where they were kept at -80 °C. The process of FMT has been described in detail previously[12]. In brief, the patients randomized to the placebo FMT group received 30 g of their own faeces (autologous), while those in the 30-g and 60-g FMT groups received 30 g and 60 g of the superdonor’s faeces (allogenic), respectively. The transplant was administered to the distal duodenum via a gastroscope[12].
Symptoms were assessed using the IBS-SSS and the Fatigue Assessment Scale (FAS)[27-31]. Quality of life was measured using the IBS quality of life scale (IBS-QoL)[32-34]. Response was defined as a decrease of ≥ 50 points in the IBS-SSS total score after FMT.
The faecal bacteria profile and dysbiosis were determined by the GA-map Dysbiosis Test (Genetic Analysis, Oslo, Norway) using the 16S rRNA gene polymerase chain reaction DNA amplification covering V3-V9; probe labelling by single nucleotide extension and signal detection by BioCode 1000A 128-Plex Analyzer (Applied BioCode, Santa Fe Springs, CA, United States)[6]. The bacterial markers used detected bacteria within 5 phyla (Firmicutes, Proteobacteria, Bacteroidetes, Tenericutes and Verrucomicrobia) that cover 10 bacterial classes, 36 genera and 32 species[6]. This test assesses > 300 bacteria at different taxonomic levels[9]. The dysbiosis index (DI) was measured on a 5-point scale from 1 to 5, where DI values 1-2 indicates normobiosis, 3-5 indicates dysbiosis[6].
The method used to determine faecal SCFA levels has been described in detail previously[25]. Briefly, the faecal samples were homogenized with a solution containing 3 mmol/L 2-ethylbutyric acid and 0.5 mmol/L H2SO4. The homogenate was vacuum distilled, and the SCFA levels were determined by gas chromatography (Agilent 7890 A, Agilent, CA, United States) using a capillary column (serial no. USE400345H, Agilent J&W GC columns, Agilent) and flame ionization[35,36] levels of total SCFAs, acetic, propionic, iso-butyric, n-butyric, iso-valeric, n-valeric acid, isocapronic and n-capronic acids, were determined and were expressed in units of mmol/kg wet weight.
The sample size required in each arm of the previously published trial was calculated by assuming that a placebo effect was 40% and an effect response was 80%. The total sample size was estimated to be 60 patients, with 20 in each arm (α = 0.05, 1-β = 0.80)[12]. In the present study a new calculation for the sample size was done based on the response rates obtained from our previous RCT[12]. Thus, assuming that the females’ response is 90% and males’ response is 60%, The total sample size was estimated to be 22 with 11 females and 11 males (α = 0.05, 1-β = 0.80). The 30- and 60-g superdonor-faeces groups were pooled together and called the active treated group in order to increase the sample size and reduce the probability of type-II statistical errors. Differences in response and dysbiosis between females and males in the placebo and the active treated group were analyzed using the χ2test. Differences between females and males in the total scores on the IBS-SSS, FAS and IBS-QoL, and in faecal bacteria and SCFA levels were analyzed using the Mann-Whitney test. These analyses were performed using GraphPad Prism (version 8, La Jolla, CA, United States).
The Regional Committee for Medical and Health Research Ethics West, Bergen, Norway approved the study (approval No. 2017/1197/REK vest). All subjects provided both oral and written consents to participate. The study was registered at www.clinicaltrials.gov (NCT03822299) and www.cristin.no (ID657402).
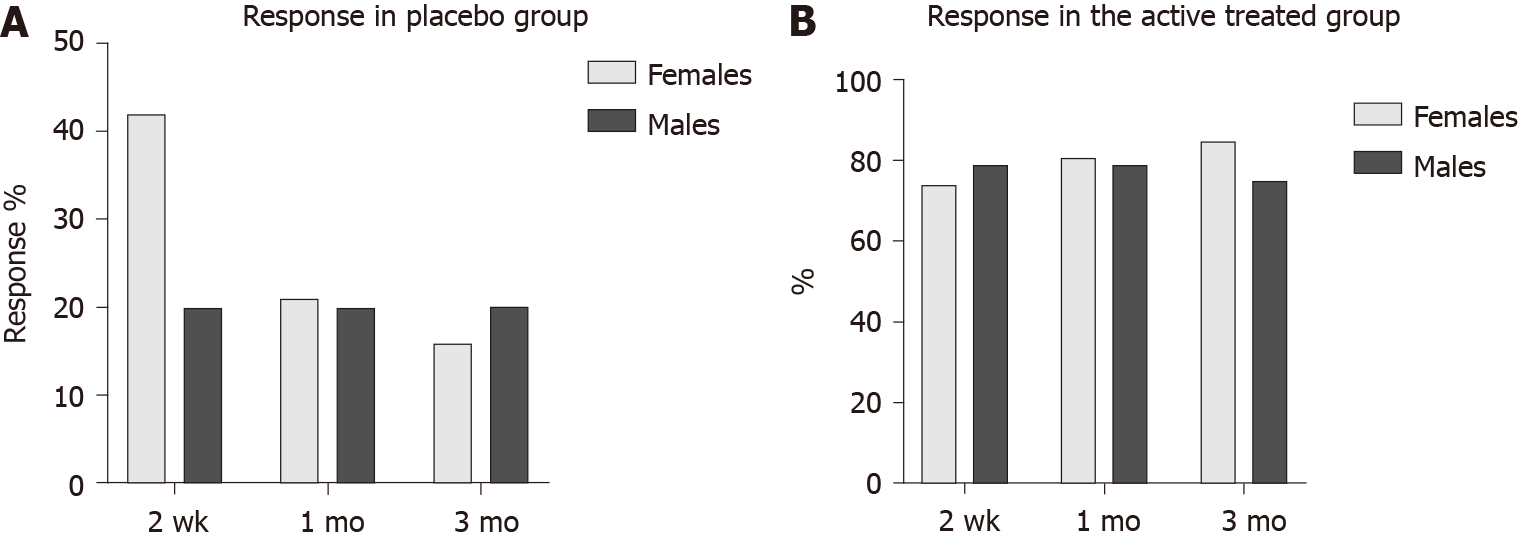
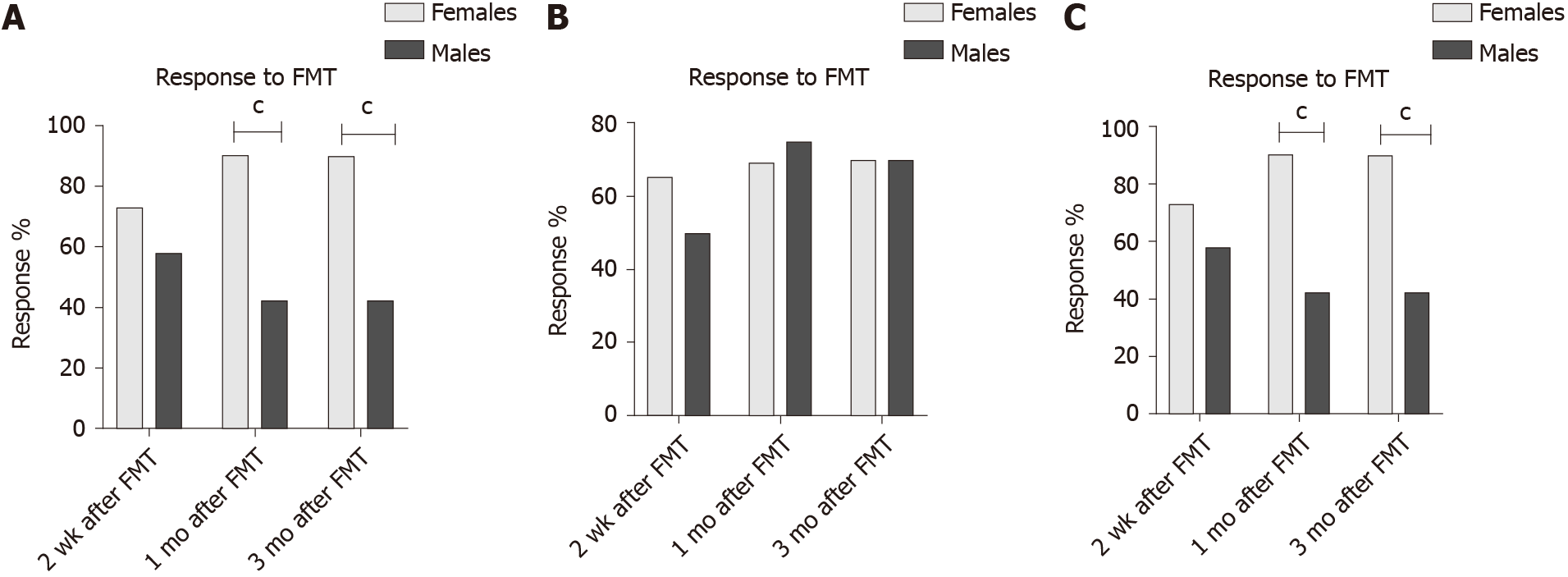
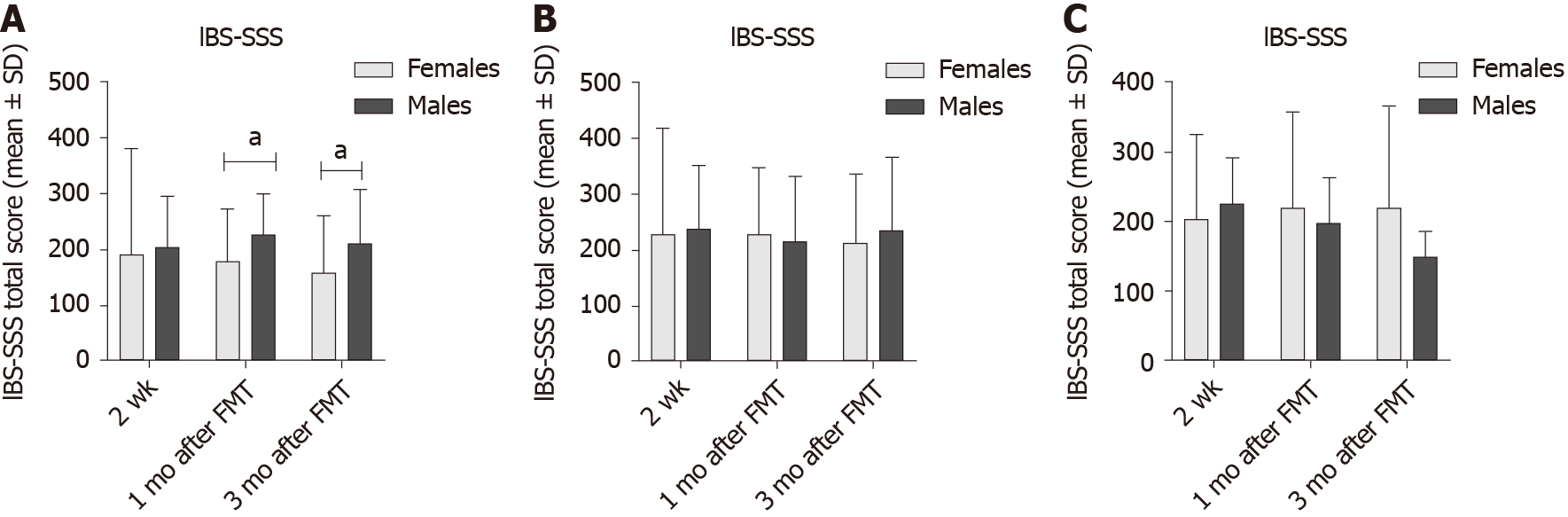
In the placebo group, the response did not differ between females and males at 2 wk, 1 mo and 3 mo after FMT (P = 0.4, 0.9 and 0.8, respectively). The responses in the active treated group did not differ between females and males after 2 wk, 1 mo and 3 mo (P = 0.6, 0.8 and 0.3, respectively) (Figure 1). The response rate was significantly higher in females with IBS-D than that of males at 1 mo, and 3 mo after FMT (Table 2 and Figure 2). There was no significant difference of response rates between female and male patients with either moderate or severe IBS symptoms (Table 3 and Figure 3).
| Time after FMT | IBS-D | IBS-C | IBS-M | ||||||
| Females | Males | P value | Females | Males | P value | Females | Males | P value | |
| 2 wk (%) | 73 | 58 | 0.3 | 65 | 50 | 0.7 | 72 | 55 | 0.3 |
| 1 mo (%) | 90 | 42 | 0.0003 | 69 | 75 | 0.7 | 65 | 60 | 0.9 |
| 3 mo (%) | 90 | 42 | 0.0003 | 70 | 75 | 0.3 | 63 | 80 | 0.6 |
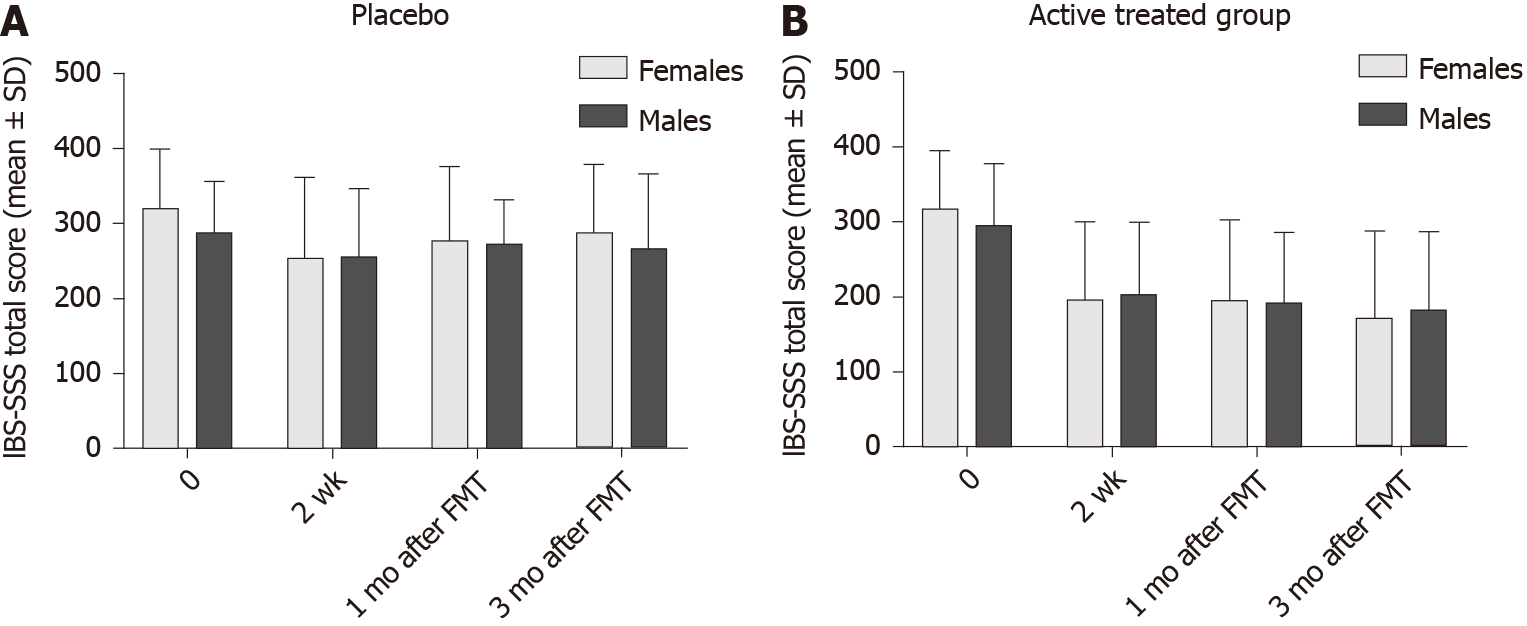
The IBS-SSS total score did not differ significantly between female and male IBS patients in either the placebo or the active treated group (Table 4 and Figure 4). However, IBS-SSS total score was significantly lower in female patients with IBS-D than that of male patients both 1 mo and 3 mo after FMT (Table 5 and Figure 5). The IBS-SSS total score did not differ significantly between females and males in patients with moderate or severe IBS symptoms (Table 6 and Figure 3).
| Time | Placebo | P value | Active treated | P value | ||
| Females | Males | Females | Males | |||
| 0 | 320 ± 78 | 287 ± 69 | 0.2 | 319 ± 77 | 297 ± 82 | 0.3 |
| 2 wk | 254 ± 106 | 256 ± 90 | 0.9 | 199 ± 102 | 205 ± 95 | 0.6 |
| 1 mo | 277 ± 98 | 272 ± 89 | 0.8 | 196 ± 108 | 193 ± 94 | 0.9 |
| 3 mo | 288 ± 90 | 266 ± 100 | 0.6 | 173 ± 116 | 183 ± 105 | 0.5 |
| Time after FMT | IBS-D | IBS-C | IBS-M | ||||||
| Females | Males | P value | Females | Males | P value | Females | Males | P value | |
| 2 wk | 190.5 ± 191.4 | 204.0 ± 92.2 | 0.6 | 228.1 ± 116.2 | 239.2 ± 113.8 | 0.5 | 202.8 ± 121.3 | 225.0 ± 65.8 | 0.5 |
| 1 mo | 177.8 ± 94.9 | 226.9 ± 73.3 | 0.02 | 228.8 ± 118.1 | 215.8 ± 115.7 | 0.6 | 219.9 ± 136.6 | 197.0 ± 65.2 | 0.8 |
| 3 mo | 157.8 ± 102.9 | 212.3 ± 96.9 | 0.03 | 212.8 ± 124.0 | 234.6 ± 131.8 | 0.5 | 219.2 ± 146.3 | 149.0 ± 36.0 | 0.5 |
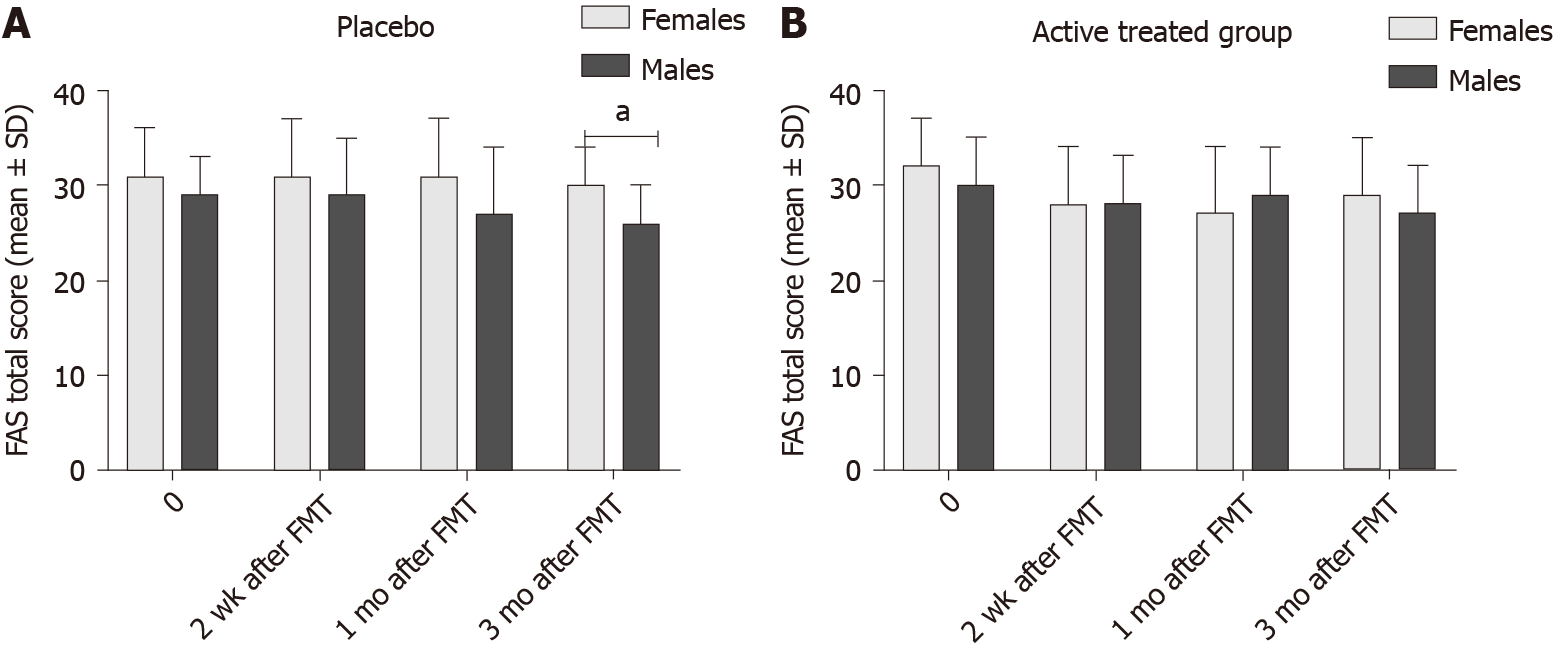
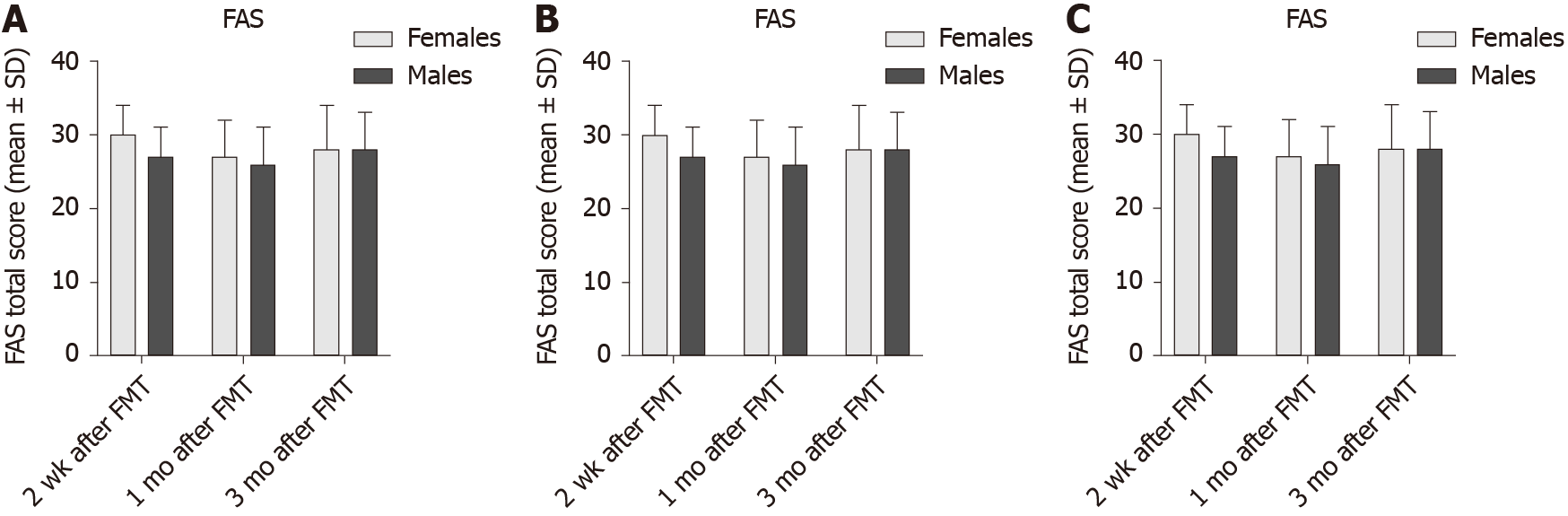
The FAS total score also did not differ significantly between female and male IBS patients in the active treated group (Table 7 and Figure 6), but it was lower in males than females in the placebo group at 3 mo after FMT. This could have been due to a type-I statistical error. There was no significant difference between female and male IBS patients belonging to different IBS-subtypes IBS symptoms (Table 8 and Figure 7). However, the FAS total score was lower in males IBS patients with IBS-D than that of females 2 wk after FMT.
| Time | Placebo | Active treated | ||||
| Females | Males | P value | Females | Males | P value | |
| 0 | 31 ± 5 | 29 ± 4 | 0.3 | 32 ± 5 | 30 ± 5 | 0.09 |
| 2 wk | 31 ± 6 | 29 ± 6 | 0.2 | 28 ± 6 | 28 ± 5 | 0.5 |
| 1 mo | 31 ± 6 | 27 ± 7 | 0.1 | 27 ± 7 | 29 ± 5 | 0.5 |
| 3 mo | 30 ± 4 | 26 ± 4 | 0.01 | 29 ± 6 | 27 ± 5 | 0.7 |
| Time after FMT | IBS-D | IBS-C | IBS-M | ||||||
| Females | Males | P value | Females | Males | P value | Females | Males | P value | |
| 2 wk | 30.1 ± 3.6 | 27.0 ± 3.6 | 0.04 | 28.0 ± 6.3 | 29.3 ± 7.4 | 0.5 | 26.7 ± 5.4 | 26.3 ± 2.1 | 0.9 |
| 1 mo | 27.1 ± 5.2 | 26.6 ± 4.6 | 0.6 | 27.1 ± 6.7 | 31.3 ± 6.1 | 0.1 | 28.3 ± 8.3 | 28.3 ± 4.0 | 0.9 |
| 3 mo | 27.5 ± 5.7 | 27.8 ± 5.0 | 0.4 | 26.0 ± 6.2 | 29.2 ± 5.0 | 0.2 | 26.8 ± 7.6 | 24.8 ± 2.2 | 0.7 |
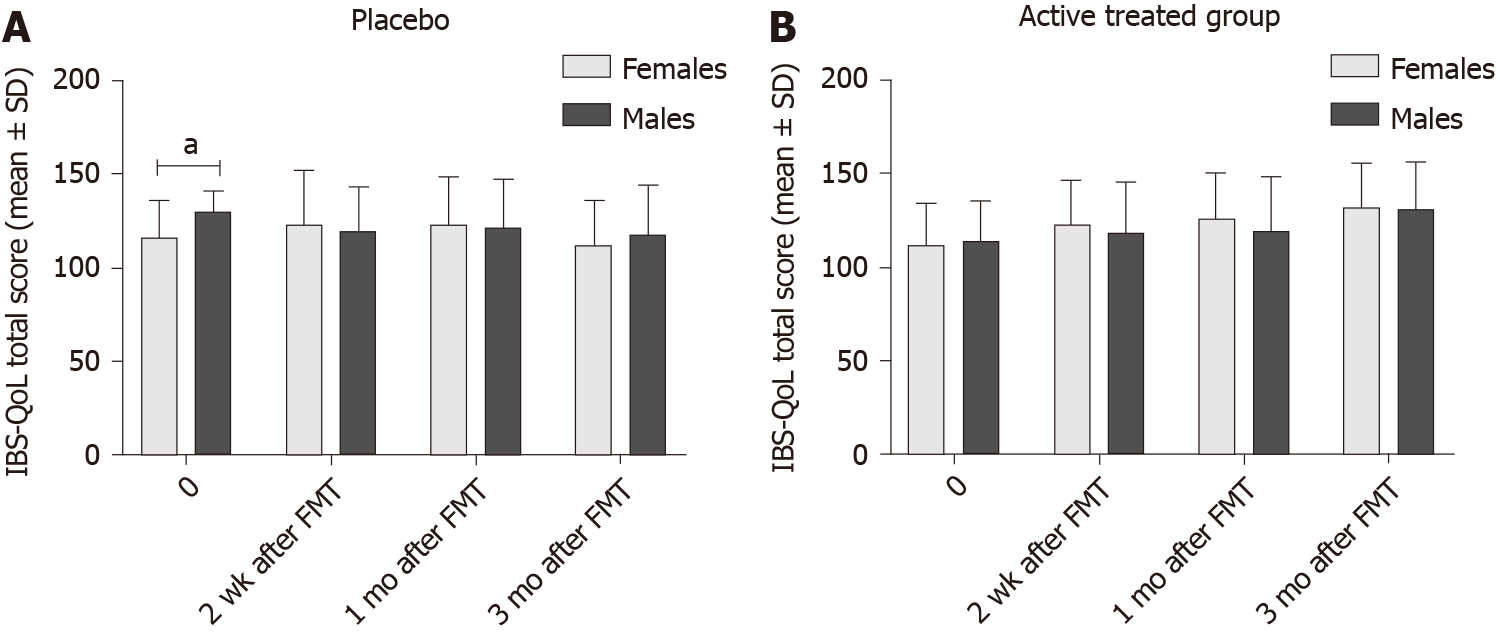
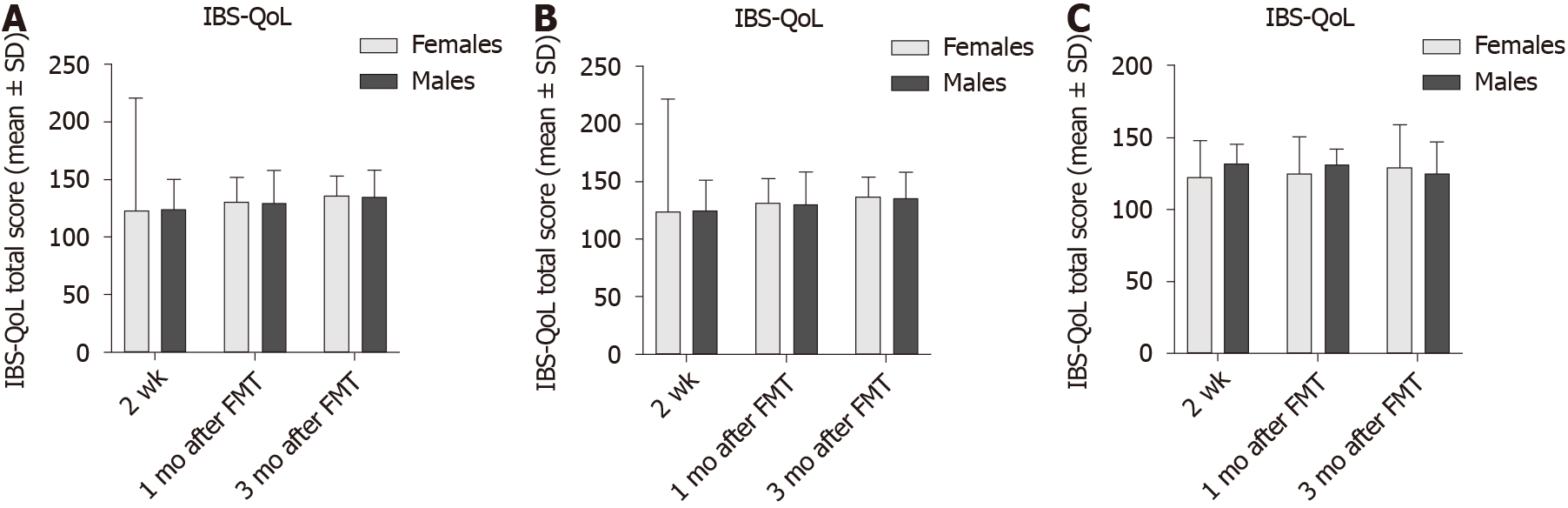
The IBS-QoL total score did differ between females and males in both the placebo and active treated groups (Table 9 and Figure 8), being higher in males than in females at the baseline. IBS-QoL total scores did not differ significantly between female and male patients belonging to different IBS-subtypes (Table 10 and Figure 9).
| Time | Placebo | Active treated | ||||
| Females | Males | P value | Females | Males | P value | |
| 0 | 116 ± 20 | 130 ± 11 | 0.03 | 111 ± 23 | 114 ± 21 | 0.9 |
| 2 wk | 123 ± 29 | 120 ± 23 | 0.7 | 122 ± 24 | 118 ± 27 | 0.6 |
| 1 mo | 123 ± 26 | 121 ± 26 | 0.8 | 126 ± 24 | 119 ± 29 | 0.4 |
| 3 mo | 112 ± 24 | 118 ± 26 | 0.2 | 132 ± 23 | 131 ± 25 | 0.9 |
| Time after FMT | IBS-D | IBS-C | IBS-M | ||||||
| Females | Males | P value | Females | Males | P value | Females | Males | P value | |
| 2 wk | 123.3 ± 98 | 123.7 ± 25.5 | 0.8 | 120.5 ± 23.1 | 102.4 ± 28.0 | 0.06 | 123.1 ± 24.6 | 131.5 ± 13.0 | 0.4 |
| 1 mo | 131.3 ± 20.8 | 129.6 ± 28.2 | 0.9 | 121.9 ± 24.4 | 111.4 ± 29.1 | 01 | 125.4 ± 25.2 | 130.5 ± 11.3 | 0.8 |
| 3 mo | 136.4 ± 16.6 | 134.5 ± 22.7 | 0.9 | 128.7 ± 24.4 | 129.1 ± 31.3 | 0.6 | 129.5 ± 28.6 | 124.8 ± 22.0 | 0.5 |
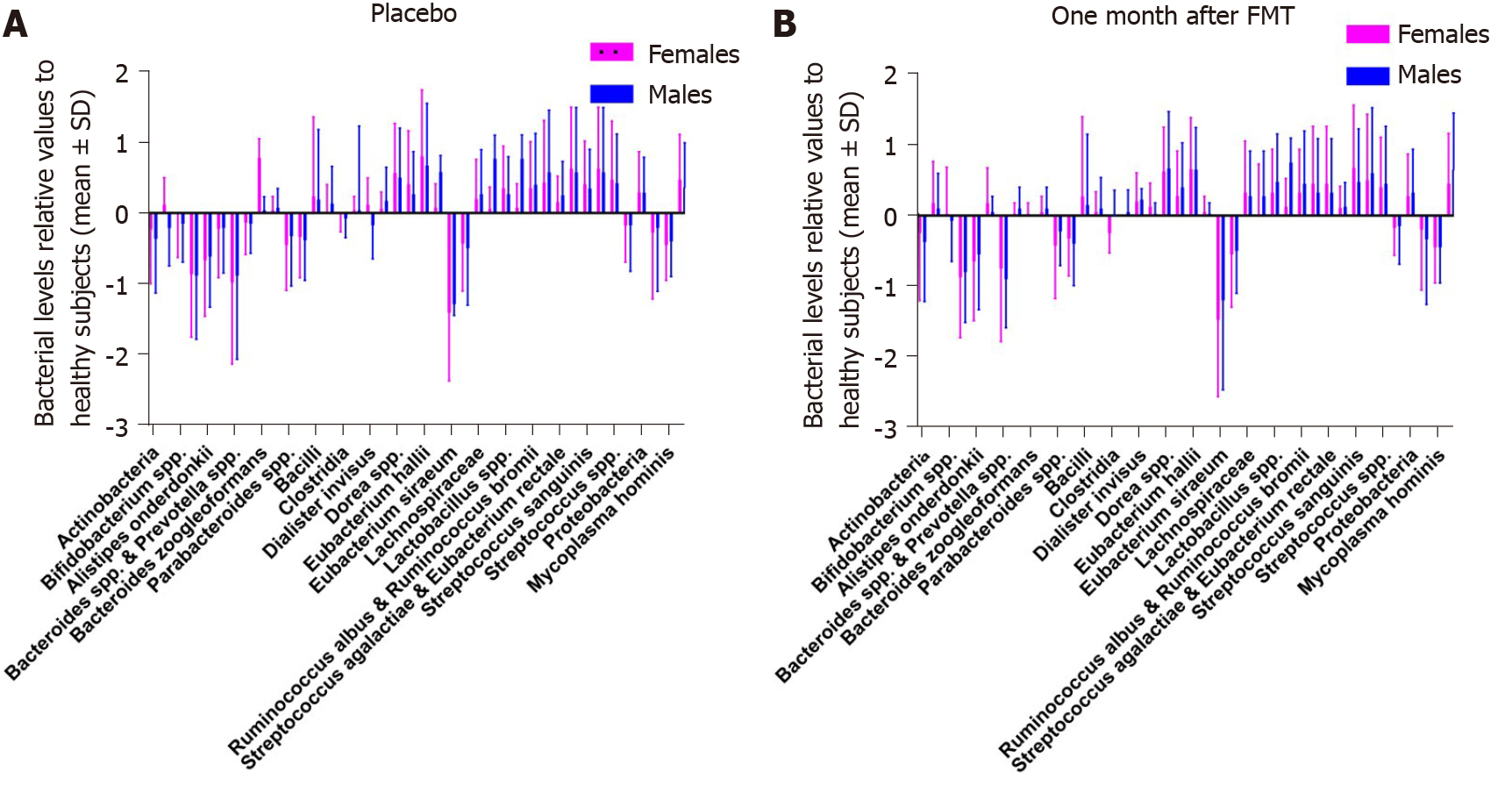
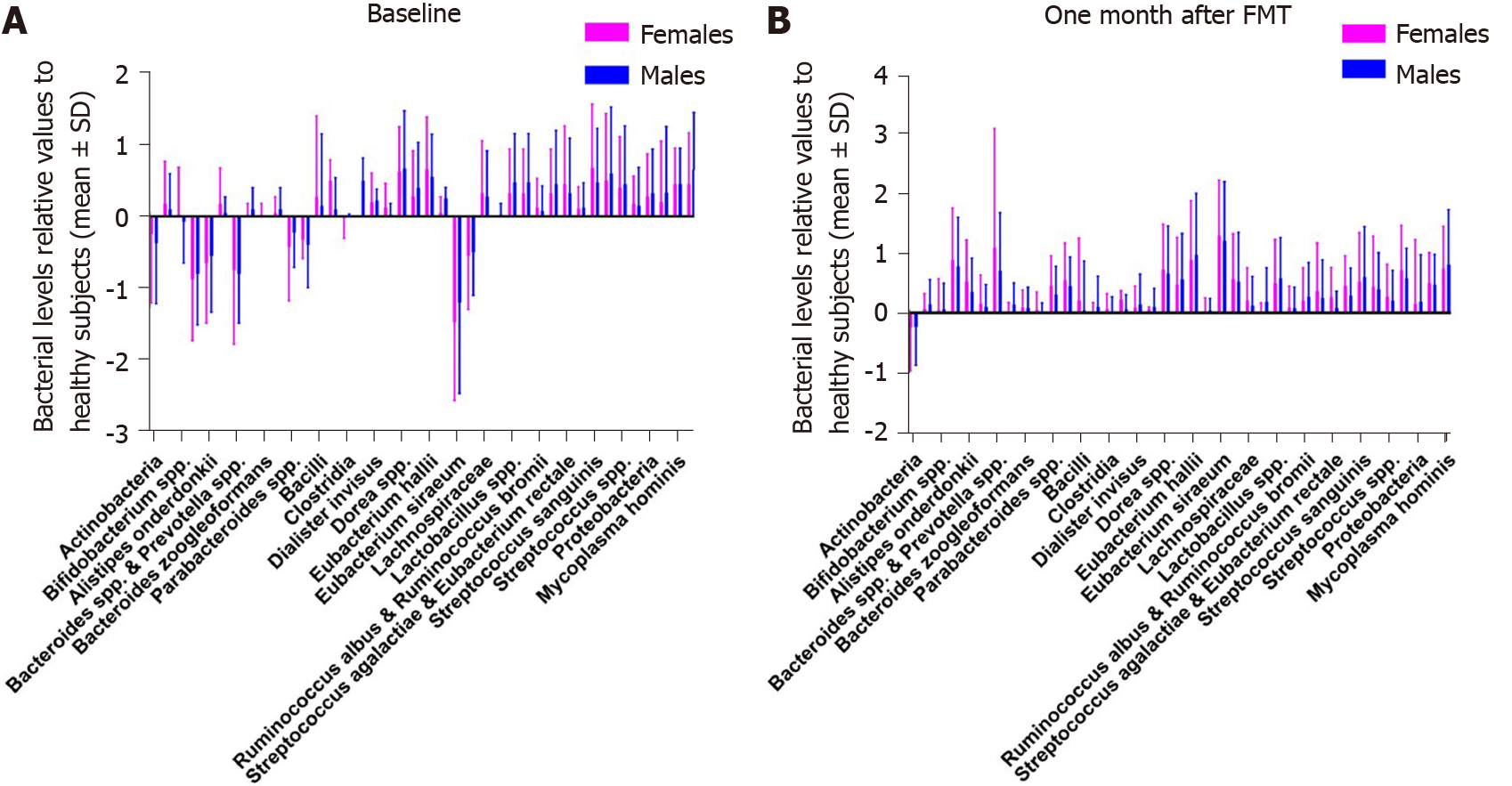
The faecal bacteria levels in the placebo group did not differ between female and male IBS patients at the baseline and 1 mo after FMT (Table 11 and Figure 10). Similarly, there were no differences in the faecal bacteria levels between female and male IBS patients in the active treated group (Table 12 and Figure 11).
| Bacteria | Baseline | 1 mo after FMT | ||
| Females | Males | Females | Males | |
| Actinobacteria | -0.235 ± 0.763 | -0.365 ± 0.768 | -0.250 ± 0.954 | -0.375 ± 0.838 |
| Actinomycetales | 0.118 ± 0.382 | -0.212 ± 0.536 | 0.175 ± 0.594 | 0.100 ± 0.496 |
| Bifidobacterium spp. | -0.020 ± 0.607 | -0.154 ± 0.539 | 0.025 ± 0.660 | -0.075 ± 0.572 |
| Alistipes | -0.863 ± 0.895 | -0.885 ± 0.900 | -0.875 ± 0.853 | -0.800 ± 0.709 |
| Alistipes onderdonkii | -0.667 ± 0.792 | -0.615 ± 0.718 | -0.650 ± 0.834 | -0.550 ± 0.783 |
| Bacteroides fragilis | -0.255 ± 0.689 | -0.212 ± 0.637 | 0.175 ± 0.501 | 0.050 ± 0.221 |
| Bacteroides spp. and Prevotella spp. | -0.980 ± 1.157 | -0.885 ± 1.182 | -0.750 ± 1.032 | -0.900 ± 0.687 |
| Bacteroides stercoris | -0.137 ± 0.448 | -0.154 ± 0.415 | 0.025 ± 0.158 | 0.100 ± 0.304 |
| Bacteroides zoogleoformans | 0.078 ± 0.272 | 0.038 ± 0.194 | 0.025 ± 0.158 | 0 ± 0 |
| Parabacteroides johnsonii | 0.039 ± 0.196 | 0.077 ± 0.269 | 0.050 ± 0.221 | 0.100 ± 0.304 |
| Parabacteroides spp. | -0.451 ± 0.642 | -0.327 ± 0.706 | -0.425 ± 0.747 | -0.225 ± 0.480 |
| Firmicutes | -0.431 ± 0.575 | -0.385 ± 0.566 | -0.325 ± 0.526 | -0.400 ± 0.591 |
| Bacilli | 0.235 ± 1.124 | 0.192 ± 0.991 | 0.271 ± 1.132 | 0.150 ± 1.001 |
| Catenibacterium mitsuokai | 0.000 ± 0.400 | 0.135 ± 0.525 | 0.050 ± 0.289 | 0.100 ± 0.441 |
| Clostridia | -0.020 ± 0.244 | -0.077 ± 0.269 | -0.025 ± 0.276 | 0.0 ± 0.036 |
| Clostridium spp. | 0.039 ± 0.196 | 0.038 ± 0.194 | 0.0 ± 0.0 | 0.050 ± 0.316 |
| Dialister invisus | 0.118 ± 0.381 | -0.173 ± 0.474 | 0.200 ± 0.405 | 0.225 ± 0.158 |
| Dialister invisus and Megasphaera micronuciformis | 0.059 ± 0.238 | 0.173 ± 0.474 | 0.125 ± 0.335 | 0.025 ± 0.158 |
| Dorea spp. | 0.569 ± 0.700 | 0.500 ± 0.700 | 0.625 ± 0.628 | 0.667 ± 0.806 |
| Eubacterium biforme | 0.412 ± 0.753 | 0.269 ± 0.598 | 0.275 ± 0.640 | 0.400 ± 0.633 |
| Eubacterium hallii | 0.804 ± 0.939 | 0.673 ± 0.879 | 0.655 ± 0.730 | 0.650 ± 0.597 |
| Eubacterium rectale | 0.078 ± 0.337 | 0.058 ± 0.235 | 0.050 ± 0.221 | 0.025 ± 0.158 |
| Eubacterium siraeum | -1.412 ± 0.963 | -1.288 ± 0.161 | -1.475 ± 1.086 | -1.200 ± 1.265 |
| Faecalibacterium prausnitzii | -0.431 ± 0.671 | -0.500 ± 0.804 | -0.550 ± 0.745 | -0.500 ± 0.599 |
| Lachnospiraceae | 0.196 ± 0.566 | 0.269 ± 0.630 | 0.325 ± 0.730 | 0.275 ± 0.640 |
| Lactobacillus ruminis and Pediococcus acidilactici | 0.059 ± 0.311 | 0.077 ± 0.334 | 0.0 ± 0.0 | 0.025 ± 0.158 |
| Lactobacillus spp. | 0.353 ± 0.594 | 0.269 ± 0.528 | 0.325 ± 0.616 | 0.475 ± 0.680 |
| Phascolarctobacterium spp. | 0.078 ± 0337 | 0.077 ± 0.337 | 0.125 ± 0.404 | 0.075 ± 0.350 |
| Ruminococcus albus and Ruminococcus bromii | 0.353 ± 0.658 | 0.404 ± 0.721 | 0.325 ± 0.616 | 0.450 ± 0.749 |
| Ruminococcus gnavus | 0.431 ± 0.878 | 0.577 ± 0.878 | 0.450 ± 0.815 | 0.325 ± 0.764 |
| Streptococcus agalactiae & Eubacterium rectale | 0.157 ± 0.367 | 0.250 ± 0.480 | 0.110 ± 0.304 | 0.125 ± 0.345 |
| Streptococcus salivarius ssp. Thermophilus and Streptococcus sanguinis | 0.412 ± 0.606 | 0.346 ± 0.556 | 0.675 ± 0.888 | 0.475 ± 0.751 |
| Streptococcus salivarius ssp. thermophilus | 0.628 ± 0.871 | 0.577 ± 0.915 | 0.500 ± 0.934 | 0.600 ± 0.928 |
| Streptococcus spp. | 0.471 ± 0.833 | 0.423 ± 0.696 | 0.400 ± 0.709 | 0.450 ± 0.815 |
| Veillonella spp. | -0.177 ± 0.518 | -0.173 ± 0.648 | -0.175 ± 0.385 | -0.150 ± 0.534 |
| Proteobacteria | 0.294 ± 0.576 | 0.289 ± 0.499 | 0.275 ± 0.599 | 0.325 ± 0.616 |
| Shigella spp. and Escherichia spp. | -0.275 ± 0.940 | -0.212 ± 0.893 | -0.200 ± 0.853 | -0.335 ± 0.920 |
| Mycoplasma hominis | -0.451 ± 0.503 | -0.404 ± 0.496 | -0.450 ± 0.504 | -0.450 ± 0.503 |
| Akkermansia muciniphila | 0.471 ± 0.644 | 0.365 ± 0.627 | 0.450 ± 0.714 | 0.650 ± 0.802 |
| Bacteria | Baseline | 1 mo after FMT | ||
| Females | Males | Females | Males | |
| Actinobacteria | -0.250 ± 0.954 | -0.375 ± 0.838 | -0.250 ± 0.719 | -0.2350 ± 0.636 |
| Actinomycetales | 0.175 ± 0.594 | 0.100 ± 0.496 | 0.068 ± 0255 | 0.145 ± 0.412 |
| Bifidobacterium spp. | 0.025 ± 0.660 | -0.075 ± 0.572 | -0.045 ± 0.526 | -0.063 ± 0.433 |
| Alistipes | -0.875 ± 0.853 | -0.800 ± 0.709 | -0.886 ± 0.869 | -0.783 ± 0.821 |
| Alistipes onderdonkii | -0.650 ± 0.834 | -0.550 ± 0.783 | -0.523 ± 0.699 | -0.354 ± 0.565 |
| Bacteroides fragilis | 0.175 ± 0.501 | 0.050 ± 0.221 | 0.159 ± 0.480 | 0.104 ± 0.371 |
| Bacteroides spp. and Prevotella spp. | -0.750 ± 1.032 | -0.800 ± 0.687 | -1.091 ± 1.996 | -0.708 ± 0.967 |
| Bacteroides stercoris | 0.025 ± 0.158 | 0.100 ± 0.304 | 0.023 ± 0.151 | 0.146 ± 0.357 |
| Bacteroides zoogleoformans | 0.025 ± 0.158 | 0 ± 0 | 0.091 ± 0.291 | 0.083 ± 0.347 |
| Parabacteroides johnsonii | 0.050 ± 0.221 | 0.100 ± 0.304 | 0.045 ± 0.302 | 0.021 ± 0.144 |
| Parabacteroides spp. | -0.425 ± 0.747 | -0.225 ± 0.480 | -0.455 ± 0.504 | -0.313 ± 0.468 |
| Firmicutes | -0.325 ± 0.526 | -0.400 ± 0.591 | -0.546 ± 0.627 | -0.454 ± 0.483 |
| Bacilli | 0.271 ± 1.132 | 0.150 ± 1.001 | 0.205 ± 1.047 | 0.042 ± 0.824 |
| Catenibacterium mitsuokai | 0.050 ± 0.289 | 0.100 ± 0.441 | 0.023 ± 0.151 | 0.104 ± 0.515 |
| Clostridia | -0.025 ± 0.276 | 0.0 ± 0.036 | 0.068 ± 0.255 | 0.021 ± 252 |
| Clostridium spp. | 0.0 ± 0.0 | 0.050 ± 0.316 | 0.223 ± 0.151 | 0.063 ± 0.245 |
| Dialister invisus | 0.200 ± 0.405 | 0.225 ± 0.158 | 0.091 ± 0.362 | 0.146 ± 0.505 |
| Dialister invisus and Megasphaera micronuciformis | 0.125 ± 0.335 | 0.025 ± 0.158 | 0.068 ± 0.034 | 0.104 ± 0.308 |
| Dorea spp. | 0.625 ± 0.628 | 0.667 ± 0.806 | 0.727 ± 0.758 | 0.663 ± 0.796 |
| Eubacterium biforme | 0.275 ± 0.640 | 0.400 ± 0.633 | 0.477 ± 0.791 | 0.563 ± 0.769 |
| Eubacterium hallii | 0.655 ± 0.730 | 0.550 ± 0.597 | 0.886 ± 0.993 | 0.979 ± 1.021 |
| Eubacterium rectale | 0.050 ± 0.221 | 0.025 ± 0.158 | 0-068 ± 0.255 | 0.042 ± 0.202 |
| Eubacterium siraeum | -1.475 ± 1.086 | -1.200 ± 1.265 | -1.295 ± 0.930 | -1.208 ± 0.988 |
| Faecalibacterium prausnitzii | -0.550 ± 0.745 | -0.500 ± 0.599 | -0.568 ± 0.759 | -0.521 ± 0.825 |
| Lachnospiraceae | 0.325 ± 0.730 | 0.275 ± 0.640 | 0.205 ± 0.553 | 0.125 ± 0.489 |
| Lactobacillus ruminis and Pediococcus acidilactici | 0.0 ± 0.0 | 0.025 ± 0.158 | 0.021 ± 0.146 | 0.188 ± 0.571 |
| Lactobacillus spp. | 0.325 ± 0.616 | 0.475 ± 0.680 | 0.500 ± 0.731 | 0.583 ± 0.679 |
| Phascolarctobacterium spp. | 0.125 ± 0.404 | 0.075 ± 0.350 | 0.091 ± 0.362 | 0.083 ± 0.347 |
| Ruminococcus albus and Ruminococcus bromii | 0.325 ± 0.616 | 0.450 ± 0.749 | 0.205 ± 0.553 | 0.271 ± 0.574 |
| Ruminococcus gnavus | 0.450 ± 0.815 | 0.325 ± 0.764 | 0.364 ± 0.810 | 0.250 ± 0.636 |
| Streptococcus agalactiae & Eubacterium rectale | 0.110 ± 0.304 | 0.125 ± 0.345 | 0.267 ± 0.495 | 0.083 ± 0.279 |
| Streptococcus salivarius ssp. thermophilus and Streptococcus sanguinis | 0.675 ± 0.888 | 0.475 ± 0.751 | 0.455 ± 0.504 | 0.292 ± 0.459 |
| Streptococcus salivarius ssp. thermophilus | 0.500 ± 0.934 | 0.600 ± 0.928 | 0.523 ± 0.821 | 0.604 ± 0.844 |
| Streptococcus spp. | 0.400 ± 709 | 0.450 ± 0.815 | 0.444 ± 0.841 | 0.396 ± 0.610 |
| Veillonella spp. | -0.175 ± 0.385 | -0.150 ± 0.534 | -0.273 ± 0.544 | -0.208 ± 0.504 |
| Proteobacteria | 0.275 ± 0.599 | 0.325 ± 0.616 | 0.717 ± 0.750 | 0.583 ± 0.498 |
| Shigella spp. and Escherichia spp. | -0.200 ± 0.853 | -0.335 ± 0.920 | -0.151 ± 1.077 | -0.188 ± 0.790 |
| Mycoplasma hominis | -0.450 ± 0.504 | -0.450 ± 0.503 | -0.500 ± 0.506 | -0.479 ± 0.505 |
| Akkermansia muciniphila | 0.450 ± 0.714 | 0.650 ± 0.802 | 0.741 ± 0.713 | 0.813 ± 0.915 |
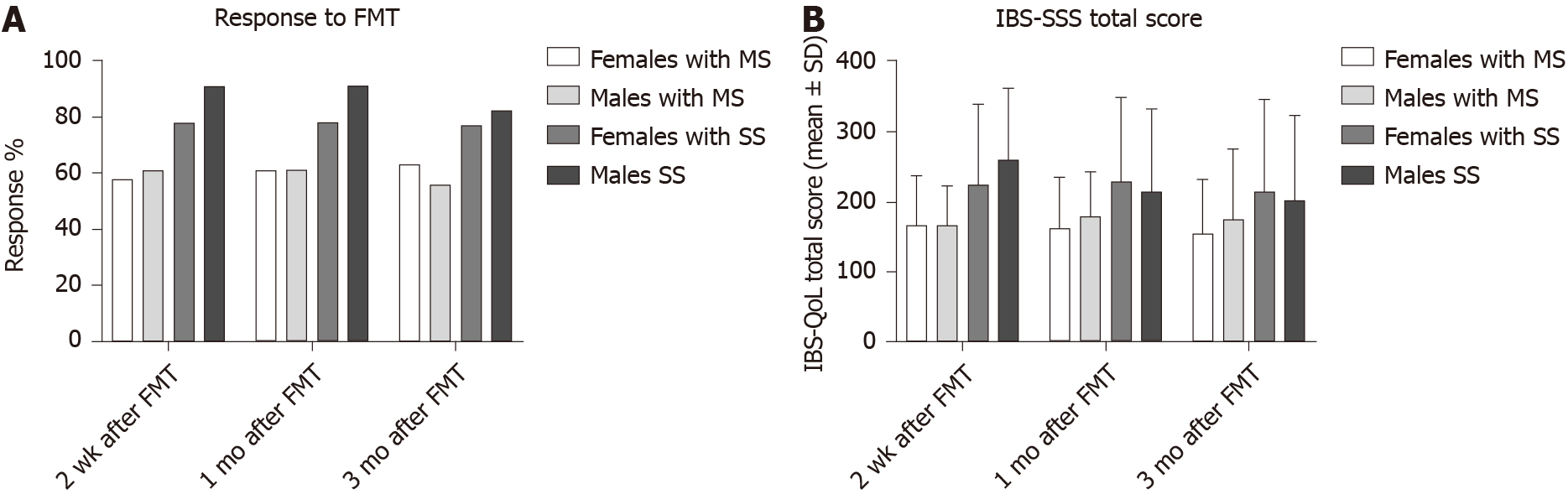
In the placebo group, 26 females (55%) and 4 males (50%) had dysbiosis (P = 0.8) at the baseline, while 25 females (53%) and 4 males (50%) had dysbiosis (P = 0.9) at 1 mo after FMT. In the active treated group, 52 females (61%) and 13 males (54%) had dysbiosis (P = 0.3) at the baseline, while 41 females (48%) and 9 males (38%) had dysbiosis (P = 0.2) at 1 mo after FMT.
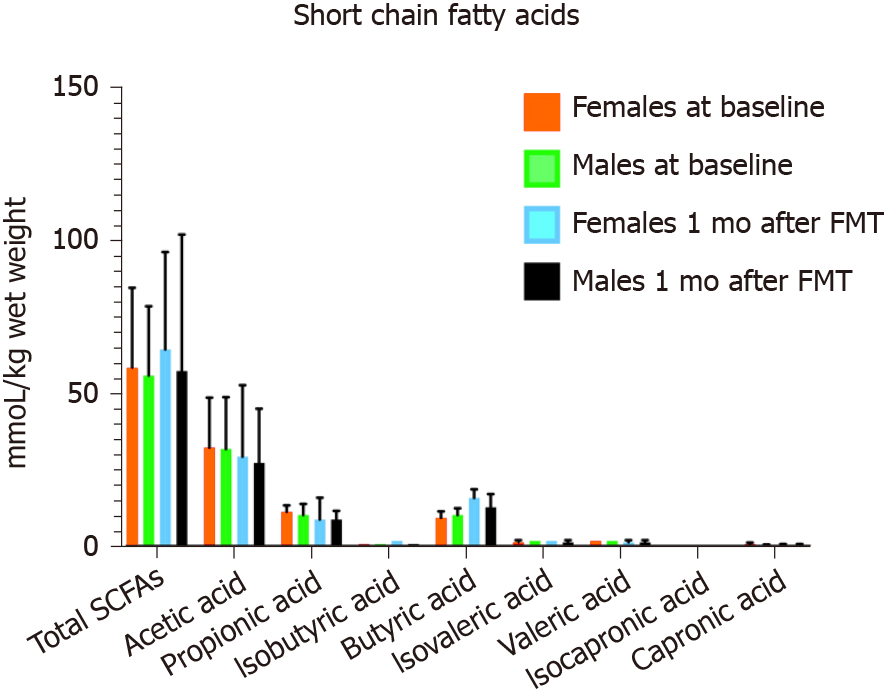
The faecal levels of total SCFAs and acetic, propionic, isobutyric, butyric, isovaleric, valeric, isocapronic and capronic acids did not differ between female and males IBS patients in both the placebo and active treated groups at the baseline and 1 mo after FMT (Table 13 and Figure 12).
| Acids | Placebo | Active treated | ||||||
| Baseline | 1 mo after FMT | Baseline | 1 mo after FMT | |||||
| Females | Males | Females | Males | Females | Males | Females | Males | |
| Total SCFAs | 72 ± 37 | 69 ± 23. | 73 ± 37 | 69 ± 23 | 77 ± 40 | 72 ± 40 | 87 ± 42 | 89 ± 26 |
| Acetic acid | 42 ± 18 | 40 ± 15 | 41 ± 17 | 40 ± 14 | 44 ± 21 | 44 ± 20 | 46 ± 13 | 40.2 ± 15.0 |
| Propionic acid | 12 ± 8 | 11 ± 5 | 12 ± 8 | 11 ± 5 | 13 ± 10 | 13 ± 8 | 14 ± 4 | 11 ± 7 |
| Iso-butyric acid | 2 ± 2 | 1 ± 1 | 1 ± 2 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 2 ± 2 | 1 ± 1 |
| Butyric acid | 14 ± 9 | 12 ± 6 | 13 ± 8 | 12 ± 6 | 11 ± 8 | 12 ± 9 | 18 ± 14 | 16 ± 10 |
| Iso-valeric acid | 2 ± 2 | 2 ± 1 | 2 ± 2 | 2 ± 1 | 2 ± 1 | 2 ± 2 | 2 ± 2 | 2 ± 1 |
| Valeric acid | 2 ± 2 | 1 ± 1 | 2 ± 2 | 1 ± 1 | 2 ± 2 | 2 ± 2 | 2 ± 1 | 2 ± 1 |
| Iso-capronic acid | 0.1 ± 0.04 | 0.01 ± 0.07 | 0.5 ± 0.7 | 0.4 ± 0.7 | 0.0 ± 0.0 | 0.02 ± 0.08 | 0.01 ± 0.04 | 0.0 ± 0.0 |
| Capronic acid | 0.5 ± 0.7 | 0.5 ± 0.7 | 0.01 ± 0.04 | 0.1 ± 0.08 | 0.7 ± 1.3 | 0.6 ± 0.8 | 0.6 ± 0.9 | 0.5 ± 0.9 |
The present study found that the response to FMT did not differ between females and males. Furthermore, the total scores on the IBS-SSS, FAS and IBS-QoL did not differ between females and males in the active treated groups before FMT and at different times after FMT. In the placebo group, the total score of IBS-QoL was higher in males than males and the FAS total score was lower in males than females 3 mo after FMT. This indicates that the effects of active treated FMT did not differ between males and females regarding IBS symptoms, fatigue and quality of life. Moreover, there was no difference between females and males regarding dysbiosis or the faecal bacteria.
SCFA profiles following FMT did not differ between females and males in both the placebo and the active treated groups. SCFAs regulate intestinal motility and the secretion and absorption of water and electrolytes[37,38]. Moreover, SCFAs increase also the secretion and up-regulate the gene expression of peptide YY (PYY)[39,40]. PYY is a mediator of the ileal brake and stimulates the absorption of water and electrolytes in the colon[37]. The faecal level of total SCFAs increased significantly in IBS patients after 1 mo and remained elevated at 1 year after FMT (unpublished data)[25]. Similarly, the faecal level of butyric acid increased in IBS patients after 1 mo and remained elevated at 1 year after FMT (unpublished data)[25]. Butyrate is a major energy source for colonic epithelial cells, interacts with the immune response, modulates the oxidative stress, and decreases both intestinal-cell permeability and intestinal motility[41]. Butyrate modulates also colonic hypersensitivity[42-44]. At 1 year after FMT levels of isobutyric and isovaleric acids were increased in IBS patients, indicating a shift in microbial fermentation from a saccharolytic to a proteolytic pattern[45]. It is worthy of note that in IBS patients, who adhered to a low-FODMAPs (fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) diet an increase in the levels of isobutyric and isovaleric acids have been found[46]. Moreover, the level of acetic acid which induces visceral hypersensitivity decreased significantly at 1 year after FMT[47].These changes in SCFAs after FMT appear to be one of the mechanisms underlying the effects seen in IBS patients after FMT. That is why the difference between females and males regarding the changes in SCFAs was assessed.
Holvoet et al[16] reported that females responded better to FMT than did males. That RCT differed from the present study in terms of the characteristics of the included patients, the size of the patient cohort and the dose of the faecal transplants[16]. The trial of Holvoet et al[16] included a subgroup of refractory IBS patients with severe bloating who had not responded to at least three conventional therapies for IBS. This subgroup contained only patients with the IBS-D or mixed (IBS-M) subtypes. The patients included in the present study had moderate-to-severe IBS symptoms belonging to the IBS-D, IBS-C and IBS-M subtypes, and had not responded to the NICE-modified diet. The patient cohort investigated by Holvoet et al[16] included 62 patients: 19 in the placebo group and 43 in the active treated group. The present study investigated a cohort of 164 patients: 55 in the placebo group and 109 in the active treated group. It is worthy of note that in the trial of Holvoet et al[16] included 30 females and 13 males in the active treated group and 8 females and 11 males in the placebo group. The present study included 85 females and 24 males in the active treated group and included 47 females and 8 males in the placebo group. Thus, this makes the present study less constrained than the RCT of Holvoet et al[16] regarding power and sample sizes. Moreover, the dose of the faecal transplant from the donor was not reported for the RCT by Holvoet et al[16], while in the present study the active treated group received either 30 g or 60 g of a superdonor transplant. In our previously published RCT we showed that the response rates increased with increased dose[12]. These differences make it difficult to compare the outcomes of the present study with those of Holvoet et al[16]. However, in the present study, female patients with IBS-D had a significant higher response rate to FMT and lower IBS-SSS score after FMT than males. These observations could explain the discrepancy between the findings of Holvoet et al[16] and the present study as in Holvoet et al[16] study the cohort of patients included were only IBS-D and IBS-M IBS-subtypes.
In conclusion, there is no sex difference in the response to FMT in IBS patients with moderate-to-severe IBS symptoms belonging to the three of IBS subtypes of IBS-C and IBS-M in patients who did not responded to NICE-modified diet. Female patients with IBS-D had a significant higher response rate to FMT and lower IBS-SSS score after FMT than males.
Irritable bowel syndrome (IBS) is a common chronic disorder, where intestinal microbiota plays a pivotal role in its pathophysiology. Faecal microbiota transplantation for IBS appears to be a promising treatment of IBS.
In Western countries, there is a female predominance in IBS with female:male ratio of 2:1. In a recent randomized double-blind placebo-controlled trial on faecal microbiota transplantation (FMT) in IBS females responded better to FMT than did males.
We aimed to investigate whether there is a sex difference in the response to FMT in terms of symptoms, dysbiosis, and bacteria and short-chain fatty acids (SCFAs) profiles in the same cohort of patients that we had investigated in our previous randomized controlled trial.
This study included 164 patients who fulfilled the Rome IV criteria for the diagnosis of IBS. These patient’s cohort included IBS diarrhoea-predominant (IBS-D), IBS-constipation predominant (IBS-C) and mixed diarrhoea and constipation (IBS-M) subtypes. They were randomized to placebo (own faeces), 30 g or 60 g donor’s faeces at a ratio of 1:1:1. The faecal transplant was administered via gastroscope to the duodenum. Patients completed IBS severity scoring system (IBS-SSS), the Fatigue Assessment Scale (FAS) and the IBS quality of life scale (IBS-QoL) questionnaires at the baseline and 2 wk, 1 mo and 3 mo after FMT. They also provided faecal samples at the baseline and 1 mo after FMT. Response was defined as a decrease of ≥ 50 points in the IBS-SSS total score after FMT. The faecal bacteria profile and dysbiosis were determined by the GA-map Dysbiosis Test (Genetic Analysis, Oslo, Norway) using the 16S rRNA gene. The levels of faecal SCFAs were determined by gas chromatography.
There was no sex difference in the response to FMT either in the placebo group or active treated group. There was no difference between females and males in either the placebo group or actively treated groups in the total score on the IBS-SSS, FAS or IBS-QoL, in dysbiosis, or in the faecal bacteria or SCFA level. However, the response rate was significantly higher in females with IBS-D than that of males at 1 mo, and 3 mo after FMT. Moreover, IBS-SSS total score was significantly lower in female patients with IBS-D than that of male patients both 1 mo and 3 mo after FMT.
There is no sex difference in the response to FMT in IBS patients with moderate-to-severe IBS symptoms belonging to the three of IBS subtypes of IBS-C and IBS-M in patients who did not responded to National Institute for Health and Care Excellence-modified diet. However, female patients with IBS-D had a significant higher response rate to FMT and lower IBS-SSS score after FMT than males.
The present observation that female patients with IBS-D respond better to FMT than males raise several questions as to the cause of this difference. Further studies are needed to explore the difference in diet and life style between females and males as possible causes for this difference.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: Norway
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Huang MC, Melchior C S-Editor: Gao CC L-Editor: A P-Editor: Liu JH
| 1. | El-Salhy M. Recent developments in the pathophysiology of irritable bowel syndrome. World J Gastroenterol. 2015;21:7621-7636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 72] [Cited by in F6Publishing: 67] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 2. | Ohman L, Simrén M. Intestinal microbiota and its role in irritable bowel syndrome (IBS). Curr Gastroenterol Rep. 2013;15:323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Öhman L, Törnblom H, Simrén M. Crosstalk at the mucosal border: importance of the gut microenvironment in IBS. Nat Rev Gastroenterol Hepatol. 2015;12:36-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 4. | Wilson BC, Vatanen T, Cutfield WS, O'Sullivan JM. The Super-Donor Phenomenon in Fecal Microbiota Transplantation. Front Cell Infect Microbiol. 2019;9:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 5. | Maier L, Pruteanu M, Kuhn M, Zeller G, Telzerow A, Anderson EE, Brochado AR, Fernandez KC, Dose H, Mori H, Patil KR, Bork P, Typas A. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature. 2018;555:623-628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1160] [Cited by in F6Publishing: 1076] [Article Influence: 179.3] [Reference Citation Analysis (0)] |
| 6. | Casén C, Vebø HC, Sekelja M, Hegge FT, Karlsson MK, Ciemniejewska E, Dzankovic S, Frøyland C, Nestestog R, Engstrand L, Munkholm P, Nielsen OH, Rogler G, Simrén M, Öhman L, Vatn MH, Rudi K. Deviations in human gut microbiota: a novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Aliment Pharmacol Ther. 2015;42:71-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 179] [Cited by in F6Publishing: 207] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 7. | Pozuelo M, Panda S, Santiago A, Mendez S, Accarino A, Santos J, Guarner F, Azpiroz F, Manichanh C. Reduction of butyrate- and methane-producing microorganisms in patients with Irritable Bowel Syndrome. Sci Rep. 2015;5:12693. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 220] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 8. | Chong PP, Chin VK, Looi CY, Wong WF, Madhavan P, Yong VC. The Microbiome and Irritable Bowel Syndrome - A Review on the Pathophysiology, Current Research and Future Therapy. Front Microbiol. 2019;10:1136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 9. | Enck P, Mazurak N. Dysbiosis in Functional Bowel Disorders. Ann Nutr Metab. 2018;72:296-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Johnsen PH, Hilpüsch F, Cavanagh JP, Leikanger IS, Kolstad C, Valle PC, Goll R. Faecal microbiota transplantation vs placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol. 2018;3:17-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 248] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 11. | Halkjær SI, Christensen AH, Lo BZS, Browne PD, Günther S, Hansen LH, Petersen AM. Faecal microbiota transplantation alters gut microbiota in patients with irritable bowel syndrome: results from a randomised, double-blind placebo-controlled study. Gut. 2018;67:2107-2115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 207] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 12. | El-Salhy M, Hatlebakk JG, Gilja OH, Bråthen Kristoffersen A, Hausken T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut. 2020;69:859-867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 245] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 13. | Aroniadis OC, Brandt LJ, Oneto C, Feuerstadt P, Sherman A, Wolkoff AW, Kassam Z, Sadovsky RG, Elliott RJ, Budree S, Kim M, Keller MJ. Faecal microbiota transplantation for diarrhoea-predominant irritable bowel syndrome: a double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2019;4:675-685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 84] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 14. | Holster S, Lindqvist CM, Repsilber D, Salonen A, de Vos WM, König J, Brummer RJ. The Effect of Allogenic Versus Autologous Fecal Microbiota Transfer on Symptoms, Visceral Perception and Fecal and Mucosal Microbiota in Irritable Bowel Syndrome: A Randomized Controlled Study. Clin Transl Gastroenterol. 2019;10:e00034. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 15. | Lahtinen P, Jalanka J, Hartikainen A, Mattila E, Hillilä M, Punkkinen J, Koskenpato J, Anttila VJ, Tillonen J, Satokari R, Arkkila P. Randomised clinical trial: faecal microbiota transplantation vs autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment Pharmacol Ther. 2020;51:1321-1331. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 56] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 16. | Holvoet T, Joossens M, Vázquez-Castellanos JF, Christiaens E, Heyerick L, Boelens J, Verhasselt B, van Vlierberghe H, De Vos M, Raes J, De Looze D. Fecal Microbiota Transplantation Reduces Symptoms in Some Patients With Irritable Bowel Syndrome With Predominant Abdominal Bloating: Short- and Long-term Results From a Placebo-Controlled Randomized Trial. Gastroenterology 2021; 160: 145-157. e8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 92] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 17. | Benech N, Sokol H. Fecal microbiota transplantation in gastrointestinal disorders: time for precision medicine. Genome Med. 2020;12:58. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Grundmann O, Yoon SL. Irritable bowel syndrome: epidemiology, diagnosis and treatment: an update for health-care practitioners. J Gastroenterol Hepatol. 2010;25:691-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 19. | Camilleri M. Diagnosis and Treatment of Irritable Bowel Syndrome: A Review. JAMA. 2021;325:865-877. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 20. | Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 360] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 21. | Kwan AC, Hu WH, Chan YK, Yeung YW, Lai TS, Yuen H. Prevalence of irritable bowel syndrome in Hong Kong. J Gastroenterol Hepatol. 2002;17:1180-1186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Husain N, Chaudhry IB, Jafri F, Niaz SK, Tomenson B, Creed F. A population-based study of irritable bowel syndrome in a non-Western population. Neurogastroenterol Motil. 2008;20:1022-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Xiong LS, Chen MH, Chen HX, Xu AG, Wang WA, Hu PJ. A population-based epidemiologic study of irritable bowel syndrome in South China: stratified randomized study by cluster sampling. Aliment Pharmacol Ther. 2004;19:1217-1224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Chang FY, Lu CL, Chen TS. The current prevalence of irritable bowel syndrome in Asia. J Neurogastroenterol Motil. 2010;16:389-400. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | El-Salhy M, Valeur J, Hausken T, Gunnar Hatlebakk J. Changes in fecal short-chain fatty acids following fecal microbiota transplantation in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2021;33:e13983. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 26. | Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A, Segal J, Aloi M, Masucci L, Molinaro A, Scaldaferri F, Gasbarrini G, Lopez-Sanroman A, Link A, de Groot P, de Vos WM, Högenauer C, Malfertheiner P, Mattila E, Milosavljević T, Nieuwdorp M, Sanguinetti M, Simren M, Gasbarrini A; European FMT Working Group. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569-580. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 601] [Cited by in F6Publishing: 662] [Article Influence: 94.6] [Reference Citation Analysis (0)] |
| 27. | Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 973] [Cited by in F6Publishing: 1073] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 28. | Roalfe AK, Roberts LM, Wilson S. Evaluation of the Birmingham IBS symptom questionnaire. BMC Gastroenterol. 2008;8:30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 69] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 29. | Hendriks C, Drent M, Elfferich M, De Vries J. The Fatigue Assessment Scale: quality and availability in sarcoidosis and other diseases. Curr Opin Pulm Med. 2018;24:495-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 30. | Drent M, Lower EE, De Vries J. Sarcoidosis-associated fatigue. Eur Respir J. 2012;40:255-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 31. | Atkins C, Fordham R, Clark AB, Stockl A, Jones AP, Wilson AM. Feasibility study of a randomised controlled trial to investigate the treatment of sarcoidosis-associated fatigue with methylphenidate (FaST-MP): a study protocol. BMJ Open. 2017;7:e018532. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Drossman DA, Patrick DL, Whitehead WE, Toner BB, Diamant NE, Hu Y, Jia H, Bangdiwala SI. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol. 2000;95:999-1007. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 266] [Cited by in F6Publishing: 280] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 33. | Wong RK, Drossman DA. Quality of life measures in irritable bowel syndrome. Expert Rev Gastroenterol Hepatol. 2010;4:277-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 34. | Arslan G, Lind R, Olafsson S, Florvaag E, Berstad A. Quality of life in patients with subjective food hypersensitivity: applicability of the 10-item short form of the Nepean Dyspepsia Index. Dig Dis Sci. 2004;49:680-687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Zijlstra JB, Beukema J, Wolthers BG, Byrne BM, Groen A, Dankert J. Pretreatment methods prior to gaschromatographic analysis of volatile fatty acids from faecal samples. Clin Chim Acta. 1977;78:243-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 138] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 36. | Høverstad T, Fausa O, Bjørneklett A, Bøhmer T. Short-chain fatty acids in the normal human feces. Scand J Gastroenterol. 1984;19:375-381. [PubMed] [Cited in This Article: ] |
| 37. | Soret R, Chevalier J, De Coppet P, Poupeau G, Derkinderen P, Segain JP, Neunlist M. Short-chain fatty acids regulate the enteric neurons and control gastrointestinal motility in rats. Gastroenterology. 2010;138:1772-1782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 298] [Cited by in F6Publishing: 317] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 38. | Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1657] [Cited by in F6Publishing: 1670] [Article Influence: 104.4] [Reference Citation Analysis (0)] |
| 39. | Zhou J, Martin RJ, Tulley RT, Raggio AM, McCutcheon KL, Shen L, Danna SC, Tripathy S, Hegsted M, Keenan MJ. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am J Physiol Endocrinol Metab. 2008;295:E1160-E1166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 297] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 40. | Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324:353-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 336] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 41. | Zhou J, Martin RJ, Raggio AM, Shen L, McCutcheon K, Keenan MJ. The importance of GLP-1 and PYY in resistant starch's effect on body fat in mice. Mol Nutr Food Res. 2015;59:1000-1003. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Zhang J, Song L, Wang Y, Liu C, Zhang L, Zhu S, Liu S, Duan L. Beneficial effect of butyrate-producing Lachnospiraceae on stress-induced visceral hypersensitivity in rats. J Gastroenterol Hepatol. 2019;34:1368-1376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 113] [Cited by in F6Publishing: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 43. | Long X, Li M, Li LX, Sun YY, Zhang WX, Zhao DY, Li YQ. Butyrate promotes visceral hypersensitivity in an IBS-like model via enteric glial cell-derived nerve growth factor. Neurogastroenterol Motil. 2018;30:e13227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 44. | Banasiewicz T, Krokowicz Ł, Stojcev Z, Kaczmarek BF, Kaczmarek E, Maik J, Marciniak R, Krokowicz P, Walkowiak J, Drews M. Microencapsulated sodium butyrate reduces the frequency of abdominal pain in patients with irritable bowel syndrome. Colorectal Dis. 2013;15:204-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 45. | Tana C, Umesaki Y, Imaoka A, Handa T, Kanazawa M, Fukudo S. Altered profiles of intestinal microbiota and organic acids may be the origin of symptoms in irritable bowel syndrome. Neurogastroenterol Motil. 2010;22:512-519, e114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 186] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 46. | Valeur J, Røseth AG, Knudsen T, Malmstrøm GH, Fiennes JT, Midtvedt T, Berstad A. Fecal Fermentation in Irritable Bowel Syndrome: Influence of Dietary Restriction of Fermentable Oligosaccharides, Disaccharides, Monosaccharides and Polyols. Digestion. 2016;94:50-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 47. | Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615-627. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |