Published online Jan 28, 2015. doi: 10.3748/wjg.v21.i4.1189
Peer-review started: June 2, 2014
First decision: June 27, 2014
Revised: August 25, 2014
Accepted: September 29, 2014
Article in press: September 30, 2014
Published online: January 28, 2015
AIM: To determine characteristics and prognostic predictors of patients with hepatocellular carcinoma (HCC) in association with non-alcoholic fatty liver disease (NAFLD).
METHODS: We reviewed the records of all patients with NAFLD associated HCC between 2000 and 2012. Data collected included demographics; histology; presence or absence of cirrhosis, size and number of HCC, alpha-fetoprotein, body mass index (BMI), and the presence of diabetes, hypertension, or dyslipidaemia.
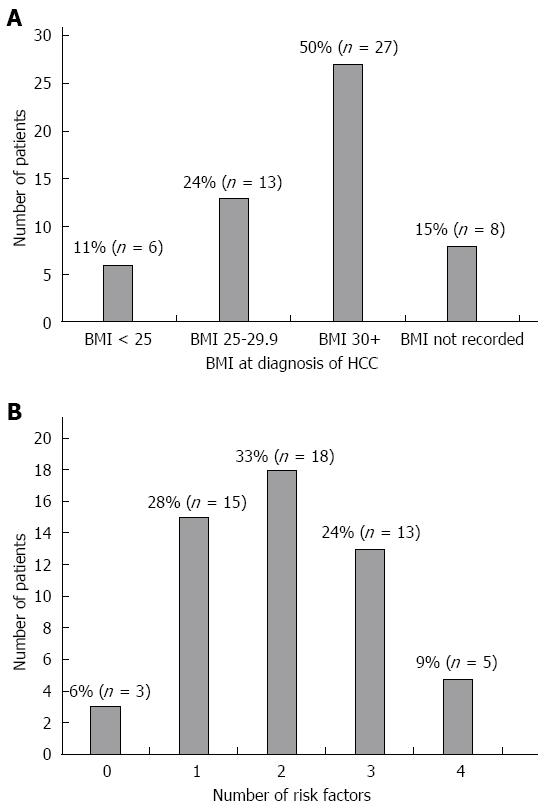
RESULTS: Fifty-four patients with NAFLD associated HCC were identified. Mean age was 64 years with 87% male. Fifteen percent (8/54) were not cirrhotic. 11%, 24% and 50% had a BMI of < 25 kg/m2, 25-29 kg/m2 and ≥ 30 kg/m2 respectively. Fifty-nine percent were diabetic, 44% hypertensive and 26% hyperlipidaemic. Thirty-four percent of the patients had ≤ 1 of these risk factors. Non-cirrhotics had a significantly larger mean tumour diameter at diagnosis than cirrhotics (P = 0.041). Multivariate analysis did not identify any other patient characteristics that predicted the size or number of HCC.
CONCLUSION: HCC can develop in NAFLD without cirrhosis. At diagnosis such tumours are larger than those in cirrhotics, conferring a poorer prognosis.
Core tip: Our study confirms that hepatocellular carcinoma can occur in non-cirrhotic non-alcoholic fatty liver disease, the incidence of which is rising worldwide. Moreover, these cancers were found to be significantly larger and more likely to be beyond Milan criteria for liver transplantation than those occurring in cirrhotic patients. Further research is needed to identify clinical risk factor profiles predisposing to cancer development in patients with non-alcoholic fatty liver disease such that screening if implemented can be appropriately targeted.
- Citation: Leung C, Yeoh SW, Patrick D, Ket S, Marion K, Gow P, Angus PW. Characteristics of hepatocellular carcinoma in cirrhotic and non-cirrhotic non-alcoholic fatty liver disease. World J Gastroenterol 2015; 21(4): 1189-1196
- URL: https://www.wjgnet.com/1007-9327/full/v21/i4/1189.htm
- DOI: https://dx.doi.org/10.3748/wjg.v21.i4.1189
Non-alcoholic fatty liver disease (NAFLD) affects up to 30% of the population in industrialised countries and is becoming more prevalent in the developing world as living standards rise and dietary habits change[1,2]. Although most individuals with this condition do not develop serious liver disease, it is associated with an increased annual incidence of hepatocellular carcinoma (HCC) of 76-201 per 100000[3,4] compared to a background incidence of sporadic HCC of 4.9-16 per 100000[5,6]. Recent studies identified NAFLD or cryptogenic cirrhosis (which is thought to often represent end-stage NAFLD[7]) as the underlying cause in 13% to 38.2% of patients presenting with HCC[8-10].
Diabetes and obesity have been suggested as risk factors for HCC in large cohort and case-control studies[11-13] both with and without pre-existing NAFLD. A meta-analysis by Larsson et al[14] of 10 cohort studies found a relative risk of HCC of 1.89 in obese patients. Another meta-analysis of 17 case control studies and 32 cohort studies by Wang et al[15] found a relative risk of HCC of 2.31 in diabetics.
Whether other features of the metabolic syndrome such as hypertension and dyslipidaemia may also contribute to HCC risk is less well studied, though these conditions have been shown to independently correlate with NAFLD fibrotic severity which itself may increase HCC risk[16-18].
2012 American Association for the Study of Liver Diseases (AASLD) guidelines state that the risk of HCC in NAFLD is “likely limited to those with advanced fibrosis and cirrhosis”[19] and as such there are no established HCC screening guidelines for NAFLD patients in general[20]. However, recent international reports that HCC occurs in non cirrhotic patients with NAFLD[21] suggest that work is needed to identify factors other than severe fibrosis or cirrhosis that could be used to identify patients in whom HCC screening may be justified.
The aims of this study in a cohort of patients with NAFLD associated HCC were: (1) to describe the risk factor profile of these patients focussing on features of the metabolic syndrome; (2) to determine any demographic or risk factor profile differences between cirrhotics and non-cirrhotics; and (3) to determine if any risk factors correlated with poorer prognosis, in terms of HCC size and number.
This retrospective study was conducted at the Victorian Liver Transplant Unit which provides all liver transplant services to the states of Victoria and Tasmania covering a population of approximately 5 million people. As the sole liver transplant referral centre for this population, it has become the major tertiary referral centre for patients with HCC. The records of patients with “hepatocellular carcinoma”, “HCC”, “liver cell cancer” or “hepatoma” and “NAFLD”, “fatty liver”, “NASH” or “cryptogenic cirrhosis” between 2000 and 2012 based on International Classification of Diseases 10 coding were audited, using hospital medical records and pathology department databases.
Patients were considered to have HCC if they had radiological and/or histological diagnoses according to AASLD guidelines[22]. Patients were included if they had characteristic radiological or histological features of NAFLD as recommended in Asia-Pacific Guidelines[23], or if the diagnosis of NAFLD or cryptogenic cirrhosis had previously been made in a patient with relevant risk factors and if concomitant causes of liver diseases including cleared or chronic hepatitis B (defined as having detectable hepatitis B core antibody, with or without positive surface antigen), chronic hepatitis C, Wilson’s disease, haemochromatosis, autoimmune hepatitis, alpha1-antitrypsin deficiency, cystic fibrosis, primary biliary cirrhosis, primary sclerosing cholangitis and other chronic biliary tract diseases and other hepatic malignancies had been excluded by relevant blood tests and/or liver histology. Patients were excluded if they had an alcohol intake of over 140 g per week for men and 70 g per week for women. These diagnostic criteria broadly concur with those in international (Chinese, Italian, European and American) guidelines as summarised by Nascimbeni et al[24].
Information was collected from the time of HCC diagnosis including patient sex, age, radiological or histological evidence of cirrhosis, Child-Pugh scores, histological NAFLD grade and stage where available according to Brunt criteria[25], size and number of HCCs, alpha-fetoprotein (AFP); metabolic profile (presence of diabetes, hypertension, dyslipidaemia and obesity) and medications. Obesity was measured via body mass index (BMI) instead of waist circumference which was not recorded in a majority of patients. No patients were of Asian background (in whom altered BMI cut-offs for overweight or obesity would have otherwise applied).
Diabetes was defined as having had a previous diagnosis of the disorder and/or being on relevant medication. Hypertension was defined as having a systolic blood pressure of over 130 mmHg and/or being on relevant medication. Dyslipidaemia was defined as being on relevant medication, having serum triglyceride levels > 2.0 mmol/L and/or total serum cholesterol levels > 5.5 mmol/L.
Univariate analysis was performed using Kruskal-Wallis testing, Fisher’s exact test and two-sample t testing to analyse for differences between cirrhotic and non-cirrhotic cohorts. Multivariate analysis was performed with Pearson correlation analysing for predictors of tumour size and number, and thus prognosis. Ethical approval was obtained by the Austin Hospital Human Research Ethics Committee.
Between 2000-2012, 39 (13%) patients with HCC had a primary diagnosis of NAFLD and 15 (5%) cryptogenic cirrhosis. Therefore 54 patients were included in our study, 47 (87%) were male and mean age was 64 years.
Liver fibrosis and cirrhosis: Thirty (57%) patients had diagnostic biopsies of non-HCC liver parenchyma available for review. Of these, 7 (23%), 11 (37%), 9 (30%) and 3 (10%) had NAFLD grade 0, 1, 2 and 3 respectively. Twenty four were cirrhotic (stage 4) on biopsy. Six were non-cirrhotic: 2 were stage 0 with NAFLD grade 1; and 4 were stage 1-2 with NAFLD grade 2. As such, all non-cirrhotic patients had some degree of inflammation. Notably, there were none with just simple steatosis (grade 0) in our cohort.
Twenty four patients did not have biopsies or had missing biopsy information, despite a previous diagnosis of either NAFLD or cryptogenic cirrhosis. Twenty two of these had evidence of cirrhosis on CT or ultrasound scanning, while another two were non-cirrhotic on imaging.
In total, forty six of the fifty four patients had cirrhosis which was diagnosed either histologically in 24 (52%) patients or radiologically in 22 (48%) patients. Of these, 18 (40%), 14 (30%) and 13 (28%) had Child-Pugh scores of A, B and C respectively at time of HCC diagnosis. One patient had missing biochemical information and thus a Child-Pugh score could not be calculated. Demographic and risk factor profiles of patients with NAFLD associated cirrhosis and cryptogenic cirrhosis were not statistically different, except for a higher prevalence of hypertension in the former cohort (Table 1).
| NAFLD cirrhotics (n = 31) | Cryptogenic cirrhotics (n = 15) | P value | |
| Median age (yr) | 65 | 65 | 0.680 |
| Median BMI (kg/m2) | 31.35 | 28 | 0.407 |
| Overweight/obesity | 72% | 73% | 0.919 |
| Diabetes | 72% | 40% | 0.058 |
| Hyperlipidaemia | 29% | 33% | 0.952 |
| Hypertension | 48% | 13% | 0.025 |
| Number of risk factors (n) | 0.310 | ||
| 0 | 2 | 1 | |
| 1 | 6 | 6 | |
| 2 | 10 | 6 | |
| 3 | 9 | 2 | |
| 4 | 4 | 0 | |
| Child-Pugh Score | 0.880 | ||
| A | 12 | 6 | |
| B | 10 | 4 | |
| C | 8 | 5 | |
Other HCC risk factors: Thirteen patients (24%) were overweight at HCC diagnosis (BMI 25-29.9 kg/m2) and 27 (50%) were obese (BMI equal to or greater than 30 kg/m2) according to World Health Organisation guidelines. Six (11%) had a BMI under 25 kg/m2. Median BMI was 31 kg/m2. In the remaining 8 patients, BMI was not recorded (Figure 1A).
Overall 40 (74%) were either overweight or obese, 32 (59%) were diabetic, 24 (44%) had hypertension and 14 (26%) had dyslipidaemia. Six percent, 28%, 33%, 24% and 9% had 0, 1, 2, 3, 4 of the above risk factors respectively (Figure 1B).
HCC characteristics: Twenty seven (60%) cirrhotic patients were diagnosed while asymptomatic by scheduled screening, with 9 (20%) diagnosed due to symptoms of hepatic decompensation and 10 (20%) diagnosed incidentally when being imaged for other reasons. Four (50%) non-cirrhotic patients were diagnosed due to symptoms and in 4 it was found incidentally. Eighteen (33%) patients had AFP levels in the normal range (< 5.8 kU/L). Median AFP was 12.2 kU/L. In twenty four patients (43%), HCC was already multifocal by time of diagnosis. The median maximum diameter was 3 cm. Twenty patients (37%) were already outside the Milan criteria[26] for transplantation at diagnosis.
Differences between non-cirrhotics and cirrhotics: Between non-cirrhotics and cirrhotics there were no statistically significant differences in BMI, age or level of HCC differentiation, number of risk factors, number of HCC’s or NAFLD grade. However non-cirrhotic patients had a higher median HCC diameter than cirrhotics at diagnosis (4.7 cm vs 3.2 cm, P = 0.041). Similarly, a greater proportion of non-cirrhotics failed the Milan criteria for transplantation (87.5% vs 28.2%, P = 0.003) (Table 2).
| Cirrhotics (n = 46) | Non-cirrhotics (n = 8) | P value | |
| Median age (yr) | 65 | 69 | 0.227 |
| Median BMI (kg/m2) | 30 | 30 | 0.939 |
| Median HCC diameter (cm) | 3.2 | 4.7 | 0.041 |
| Median number of HCCs | 1 | 2 | 0.147 |
| Number of risk factors (n) | 0.969 | ||
| 0 | 3 | 0 | |
| 1 | 12 | 3 | |
| 2 | 16 | 2 | |
| 3 | 11 | 2 | |
| 4 | 4 | 1 | |
| NAFLD grade (n) | 0.188 | ||
| 0 | 8 | 0 | |
| 1 | 9 | 2 | |
| 2 | 5 | 4 | |
| 3 | 3 | 0 | |
| Level of HCC differentation (n) | 0.317 | ||
| Well | 10 | 4 | |
| Moderate | 7 | 1 | |
| Poor | 0 | 0 | |
| Necrotic | 1 | 0 |
There were no statistically significant differences between cirrhotics and non cirrhotics in terms of the prevalence of individual HCC risk factors (Table 3).
| Cirrhotics (n = 46) | Non-cirrhotics (n = 8) | P value | |
| Overweight/obesity | 74% | 75% | 0.660 |
| Diabetes | 61% | 50% | 0.410 |
| Hyperlipidaemia | 24% | 37.50% | 0.340 |
| Hypertension | 43% | 50% | 0.767 |
Predictors of tumour characteristics: Multivariate analysis was conducted to determine if patient characteristics apart from presence or absence of cirrhosis could predict the size or number of HCCs and thereby the prognosis. Characteristics including sex, presence of diabetes, hypertension, hyperlipidaemia and/or overweight/obesity, number of risk factors, Child-Pugh scores, NAFLD grade and fibrosis stage, age at diagnosis of HCC and BMI were analysed. Only fibrosis stage significantly correlated with tumour size, with non-cirrhotics more likely to have larger tumours than cirrhotics (P = 0.03) (Table 4). No characteristics significantly correlated with tumour number (Table 5).
| Pearson correlation | P value | |
| Overweight/obesity | -0.256 | 0.093 |
| Diabetes | 0.038 | 0.791 |
| Hypertension | 0.106 | 0.470 |
| Dyslipidaemia | 0.146 | 0.510 |
| Age at diagnosis | 0.142 | 0.320 |
| Gender | 0.042 | 0.770 |
| BMI | -0.026 | 0.867 |
| Number of risk factors | -0.090 | 0.534 |
| Stage of fibrosis | -0.308 | 0.030 |
| Grade of NAFLD | 0.243 | 0.196 |
| Child-Pugh Score | -0.170 | 0.238 |
| Pearson correlation | P value | |
| Overweight/obesity | 0.174 | 0.248 |
| Diabetes | -0.270 | 0.053 |
| Hypertension | -0.040 | 0.779 |
| Dyslipidaemia | 0.080 | 0.715 |
| Age at diagnosis | -0.041 | 0.771 |
| Gender | -0.090 | 0.523 |
| BMI | 0.064 | 0.677 |
| Number of risk factors | 0.055 | 0.696 |
| Stage of fibrosis | -0.058 | 0.682 |
| Grade of NAFLD | -0.192 | 0.310 |
| Child-Pugh Score | 0.179 | 0.208 |
This is the first cohort study to examine NAFLD-associated HCC in an Australian context, where the majority were obese and diabetic. Furthermore, it is the first, to our knowledge, to include patients with cryptogenic cirrhosis as a subset of NAFLD given evidence strongly linking the two conditions. Indeed, in our cohort, patients with NAFLD cirrhosis had similar demographic and risk factor profiles to those with cryptogenic cirrhosis. This concurs well with other studies which have found that patients with cryptogenic cirrhosis are more likely to be obese and up to four times as likely to have diabetes than other forms of cirrhosis[27]. Moreover, there is a high rate of NAFLD development after transplantation for cryptogenic cirrhosis[28].
Our study also adds to the increasing body of evidence that HCC can occur in patients with non-cirrhotic NAFLD, even in those who are non-obese. This concurs well with recent studies including two large Japanese studies of of 292[29] and 87 patients[30] with NAFLD and HCC that reported non-cirrhotic patients comprised 38% and 49% respectively of these patients. Duan et al[31], pooling 169 NAFLD patients from 25 smaller cohorts in the literature, found 40% were non-cirrhotic and a French series of 31 HCC patients with at least 2 features of the metabolic syndrome (25 of whom had histological evidence of hepatic steatosis) found 66% were non-cirrhotic[32].
There are multiple postulated mechanisms for HCC occurring in non-cirrhotic NAFLD. Hepatic steatosis and concomitant insulin resistance causes oxidative (via increased reactive oxygen species[33] and carcinogenic metabolites of lipid peroxidation such as trans-4-hydroxy-nonenal[34]), inflammatory (upregulation of tumour necrosis factor-α, interleukin-6 and nuclear factor kappa-light-chain-enhancer of activated B cells[35]), apoptotic and hormonal changes. Increased activity of c-Jun amino-terminal kinase 1 and consequent phosphorylation of insulin receptor substrate-1[36] increase hepatic inflammation and apoptosis through downstream modulation of such pathways as mitogen-active protein kinase and phosphatidylinositol-3 kinase[37]. Adiponectin levels are decreased in NAFLD with subsequent loss of its anti-angiogenic and anti-inflammatory effects[38]. There are also decreased levels of binding proteins of insulin-like growth factor-1, with its subsequent increased bioavailability promoting cellular proliferation[39]. More novel pathways being investigated include the phosphorylation of adenosine monophosphate-activated protein kinase and activation of target of rapamycin complex 1 which then inhibits hepatic autophagy and its “quality control” effects in the liver to remove carcinogenic material[40].
Our finding that HCCs in non-cirrhotic patients are significantly larger at diagnosis is concerning as tumour size correlates with worse prognosis according to the Barcelona Clinic Liver Cancer staging system[6] and likely reflects lead time bias due to a lack of screening. Also, importantly, none of these patients with HCC had cholangiocarcinoma. A similar result was found by Paradis et al[32] in a French audit of 31 patients in whom 20 (65%) were non-cirrhotic with F0-F2 fibrosis scores, with larger tumours than in cirrhotics (median diameter 10.1 cm vs 6.6 cm, P = 0.05). While this suggests that HCC screening programs in non-cirrhotic NAFLD patients may prevent significant morbidity, the high prevalence of NAFLD in the general population makes such screening difficult to justify in terms of cost-effectiveness.
Furthermore, the incidence of HCC development in non-cirrhotic NAFLD appears to be low. There is limited literature addressing this issue with many longitudinal cohort studies having either focussed on NAFLD patients who are cirrhotic at baseline, or are confounded by not analysing if non-cirrhotic NAFLD patients who develop HCC had also become cirrhotic in the interim. In 399 non-cirrhotic NAFLD patients followed up over a mean of 7.6 years who did not develop cirrhosis on serial biopsies, Adams et al[41] found no HCCs. Similarly, no HCCs were found in a study of 64 non-cirrhotic patients followed up over a mean of 13.7 years[42] and a cohort of 109 patients over 16.7 years[43]. In the largest cohort of NAFLD patients prospectively studied, Arase et al[3] found in 1600 ultrasound-diagnosed NAFLD patients followed up over a median of 8.2 years, a HCC incidence of 0.6% (10 patients) was found, which is equivalent to 0.08% per year. Out of these 10 patients, 7 had histology taken at the time of HCC diagnosis with 6 being non-cirrhotic (Stage 0-3). Unfortunately, this study did not specify the proportion of cirrhotics in the total cohort. These low rates correlate with the data from our large centre study which identified only 15 non-cirrhotic NAFLD patients in 13 years. Moreover, there is no way to establish a clear causal link between steatosis and carcinogenesis in all these patients. Some may have been patients who developed sporadic HCC in whom the presence of hepatic steatosis may have been coincidental. Others may have had HCC derived from a pre-existing adenoma, since there is increasing literature postulating that the metabolic syndrome may drive malignant transformation of adenomas[44].
If HCC screening of non-cirrhotic NAFLD patients were to be viable, this would require narrowing the screened population by targeting those with specific risk factor profiles associated with HCC. Unfortunately we could not identify such a profile that defined our non-cirrhotic group. Comparisons of previous large cohort studies suggest that patients with NAFLD-associated HCC are more likely to be diabetic than the general NAFLD population (up to 70%[29] vs 46%[45], respectively). However, the proportion of non-cirrhotics who develop HCC in these studies was not clear and they were performed in Japanese cohorts making their findings potentially less generalisable to a Western population. Moreover, the high prevalence of diabetes in the NAFLD population means this cannot be used alone as an indication for cost-effective HCC screening. We have also shown that the cumulative number of HCC risk factors is not useful in determining HCC risk, as many of our non-cirrhotic cohort had less than 2 risk factors. Similarly we were unable to identify patient characteristics or risk factors correlating with poorer tumour prognosis that theoretically could guide targeted screening. Finally we have found AFP poorly sensitive for the presence of HCC, reflecting previous literature and 2011 AASLD guidelines that recommend against the use of AFP to guide screening[22].
In conclusion, this is the first Australian study describing the risk factor profile of patients with NAFLD-associated HCC. Even though we have a small study size and a significant bias toward males, we have shown that HCC occurs in non-cirrhotic NAFLD patients. However this is of uncertain clinical significance in the context of the burgeoning NAFLD epidemic as rates of this phenomenon still appear low. In such patients, tumours are larger than in cirrhotics which may be due to delayed diagnosis due to lack of screening. In regards to screening, we suggest, firstly, that novel biomarkers with more accuracy than AFP should be identified to better complement radiological screening. Secondly, of more importance, HCC screening in non-cirrhotic NAFLD cannot be deemed cost-effective without further studies identifying specific risk factor profiles that significantly restrict the potential target population.
We gratefully acknowledge Panteha Rezvan for her expert statistical assistance and Angela Li for her assistance with patient records.
The prevalence of non-alcoholic fatty liver disease (NAFLD) is rising worldwide, especially in industrialised countries such as Australia. This condition can progress to cirrhosis and the development of hepatocellular carcinoma (HCC). Furthermore, the condition of “cryptogenic cirrhosis” is often thought to represent end stage-NAFLD.
Basic science research has elucidated pro-carcinogenic mechanisms by which NAFLD could cause HCC in the absence of cirrhosis. At present, however, no guidelines recommend screening for HCC in non-cirrhotic NAFLD patients.
Of concern, there have been increasing reports of HCC occurring in non-cirrhotic NAFLD internationally over the last decade. This phenomenon, however, has not yet been described in an Australian cohort. Diabetes and obesity have been found to be independent risk factors for HCC development in NAFLD, but further research is needed to define such risk factors in specifically non-cirrhotic NAFLD cohorts that could guide cost-effective HCC screening.
This study reaffirms that HCC can develop in non-cirrhotic NAFLD, but could not identify particular risk factor profiles differentiating such patients from cirrhotic patients who develop HCC. Non-cirrhotic patients, however, had larger tumours at diagnosis than cirrhotic patients. This study thus underlines the need for further research into HCC risk factors amongst non-cirrhotic NAFLD patients, such that future HCC screening guidelines may take such patient groups into consideration in a cost-effective and targeted manner.
In this paper, the authors examined clinicopathological features of cases of NAFLD that developed HCC. As a result, they confirmed that HCC could develop in NAFLD without cirrhosis, and non-cirrhotics had a significantly larger mean tumour diameter than cirrhotics. HCC cases originating from NAFLD have been increasing, and to elucidate clinicopathological features of these cases is important. The present study elucidates a clinical aspect of carcinogenesis in NAFLD, and its results are important in establishing the screening method for non-cirrhotic NAFLD patients.
P- Reviewer: Lonardo A, Pan Q, Takahashi Y S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34:274-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2065] [Cited by in F6Publishing: 2143] [Article Influence: 164.8] [Reference Citation Analysis (0)] |
| 2. | Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, Srishord M. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524-530.e1; quiz e60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 758] [Cited by in F6Publishing: 748] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 3. | Arase Y, Kobayashi M, Suzuki F, Suzuki Y, Kawamura Y, Akuta N, Imai N, Kobayashi M, Sezaki H, Matsumoto N. Difference in malignancies of chronic liver disease due to non-alcoholic fatty liver disease or hepatitis C in Japanese elderly patients. Hepatol Res. 2012;42:264-272. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Söderberg C, Stål P, Askling J, Glaumann H, Lindberg G, Marmur J, Hultcrantz R. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595-602. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 529] [Cited by in F6Publishing: 523] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 5. | Altekruse SF, McGlynn KA, Reichman ME. Hepatocellular carcinoma incidence, mortality, and survival trends in the United States from 1975 to 2005. J Clin Oncol. 2009;27:1485-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1196] [Cited by in F6Publishing: 1285] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 6. | Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis. 2010;30:61-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 764] [Cited by in F6Publishing: 823] [Article Influence: 58.8] [Reference Citation Analysis (0)] |
| 7. | Caldwell SH, Crespo DM. The spectrum expanded: cryptogenic cirrhosis and the natural history of non-alcoholic fatty liver disease. J Hepatol. 2004;40:578-584. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 187] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Ertle J, Dechêne A, Sowa JP, Penndorf V, Herzer K, Kaiser G, Schlaak JF, Gerken G, Syn WK, Canbay A. Non-alcoholic fatty liver disease progresses to hepatocellular carcinoma in the absence of apparent cirrhosis. Int J Cancer. 2011;128:2436-2443. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 335] [Cited by in F6Publishing: 355] [Article Influence: 27.3] [Reference Citation Analysis (1)] |
| 9. | Marrero JA, Fontana RJ, Su GL, Conjeevaram HS, Emick DM, Lok AS. NAFLD may be a common underlying liver disease in patients with hepatocellular carcinoma in the United States. Hepatology. 2002;36:1349-1354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 307] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26:2183-2191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 219] [Cited by in F6Publishing: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 11. | El-Serag HB, Tran T, Everhart JE. Diabetes increases the risk of chronic liver disease and hepatocellular carcinoma. Gastroenterology. 2004;126:460-468. [PubMed] [Cited in This Article: ] |
| 12. | Kawamura Y, Arase Y, Ikeda K, Seko Y, Imai N, Hosaka T, Kobayashi M, Saitoh S, Sezaki H, Akuta N. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107:253-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 209] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 13. | Patton HM, Yates K, Unalp-Arida A, Behling CA, Huang TT, Rosenthal P, Sanyal AJ, Schwimmer JB, Lavine JE. Association between metabolic syndrome and liver histology among children with nonalcoholic Fatty liver disease. Am J Gastroenterol. 2010;105:2093-2102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer. 2007;97:1005-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 332] [Cited by in F6Publishing: 326] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 15. | Wang P, Kang D, Cao W, Wang Y, Liu Z. Diabetes mellitus and risk of hepatocellular carcinoma: a systematic review and meta-analysis. Diabetes Metab Res Rev. 2012;28:109-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 201] [Cited by in F6Publishing: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 16. | Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91-100. [PubMed] [Cited in This Article: ] |
| 17. | Hsiao PJ, Kuo KK, Shin SJ, Yang YH, Lin WY, Yang JF, Chiu CC, Chuang WL, Tsai TR, Yu ML. Significant correlations between severe fatty liver and risk factors for metabolic syndrome. J Gastroenterol Hepatol. 2007;22:2118-2123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Wang CC, Tseng TC, Hsieh TC, Hsu CS, Wang PC, Lin HH, Kao JH. Severity of fatty liver on ultrasound correlates with metabolic and cardiovascular risk. Kaohsiung J Med Sci. 2012;28:151-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2413] [Cited by in F6Publishing: 2449] [Article Influence: 204.1] [Reference Citation Analysis (0)] |
| 20. | Page JM, Harrison SA. NASH and HCC. Clin Liver Dis. 2009;13:631-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 21. | Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: an emerging menace. J Hepatol. 2012;56:1384-1391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 607] [Cited by in F6Publishing: 608] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 22. | Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 6338] [Article Influence: 487.5] [Reference Citation Analysis (1)] |
| 23. | Farrell GC, Chitturi S, Lau GK, Sollano JD. Guidelines for the assessment and management of non-alcoholic fatty liver disease in the Asia-Pacific region: executive summary. J Gastroenterol Hepatol. 2007;22:775-777. [PubMed] [Cited in This Article: ] |
| 24. | Nascimbeni F, Pais R, Bellentani S, Day CP, Ratziu V, Loria P, Lonardo A. From NAFLD in clinical practice to answers from guidelines. J Hepatol. 2013;59:859-871. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 261] [Article Influence: 23.7] [Reference Citation Analysis (1)] |
| 25. | Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467-2474. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2702] [Cited by in F6Publishing: 2730] [Article Influence: 109.2] [Reference Citation Analysis (0)] |
| 26. | Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5110] [Cited by in F6Publishing: 4991] [Article Influence: 178.3] [Reference Citation Analysis (0)] |
| 27. | Caldwell S, Argo C. The natural history of non-alcoholic fatty liver disease. Dig Dis. 2010;28:162-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 119] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Ong J, Younossi ZM, Reddy V, Price LL, Gramlich T, Mayes J, Boparai N. Cryptogenic cirrhosis and posttransplantation nonalcoholic fatty liver disease. Liver Transpl. 2001;7:797-801. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 29. | Tokushige K, Hashimoto E, Horie Y, Taniai M, Higuchi S. Hepatocellular carcinoma in Japanese patients with nonalcoholic fatty liver disease, alcoholic liver disease, and chronic liver disease of unknown etiology: report of the nationwide survey. J Gastroenterol. 2011;46:1230-1237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 30. | Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, Shima T, Kanbara Y, Saibara T, Mori T. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428-433; quiz e50. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 31. | Duan XY, Qiao L, Fan JG. Clinical features of nonalcoholic fatty liver disease-associated hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2012;11:18-27. [PubMed] [Cited in This Article: ] |
| 32. | Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, Bedossa P, Belghiti J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851-859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 416] [Cited by in F6Publishing: 385] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 33. | Yang S, Zhu H, Li Y, Lin H, Gabrielson K, Trush MA, Diehl AM. Mitochondrial adaptations to obesity-related oxidant stress. Arch Biochem Biophys. 2000;378:259-268. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 263] [Cited by in F6Publishing: 272] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 34. | Hu W, Feng Z, Eveleigh J, Iyer G, Pan J, Amin S, Chung FL, Tang MS. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis. 2002;23:1781-1789. [PubMed] [Cited in This Article: ] |
| 35. | Luedde T, Beraza N, Kotsikoris V, van Loo G, Nenci A, De Vos R, Roskams T, Trautwein C, Pasparakis M. Deletion of NEMO/IKKgamma in liver parenchymal cells causes steatohepatitis and hepatocellular carcinoma. Cancer Cell. 2007;11:119-132. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 468] [Cited by in F6Publishing: 468] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 36. | Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 449] [Cited by in F6Publishing: 430] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 37. | Hashimoto E, Tokushige K. Hepatocellular carcinoma in non-alcoholic steatohepatitis: Growing evidence of an epidemic? Hepatol Res. 2012;42:1-14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 38. | Bråkenhielm E, Veitonmäki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, Funahashi T, Cao Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci USA. 2004;101:2476-2481. [PubMed] [Cited in This Article: ] |
| 39. | Ohlsson C, Mohan S, Sjögren K, Tivesten A, Isgaard J, Isaksson O, Jansson JO, Svensson J. The role of liver-derived insulin-like growth factor-I. Endocr Rev. 2009;30:494-535. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 291] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 40. | Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131-1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2517] [Cited by in F6Publishing: 2788] [Article Influence: 185.9] [Reference Citation Analysis (0)] |
| 41. | Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113-121. [PubMed] [Cited in This Article: ] |
| 42. | Ekstedt M, Franzén LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1647] [Cited by in F6Publishing: 1624] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 43. | Dam-Larsen S, Franzmann M, Andersen IB, Christoffersen P, Jensen LB, Sørensen TI, Becker U, Bendtsen F. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53:750-755. [PubMed] [Cited in This Article: ] |
| 44. | Farges O, Ferreira N, Dokmak S, Belghiti J, Bedossa P, Paradis V. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011;60:85-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 235] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 45. | Hamaguchi M, Takeda N, Kojima T, Ohbora A, Kato T, Sarui H, Fukui M, Nagata C, Takeda J. Identification of individuals with non-alcoholic fatty liver disease by the diagnostic criteria for the metabolic syndrome. World J Gastroenterol. 2012;18:1508-1516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 67] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |