Published online Nov 7, 2017. doi: 10.3748/wjg.v23.i41.7459
Peer-review started: March 29, 2017
First decision: April 20, 2017
Revised: May 22, 2017
Accepted: June 18, 2017
Article in press: June 19, 2017
Published online: November 7, 2017
To demonstrate the non-inferiority (15% non-inferiority limit) of monotherapy with tenofovir disoproxil fumarate (TDF) vs the combination of lamivudine (LAM) plus adefovir dipivoxil (ADV) in the maintenance of virologic response in patients with chronic hepatitis B (CHB) and prior failure with LAM.
This study was a Phase IV prospective, randomized, open, controlled study with 2 parallel groups (TDF and LAM+ADV) of adult patients with hepatitis B e antigen (HBeAg)-negative CHB, prior failure with LAM, on treatment with LAM+ADV for at least 6 mo, without prior resistance to ADV and with an undetectable viral load at the start of the study, in 14 Spanish hospitals. The follow-up time for each patient was 48 wk after randomization, with quarterly visits in which the viral load, biochemical and serological parameters, adverse effects, adherence to treatment and consumption of hospital resources were analysed.
Forty-six patients were evaluated [median age: 55.4 years (30.2-75.2); 84.8% male], including 22 patients with TDF and 24 with LAM+ADV. During study development, hepatitis B virus DNA (HBV-DNA) remained undetectable, all patients remained HBeAg negative, and hepatitis B surface antigen (HBsAg) positive. Alanine aminotransferase (ALT) values at the end of the study were similar in the 2 groups (25.1 ± 7.65, TDF vs 24.22 ± 8.38, LAM+ADV, P = 0.646). No significant changes were observed in creatinine or serum phosphorus values in either group. No significant differences between the 2 groups were noted in the identification of adverse effects (AEs) (53.8%, TDF vs 37.5%, LAM+ADV, P = 0.170), and none of the AEs which occurred were serious. Treatment adherence was 95.5% and 83.3% in the TDF and the LAM+ADV groups, respectively (P = 0.488). The costs associated with hospital resource consumption were significantly lower with the TDF treatment than the LAM+ADV treatment (€4943 ± 1059 vs €5811 ± 1538, respectively, P < 0.001).
TDF monotherapy proved to be safe and not inferior to the LAM+ADV combination therapy in maintaining virologic response in patients with CHB and previous LAM failure. In addition, the use of TDF generated a significant savings in hospital costs.
Core tip: The Tenosimp-B study was performed to demonstrate the non-inferiority (15% non-inferiority limit) of tenofovir disoproxil fumarate (TDF) monotherapy versus the combination of lamivudine+adefovir (LAM+ADF) in 46 patients with chronic hepatitis B (CHB) and resistance to LAM (22 with TDF and 24 with LAM+ADV). TDF demonstrated its safety (no significant differences in adverse events (AEs), kidney function or liver function) and non-inferiority in maintaining virologic response [undetectable hepatitis B virus DNA (HBV-DNA) and negative for hepatitis B e antigen (HBeAg)] during the study, without differences in adherence to treatment. Additionally, the use of TDF resulted in significant savings in hospital costs.
- Citation: Rodríguez M, Pascasio JM, Fraga E, Fuentes J, Prieto M, Sánchez-Antolín G, Calleja JL, Molina E, García-Buey ML, Blanco MÁ, Salmerón J, Bonet ML, Pons JA, González JM, Casado MÁ, Jorquera F, the TENOSIMP-B Research Group. Tenofovir vs lamivudine plus adefovir in chronic hepatitis B: TENOSIMP-B study. World J Gastroenterol 2017; 23(41): 7459-7469
- URL: https://www.wjgnet.com/1007-9327/full/v23/i41/7459.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i41.7459
Approximately 400 million people worldwide are infected by the hepatitis B virus (HBV)[1]. Given that a significant portion of these patients receive treatment for management of chronic hepatitis B (CHB) over a long period of time[2-4], it is necessary to use new antivirals with potent action and an adequate long-term safety profile. Likewise, it is important that these medications possess a high genetic barrier that will lead to reduced HBV resistance rates[5]. The establishment of CHB treatment has, as its objective, the sustained suppression of virus replication to prevent disease progression and increase survival.
Sustained viral suppression with lamivudine (LAM) has been shown to reduce the progression of the disease, preventing the development of cirrhosis and the occurrence of complications, which include liver failure, hepatocellular carcinoma and liver-related mortality[6]. However, while LAM is an effective medication, its use has been limited due to the development of mutations in the polymerase region of HBV that promote resistance, causing loss of antiviral activity and its clinical benefit[7]. The rate of appearance of resistance is in the range of 15%-20% per year of treatment and may increase to up to 80% at 5 years after the start of treatment[8]. Management of LAM resistance has evolved in recent years. Initially, the recommended therapeutic option was to switch to adefovir dipivoxil (ADV)[9]. However, it was found that this strategy favoured the appearance of ADV resistance[10]. One randomized study demonstrated that the probability of developing ADV resistance at 3 years of treatment in patients with resistance to LAM was significantly higher in the patient group treated with ADV monotherapy than in the group receiving a combination of LAM plus ADV[11]. The Clinical Guidelines of the European Association for the Study of the Liver (EASL) recommend that patients with CHB and LAM resistance be changed to tenofovir disoproxil fumarate (TDF) or have ADV added if TDF is not available[12].
TDF, a prodrug of tenofovir, is a potent nucleotide analogue with high efficacy in CHB treatment[13]. Phase III clinical trials showed that TDF is superior to ADV in the suppression of viral replication and in histological improvement in both hepatitis B e antigen- (HBeAg-) positive and HBeAg-negative patients[5]. Furthermore, it is a drug with a high genetic barrier to resistance, with no reported cases of resistance during 6 years of treatment[14]. Additionally, the antiviral activity of TDF is maintained against HBV strains resistant to LAM[5,15,16].
The antiviral efficacy of TDF monotherapy has been demonstrated in patients with HBV infection and a partial prior response to ADV, including patients with a history of LAM resistance; its efficacy is similar to that of TDF in combination with emtricitabine (FTC) [17]. Based on these results and on the fact that, at the current time, there are no reports of TDF resistance, several authors have purposed TDF monotherapy treatment in patients with LAM resistance[12,18].
Patients who have previously failed LAM treatment and who have previously received combination treatment with LAM+ADV would be the ideal population to determine whether TDF monotherapy is equivalent to standard treatment (LAM+ADV) in maintaining a virologic response. As a result, the primary objective of the TENOSIMP-B study was to demonstrate the non-inferiority (with a limit of 15%) of TDF monotherapy in maintaining an undetectable viral load vs the combination LAM+ADV treatment in patients with CHB and prior failure of LAM. The secondary objectives of the study were to compare the safety profiles in each treatment group, especially the incidence of renal safety; to calculate adherence to treatment; and to determine the differences in hospital expenses for patients assigned to each of the treatment strategies.
TENOSIMP-B is a Phase IV open, randomized, controlled study of non-inferiority with 2 parallel arms (TDF and LAM+ADV) and prospective follow-up. The trial was performed in 14 Spanish public hospitals with the participation of adult patients with chronic HBV infection. The recruitment period was between August 2011 and January 2013. Patients were followed for 48 wk with intermediate visits during weeks 12, 24 and 36.
During the study period, patients were treated with the medication to be studied, and data were collected every 12 wk using an electronic case report form (eCRF). Variables collected included those related to the patient’s clinical data (HBV-DNA load and hepatitis B biochemical and serological parameters), information on possible adverse events (AEs) during study tracking and adherence to therapy using dispensation records provided by Hospital Pharmacy Services. In addition, through the use of a patient diary, information was compiled at each visit regarding the use of hospital resources by patients during the study period.
The randomization process was performed in blocks to ensure that each centre had a 1:1 ratio of patients with TDF (300 mg/d) or LAM (100 mg/d) plus ADV (10 mg/d). All drugs were administered orally.
The study was authorized by the Medication Research Ethics Committee (MREC) of the Hospital Universitario Central de Asturias (Oviedo, Asturias, Spain), which acted as the reference committee in coordination with committees from the other participating centres. The study was developed in accordance with the ethical principles stated in the Declaration of Helsinki and was consistent with the guidelines for good clinical practice and the applicable local regulatory requirements. All patients gave informed written consent for participation in the study.
Included in the study were patients with HBV infection with previous LAM failure who were rescued with LAM+ADV, who received this treatment for at least 6 mo and with undetectable viral load [HBV-DNA below the lower limit of quantification (LOQ)] before randomization, with compensated liver disease and with positive hepatitis B surface antigen (HBsAg) in the baseline visit.
Patients who were co-infected with another virus (hepatitis C, hepatitis D or HIV); were intolerant to one of the components of the therapeutic regimen; had HBV mutations associated with ADV resistance (as evidenced by resistance tests or history of virologic rebound reported in the case history); had hepatocellular carcinoma; had a liver or kidney transplant; had serious pulmonary or neurologic disease that might interfere with their participation in the study; were pregnant or lactating; were undergoing treatment with any experimental (unapproved) medications 30 d prior to the baseline visit; and/or had moderate to severe renal insufficiency with one of the following conditions were not included in this study: a glomerular filtration rate (GFR) ≤ 60 mL/min [using the abbreviated Modification of Diet in Renal Disease (MDRD) formula] and/or creatinine clearance (CrCl) ≤ 60 mL/min (according to the Cockcroft-Gault equation).
The primary variable in this study was the proportion of patients who maintained a sustained virologic response, defined as HBV-DNA levels that were undetectable (below the LOQ) at 48 wk of study using the quantitative HBV-DNA detection technique used in routine clinical practice in each of the participating centres. Additionally, during weeks 12, 24 and 36, the following parameters were evaluated: percentage of patients with sustained virologic response, percentage of patients with virologic rebound in each arm of treatment who developed resistance to ADV or TDF, percentage of patients with loss of HBsAg and percentage of patients with seroconversion to anti-HBs.
During each visit, the patient’s levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine, CrCl and serum phosphate were measured. Other measured parameters included safety, using the registry of serious AEs and the relationship of AEs to drugs administered in each visit; the degree of therapeutic compliance in each group; and the costs associated with each treatment group.
Safety: Safety analyses included all patients who received at least 1 drug dose during the study and all events that occurred during treatment. The safety parameters evaluated in this study included all AEs and all anomalies (in laboratory parameters) that were symptomatic or clinically significant (documented as AEs). All AEs were registered in the patient’s clinical record and in the eCRF. To assess toxicity of the AEs, the World Health Organization (WHO) recommendations were used. Patients with adverse reactions (ARs), that is, AEs related to the studied medication, were monitored using the pertinent clinical evaluations and laboratory analyses until satisfactory resolution of the event or stabilization.
Adherence: Adherence to treatment was calculated using the Morisky-Green test[19] and the dispensation records of the Pharmacy Service. The Morisky-Green test consists of 4 questions scored as 0 (negative response) or 1 (affirmative response). The dispensation records noted, in a retrospective form, the quantity of medication dispensed to the patient at the prior visit and the units returned to the hospital Pharmacy Service following 12 wk of treatment. Patients with missing data for these variables were considered non-adherent.
The calculation of the percentage of adherence to LAM and ADV was performed separately, and the lower adherence value was used to determine whether the patient was adherent; in these patients, it was considered failure to comply if only 1 of the 2 drugs used in treatment was taken.
A patient was considered adherent when the adherence percentage in the dispensation record was greater than or equal to 80% and the score obtained from the Morisky-Green test was between 0 and 1.
Analysis of expenses: Hospital resources consumed by patients were recorded throughout the 48 wk of the study with the objective of quantifying the cost in euros associated with the management of these patients. The costs calculation was obtained by finding the product of resources consumed multiplied by the unit cost associated with each resource. Unit costs (euros, 2014) of the resources were obtained from the healthcare cost database eSalud (eHealth)[20]. Pharmaceutical costs were estimated based on laboratory sale price (LSP) established in the medication catalogue of the General Council of Official Associations of Pharmacists[21], applying the deduction corresponding to each drug established by Royal Decree-Law 8/2010[22].
Except for safety analyses, in which the safety population was used, statistical analyses were performed in the population per protocol (PPP) function of statistical software R version 3.10.0[23]. For the hypothesis contrasts, an alpha risk of 0.05 was assumed. The included P-values were calculated based on bilateral contrast without adjustment for the performance of multiple contrasts.
For comparison of the percentage of patients maintaining a sustained virologic response at 48 wk of study and to evaluate differences in AE incidence in the 2 arms, Fisher’s exact text was used.
To evaluate differences in the evolution of hepatic and renal function, differences between baseline values and those obtained at weeks 12, 24, 36 and 48 were compared by hypothesis contrast using the Friedman test.
In the cost analysis, differences between costs were compared using the Mann-Whitney U test.
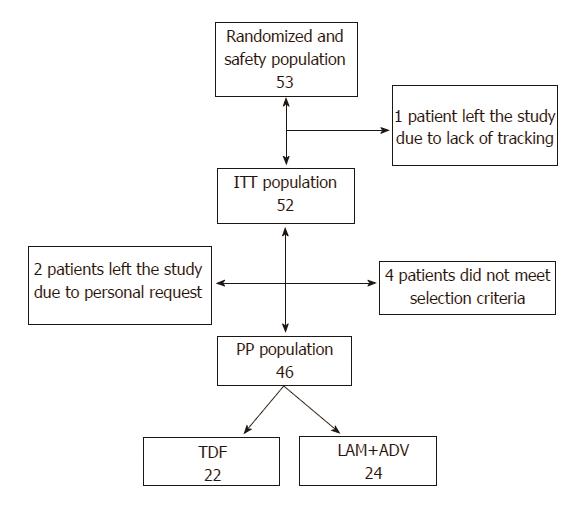
In total, 53 patients were randomized; of these, 4 patients did not comply with all study selection criteria (3 due to moderate or severe renal insufficiency and 1 due to not having a documented failure on LAM). Of the 49 remaining patients, 2 left the study by personal petition (1 after the baseline visit and another at the week-12 visit) and 1 due to lack of follow-up. For the study’s data analysis, the data of 46 patients was considered, which constituted the final PPP of the study. Of the randomized patients, 22 were assigned to the group administered 300 mg TDF/d and 24 patients were assigned to the group administered 100 mg LAM/d + 10 mg ADV/d (Figure 1).
The average age of the patients included in the analysis was 54.82 ± 11.88 years. Males comprised 84.8% of the study population. Obesity was present in 19.6% of the patients, and 8 patients (17.4%) had diabetes or hypertension. Globally, 19.6% of the patients had comorbidities in the gastrointestinal, cardiovascular or skeletal systems. A small proportion of patients (8.7%) had liver cirrhosis (Table 1).
| Characteristic | TDF | LAM+ADV | Total | P value |
| (n = 22) | (n = 24) | (n = 46) | ||
| Age - average (range) | 53.14 ± 11.95 | 56.35 ± 11.86 | 54.82 ± 11.88 | 0.377 |
| Gender | ||||
| Male | 17 (77.3) | 22 (91.7) | 39 (84.4) | |
| Female | 5 (22.7) | 2 (8.3) | 7 (15.2) | |
| Weight - average (range) | 75.37 (46 - 105) | 78.72 (62 - 107) | 77.08 (46 - 107) | 0.663 |
| Height - average (range) | 1.68 (1.48 - 1.93) | 1.70 (1.56 - 1.80) | 1.69 (1.48 - 1.93) | 0.30 |
| BMI - average (range) | 26.59 (17.10 - 32.96) | 27.17 (21.45-34.54) | 26.89 (17.1-34.54) | 0.883 |
| < 18.50 - Underweight | 2 (9.10) | 1 (4.20) | 3 (6.50) | |
| 18.50 - 24.99 - Normal | 7 (31.80) | 8 (33.30) | 15 (32.60) | |
| 25.0 - 29.9 - Overweight | 9 (40.90) | 10 (41.70) | 19 (41.30) | |
| ≥ 30.0 - Obese | 4 (18.20) | 5 (20.8) | 9 (19.60) | |
| LAM resistance establishment | 0.725 | |||
| Clinical records | 18 (81.8) | 18 (75.0) | 36 (78.3) | |
| Resistance test | 4 (18.2) | 6 (25.0) | 10 (21.7) | |
| Cirrhosis, F4 state | 3 (13.6) | 1 (4.2) | 4 (8.7) | 0.336 |
| Diabetes mellitus and/ or hypertension | 2 (9.1) | 6 (25.0) | 8 (17.4) | 0.247 |
A patient was considered to have LAM resistance when, after a negative HBV DNA result was obtained, virologic rebound was found without stopping LAM administration. All researchers verified the presence of LAM resistance in clinical records. In 21.7% of patients, LAM resistance was corroborated using a resistance test (Table 1).
No significant differences were found between both groups of therapy regarding sociodemographic, clinical and biochemical characteristics at baseline (Tables 1 and 2).
| Baseline visit | Visit at 48 wk | |||||
| TDF | LAM+ADV | P value | TDF | LAM+ADV | P value | |
| average (range) | average (range) | average (range) | average (range) | |||
| AST (UI/mL) | 26.66 (9.00-74.00) | 26.69 (15.00-69.00) | 0.904 | 26.41 (14.00-71.00) | 26.05 (18.00-52.00) | 0.916 |
| ALT (UI/mL) | 25.41 (12.00-62.00) | 23.62 (13.60-40.00) | 0.646 | 26.32 (12.00-55.00) | 27.29 (17.00-51.00) | 0.965 |
| Phosphorus (mmol/dL) | 3.00 (1.80-4.00) | 2.84 (1.90-3.80) | 0.263 | 3.08 (1.70-4.40) | 2.95 (2.00-3.70) | 0.832 |
| Urea (mg/dL) | 36.14 (20.00-53.00) | 36.96 (18.00-61.90) | 0.698 | 38.29 (25.00-57.00) | 38.69 (22.00-53.00) | 0.479 |
| Serum creatinine (mg/dL) | 0.88 (0.70-1.16) | 0.89 (0.60-1.35) | 0.912 | 0.88 (0.69-1.16) | 0.92 (0.60-1.27) | 0.396 |
| Glomerular filtration rate (mL/min/1.73 m2) | 94.13 (66.95-121.03) | 97.48 (60.47-157.83) | 0.922 | 93.11 (66.75-123.55) | 93.14 (59.14-151.26) | 0.468 |
| Creatinine clearance (mL/min) | 103.52 (65.57-185.25) | 105.43 (64.79-166.22) | 0.831 | 100.85 (60.84-149.66) | 102.32 (60.26-157.5) | 0.308 |
The HBV-DNA viral load remained below the LOQ for the length of the study (weeks 12, 24, 26 and 48) in 100% of patients in both treatment groups. As a result, no patient presented virologic rebound during the study period. HBeAg remained negative in all patients for the duration of the study. No patient cleared HBsAg during the 48 wk of the study.
Safety: Of the 53 patients evaluated in the safety analysis, none were found to have a serious AE (SAE) during study tracking, nor was there any discontinuation in either treatment group due to lack of efficacy prior to week 48.
A total of 25 AEs were evaluated during the study period; the common cold was the most frequent (10.89% of cases). Twenty-three of those cases were unrelated events, including 9 in 4 patients from the LAM+ADV group and 14 in 9 patients from the TDF group. The other 2 AEs occurred in the same patient and were considered moderate-intensity adverse reactions (RA) and not serious (digestive intolerance and muscle pains) (Table 3). In this case, the research team decided to discontinue the studied medication two weeks after randomization, abating the RA.
| Event | Intensity | TDF ( n = 26) | LAM+ADV ( n = 27) | Total (n = 53) | P value |
| Adverse reactions | 2 (7.70) | 0 | 2 (3.57) | 0.236 | |
| Digestive intolerance | moderate | 1 (3.85) | 0 | 1 (1.89) | 1.000 |
| Muscle pains | moderate | 1 (3.85) | 0 | 1 (1.89) | 1.000 |
| Adverse events | 14 (53.85) | 9 (37.5) | 23 (43.40) | 0.17 | |
| Common cold | mild | 3 (11.54) | 2 (7.41) | 5 (9.43) | 0.699 |
| Headache | mild | 1 (3.85) | 1 (3.70) | 2 (3.77) | 1.000 |
| Retrosternal oppression with heartburn | moderate | 0 | 1 (3.70) | 1 (1.89) | 1.000 |
| Otitis | mild | 1 (3.85) | 0 | 1 (1.89) | 0.491 |
| Molar extraction | mild | 1 (3.85) | 0 | 1 (1.89) | 0.491 |
| Right- and left-flank pain | mild | 1 (3.85) | 0 | 1 (1.89) | 0.491 |
| Vomiting | mild | 1 (3.85) | 0 | 1 (1.89) | 0.491 |
| Osteoarticular pain in hands | mild | 0 | 1 (3.70) | 1 (1.89) | 1.000 |
| Hypophosphatemia and hypomagnesemia | moderate | 0 | 1 (3.70) | 1 (1.89) | 1.000 |
| Renal colic | mild | 1 (3.85) | 0 | 1 (1.89) | 0.491 |
| Epigastric pain | mild | 1 (3.85) | 0 | 1 (1.89) | 0.491 |
| Back pain | mild | 1 (3.85) | 0 | 1 (1.89) | 0.491 |
| Chronic prostatitis | mild | 0 | 1 (3.70) | 1 (1.89) | 1.000 |
| Epidermoid carcinoma | moderate | 1 (3.85) | 0 | 1 (1.89) | 0.491 |
| Vertiginous syndrome | mild | 0 | 1 (3.70) | 1 (1.89) | 1.000 |
| Itching (eyes, nose and mouth) | mild | 1 (3.85) | 0 | 1 (1.89) | 0.491 |
| Vertigo | mild | 0 | 1 (3.70) | 1 (1.89) | 1.000 |
| Choluria | mild | 1 (3.85) | 0 | 1 (1.89) | 0.491 |
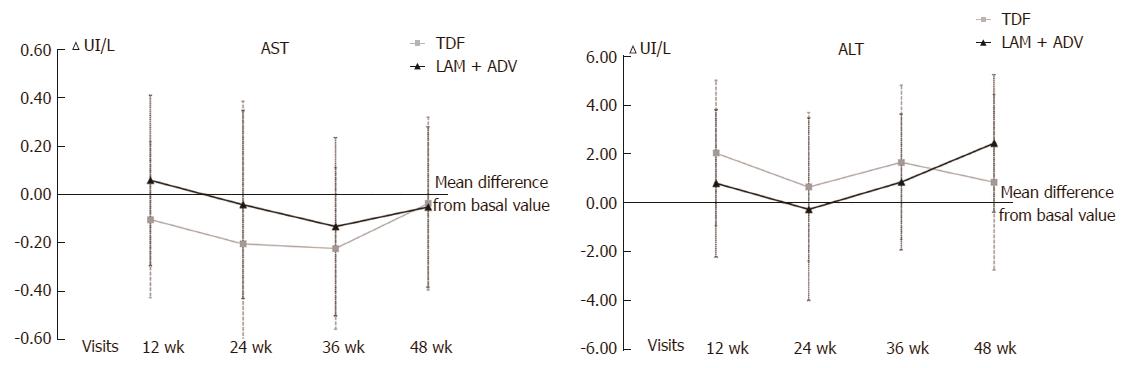
No statistically significant differences between the 2 study groups were found in the evolution of ALT and AST transaminase values (Figure 2), which were used to evaluate liver function, from the baseline visit to 48 wk of study.
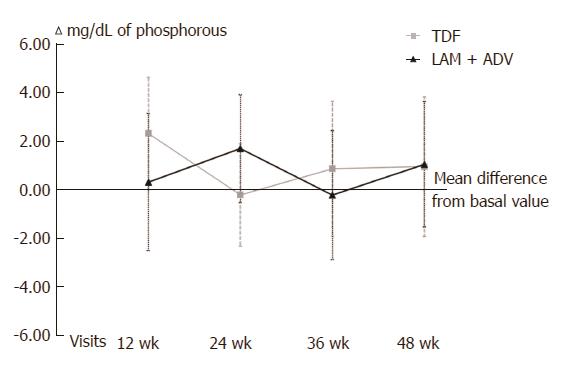
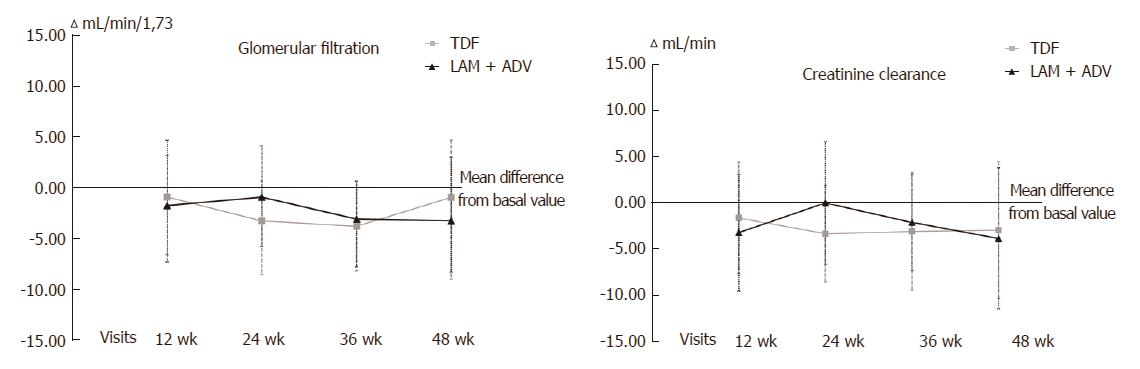
With respect to kidney function, no differences between the 2 groups were found in phosphorus levels (Figure 3), urea, serum creatinine, GFR or CrCl (Figure 4) at 48 wk of tracking (Table 2). Over the length of the study, 4 patients changed to a state of renal insufficiency. Two belonging to the LAM+ADV arm moved from moderate insufficiency (stage III) to mild (stage II) insufficiency and returned to stage III prior to the study’s end, and a third changed from stage II to stage III at the final visit. The 4th patient from the TDF group moved from stage II to stage III and back to stage II prior to the conclusion of the study.
Adherence: Overall, 89.1% of the patients in the study were considered adherent, and there was no significant difference between the 2 groups concerning adherence (TDF 95.5%; LAM+ADV 83.3%; P = 0.745). Three patients (1 in the TDF group and 2 in the LAM+ADV group) were considered non-adherent due to missing values for this variable, although they did not present with virologic rebound (their HBV-DNA was negative at the end of tracking).
Cost analysis: The total average hospital expense per patient treated with TDF and LAM+ADV was €4943 and €5811, respectively. These numbers indicate that, in patients undergoing TDF treatment, an average savings of €868 per patient was observed over the 48 wk of the study. The difference in average cost per patient between those treated with TDF and those treated with LAM+ADV was statistically significant. In concomitant medication costs and analytic and diagnostic tests, there were no statistically significant differences between the 2 treatment groups. Furthermore, there were statistically significant differences in drug costs, with an average cost savings per TDF patient vs LAM+ADV patient of €1252 (Table 4).
| Costs - average, € (SD) | TDF | LAM+ADV | Difference TDF vs LAM+ADV | P value |
| (n = 22) | (n = 24) | |||
| Studied medication | 2966 (69) | 4218 (558) | -1252 | < 0.0011 |
| Concomitant medication | 218 (599) | 82 (213) | 136 | 0.484 |
| Medical visits | 505 (158) | 500 (137) | 5 | 0.942 |
| Tests | 1388 (182) | 1363 (242) | 25 | 0.834 |
| Admissions and surgeries | 0 (0) | 0 (0) | 0 (0) | NA |
| Total | 4943 (1059) | 5811 (1538) | -868 | < 0.0011 |
LAM, the first nucleoside analogue that has been proven effective against HBV, was widely used during the 1990s in CHB treatment. As a consequence of its previous use and due to its low genetic barrier to resistance, a significant portion of patients with CHB who received this treatment have developed resistance[8]. For years, the addition of ADV to LAM has been the recommended regimen for the management of patients with LAM resistance[11]. A retrospective multi-centre study analysed the effectiveness of TDF monotherapy in patients who had failed treatment with LAM and/or ADV, noting a cumulative probability of virologic response of 79% after an average treatment duration of 23 mo. In this study, it was observed that the presence of LAM resistance did not influence the response to TDF monotherapy, whereas the presence of ADV resistance did influence the response[24]. In recent years, various randomized studies have shown that TDF monotherapy is as effective as a combined treatment with TDF and FTC or entecavir (ETV) in the rescue of patients with not only LAM resistance[25,26] but also resistance to other analogues such as ADV or ETV[27,28].
In the present study, the effectiveness and safety of a simplification of the therapeutic regimen consisting of TDF monotherapy was evaluated in patients who had failed treatment with LAM and who had been satisfactorily rescued with a combination of LAM+ADV. The simplification with TDF could, at least theoretically, encourage adherence to treatment, reduce adverse effects over the long term and decrease hospital expenses.
In this randomized study, it has been demonstrated that TDF monotherapy is as effective as the combination of LAM+ADV in maintaining a complete virologic response. During the 48 wk of the study, all patients maintained an undetectable HBV-DNA load. The preliminary results of a similar study carried out in Taiwan showed reappearance of HBV-DNA in 9.4% of patients assigned to TDF monotherapy and in 16.7% of those assigned to LAM+ADV, although in all cases the reappearance of viremia was transitory[29]. Another prospective study conducted in China demonstrated that TDF monotherapy was superior to a combination of LAM+ADV in achieving complete virologic response in patients with LAM resistance and suboptimal response to LAM+ADV[30]. Finally, a retrospective study carried out in the United States evaluated the strategy of simplifying treatment to TDF or ETV monotherapy in patients who had not completely responded to ETV and who had been satisfactorily rescued with the ETV+TDF combination[31]. In this study, it was observed that virologic rebound rates at 6 mo from the start of monotherapy were significantly higher (88%) in patients who received ETV than in those treated with TDF (39%). One of the factors associated in this report with the risk of presenting a virologic rebound was a duration of complete virologic response of less than 12 mo prior to simplification of treatment to monotherapy[31].
Regarding the biochemical response, in our study, we observed that ALT levels at the end of the study were similar to baseline levels and that there was no difference in ALT levels between patients who received monotherapy with TDF and those who continued combined treatment with LAM+ADV. On the other hand, during the 48 wk of the study, none of the patients lost HBsAg, an unsurprising finding given the reduced clearance rate of HBsAg in patients with HBeAg-negative CHB treated with nucleos(t)ide analogues, even when a potent analogue with a high generic barrier to resistance, such as TDF, is used[13].
In the safety evaluation, no serious adverse effects were observed during the study, and the rate of adverse effects was similar in the 2 study groups, with the most frequent adverse effect being the common cold. A patient from the TDF group presented with a drug-related adverse effect (digestive intolerance and muscle pains), which resulted in the interruption of treatment. As for renal safety, although both ADV and TDF can, on rare occasions, produce a Fanconi-like renal tubular acidosis, during the present study, no significant changes in the levels of serum phosphorus were observed in either of the 2 groups. Regarding kidney function, no differences were observed between the baseline visit and the end of treatment in either group, nor were there differences at the end of the study between patients who received TDF and those treated with LAM+ADV. Three patients, 2 in the LAM+ADV group and 1 in the TDF group, displayed mild and transitory deterioration in kidney function during the study, and 1 patient belonging to the LAM+ADV group moved from stage II at the start to stage IIIa at the end of the study. An observational study conducted in France in 214 patients who were treated with analogues for a median period of 2.4 years showed that kidney function decreased significantly in patients treated with ADV in monotherapy or combination therapy, whereas kidney function remained stable in those treated with LAM, TDF or ETV[32]. Although no differences were observed between the treatment groups in our study, likely due to its short duration and to the limited number of patients, it could be inferred that changing from the LAM+ADV combination to TDF may be beneficial for kidney function over the long term. Additionally, a new formulation of tenofovir, tenofovir alafenamide (TAF), has shown lower renal toxicity than TDF[33].
Another of the potential beneficial effects of treatment simplification may be greater treatment adherence. In our study, although adherence was greater in the TDF monotherapy group (95.3%) than in the combined treatment group (87.5%), the differences were not significant. It is possible that with a larger number of patients and a longer study period, these differences might have reached statistical significance.
Among the most relevant result of this study is the significant difference in average overall cost between the TDF-monotherapy group and the LAM+ADV group due to the reduction in drug costs. This finding may have a beneficial impact on the approach to this disease from the perspective of the health system.
On the other hand, the main limitations of the present study are the small number of patients included and the relatively short period of tracking the data.
Although in randomized studies, there is an established preference for analysis of a population using intention-to-treat (ITT) vs PPP due to a reduced risk of bias; given that our study is a non-inferiority study, the more restrictive analysis were chosen because ITT analysis tends to bias the results towards a lack of difference[34]. In this study, patient assignment was performed without blinding, a factor that may be related to selection bias[34], even though assignment without blinding is closer to routine clinical practice.
The results presented here in suggest that treatment simplification from the LAM+ADV combination to TDF monotherapy, in patients with HBeAg-negative CHB, LAM-treatment failure and complete virologic response under LAM+ADV treatment, is an efficient strategy. The current results are similar to the ones obtained with combined treatment in terms of effectiveness and safety. The treatment used was shown to decrease the cost of management of patients. It is likely that this simplification strategy would be equally efficient in patients with the same characteristics who are under LAM+TDF treatment.
Approximately 400 million people worldwide are infected by the hepatitis B virus. Given that a significant portion of these patients receive treatment for management of chronic hepatitis B (CHB) over a long period of time, it is necessary to use new antivirals with potent action and an adequate long-term safety profile. Likewise, it is important that these medications possess a high genetic barrier that will lead to reduced HBV resistance rates. The establishment of CHB treatment has, as its objective, the sustained suppression of virus replication to prevent disease progression and increase survival.
Tenofovir disoproxil fumarate (TDF), a prodrug of tenofovir, is a potent nucleotide analogue with high efficacy in CHB treatment. Phase III clinical trials showed that TDF is superior to ADV in the suppression of viral replication and in histological improvement in both hepatitis B e antigen- (HBeAg-) positive and HBeAg-negative patients[5].
In this study, it was observed that virologic rebound rates at 6 mo from the start of monotherapy were significantly higher (88%) in patients who received ETV than in those treated with TDF (39%). One of the factors associated in this report with the risk of presenting a virologic rebound was a duration of complete virologic response of less than 12 mo prior to simplification of treatment to monotherapy.
The treatment used was shown to decrease the cost of management of patients. It is likely that this simplification strategy would be equally efficient in patients with the same characteristics who are under LAM+TDF treatment.
This study focused on the efficacy and safety of Tenofovir disoproxil fumarate versus Lamivudine + adefovir in patients with chronic hepatitis B with prior failure with Lamivudine.
The study’s authors would like to thank the medical department of Gilead Sciences SL for supplying the medications used in this study and its funding regardless of the study results; Miguel Ángel Casado, María Yébenes, Álvaro Muñoz, Fernando de Andrés, Eliazar Sabater and Araceli Casado of Pharmacoeconomics and Outcomes Research Iberia (PORIB) for management, coordination and monitoring of the study; Mireia Riera and Jordi Cantoni of BioClever for support and follow-up on the electronic case report forms; and the pharmacy services of the participating centres for their collaboration in obtaining dispensation records, with special thanks to University of Leon Hospital for its participation in the destruction of the study’s medications.
Research Group TENOSIMP-B: Principal researchers: Rodríguez M (Hospital Universitario de Central Asturias), Jorquera F (Complejo Asistencial Universitario de León), , Pascasio JM (Hospital Universitario Virgen del Rocío), Fraga E (Hospital Universitario Reina Sofía), Fuentes J (Hospital Universitario Miguel Servet), Prieto M (Hospital Universitari i Politècnic La Fe), Sánchez-Antolín G (Hospital Universitario Rio Hortega), Calleja JL (Hospital Universitario Puerta de Hierro de Majadahonda), Molina E (Hospital Clínico de Santiago de Compostela), García-Buey ML (Hospital Universitario de La Princesa), Blanco MÁ (Hospital General Universitario Gregorio Marañón), Salmerón J (Complejo Hospitalario de Granada), Bonet ML (Hospital Universitario Son Espases), Pons JA, (Hospital Universitario Virgen de la Arrixaca) and González JM (Hospital Clínico Universitario de Valladolid).
Collaborating researchers: González-Diéguez ML (Hospital Universitario Central de Asturias), Linares P, Olcoz JL (Complejo Asistencial Universitario de León), Cuaresma M, Ferrer MT, Giráldez A, Márquez JL, Ruiz R, Sousa JM (Hospital Universitario Virgen del Rocío), Aguilar P, Barrera P, Costan G, Mata M, Montero JL, Núñez F, Poyato A (Hospital Universitario Reina Sofía), Barrao E, Lázaro M (Hospital Universitario Miguel Servet), Aguilera V, Berenguer M, Casterá F, García M, Rubín A, Zaragoza A (Hospital Universitari i Politècnic La Fe), Almohalla C, García F (Hospital Universitario Rio Hortega), Pons F, Revilla J, Gómez M, Rodríguez LA (Hospital Universitario Puerta de Hierro de Majadahonda), Fernández J, Otero E, Martínez ST (University of Santiago Hospital Complex), Alonso MJ, Real Y (La Princesa University Hospital), Clemente G (Hospital General Universitario Gregorio Marañón), Gila A, Quintero D (Complejo Hospitalario de Granada), Aller R and Gómez S (Valladolid University Clinical Hospital).
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Jarcuska P, Tang ZH, Yang YL S- Editor: Qi Y L- Editor: A E- Editor: Huang Y
| 1. | Lai CL, Yuen MF. Chronic hepatitis B--new goals, new treatment. N Engl J Med. 2008;359:2488-2491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 92] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 2. | Feld JJ, Heathcote EJ. Hepatitis B e antigen-positive chronic hepatitis B: natural history and treatment. Semin Liver Dis. 2006;26:116-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Hadziyannis SJ, Papatheodoridis GV. Hepatitis B e antigen-negative chronic hepatitis B: natural history and treatment. Semin Liver Dis. 2006;26:130-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 4. | Zones JS, Schroeder SA. Evolving residency requirements for ambulatory care training for five medical specialties, 1961 to 1989. West J Med. 1989;151:676-678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 5. | Marcellin P, Heathcote EJ, Buti M, Gane E, de Man RA, Krastev Z, Germanidis G, Lee SS, Flisiak R, Kaita K. Tenofovir disoproxil fumarate versus adefovir dipivoxil for chronic hepatitis B. N Engl J Med. 2008;359:2442-2455. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 890] [Cited by in F6Publishing: 851] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 6. | Fung J. Management of chronic hepatitis B before and after liver transplantation. World J Hepatol. 2015;7:1421-1426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Liaw YF, Sung JJ, Chow WC, Farrell G, Lee CZ, Yuen H, Tanwandee T, Tao QM, Shue K, Keene ON. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521-1531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1739] [Cited by in F6Publishing: 1648] [Article Influence: 82.4] [Reference Citation Analysis (0)] |
| 8. | Buti M, García-Samaniego J, Prieto M, Rodríguez M, Sánchez-Tapias JM, Suárez E, Esteban R. [Consensus document of the Spanish Association for the Study of the Liver on the treatment of hepatitis B infection (2012)]. Gastroenterol Hepatol. 2012;35:512-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Peters MG, Hann Hw Hw, Martin P, Heathcote EJ, Buggisch P, Rubin R, Bourliere M, Kowdley K, Trepo C, Gray Df Df, Sullivan M, Kleber K, Ebrahimi R, Xiong S, Brosgart CL. Adefovir dipivoxil alone or in combination with lamivudine in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2004;126:91-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 448] [Cited by in F6Publishing: 469] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 10. | Lee YS, Suh DJ, Lim YS, Jung SW, Kim KM, Lee HC, Chung YH, Lee YS, Yoo W, Kim SO. Increased risk of adefovir resistance in patients with lamivudine-resistant chronic hepatitis B after 48 weeks of adefovir dipivoxil monotherapy. Hepatology. 2006;43:1385-1391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 269] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 11. | Rapti I, Dimou E, Mitsoula P, Hadziyannis SJ. Adding-on versus switching-to adefovir therapy in lamivudine-resistant HBeAg-negative chronic hepatitis B. Hepatology. 2007;45:307-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 269] [Cited by in F6Publishing: 286] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 12. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2323] [Cited by in F6Publishing: 2338] [Article Influence: 194.8] [Reference Citation Analysis (0)] |
| 13. | Marcellin P, Gane E, Buti M, Afdhal N, Sievert W, Jacobson IM, Washington MK, Germanidis G, Flaherty JF, Aguilar Schall R. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: a 5-year open-label follow-up study. Lancet. 2013;381:468-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1228] [Cited by in F6Publishing: 1226] [Article Influence: 111.5] [Reference Citation Analysis (0)] |
| 14. | Kitrinos KM, Corsa A, Liu Y, Flaherty J, Snow-Lampart A, Marcellin P, Borroto-Esoda K, Miller MD. No detectable resistance to tenofovir disoproxil fumarate after 6 years of therapy in patients with chronic hepatitis B. Hepatology. 2014;59:434-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 190] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 15. | Brunelle MN, Lucifora J, Neyts J, Villet S, Holy A, Trepo C, Zoulim F. In vitro activity of 2,4-diamino-6-[2-(phosphonomethoxy)ethoxy]-pyrimidine against multidrug-resistant hepatitis B virus mutants. Antimicrob Agents Chemother. 2007;51:2240-2243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Delaney WE, Ray AS, Yang H, Qi X, Xiong S, Zhu Y, Miller MD. Intracellular metabolism and in vitro activity of tenofovir against hepatitis B virus. Antimicrob Agents Chemother. 2006;50:2471-2477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 186] [Cited by in F6Publishing: 186] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 17. | Berg T, Marcellin P, Zoulim F, Moller B, Trinh H, Chan S, Suarez E, Lavocat F, Snow-Lampart A, Frederick D. Tenofovir is effective alone or with emtricitabine in adefovir-treated patients with chronic-hepatitis B virus infection. Gastroenterology. 2010;139:1207-1217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 18. | Lok AS. Drug therapy: tenofovir. Hepatology. 2010;52:743-747. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67-74. [PubMed] [Cited in This Article: ] |
| 20. | Oblikue Consulting. Base de datos de costes sanitarios eSalud [Internet]. Barcelona: Oblikue Consulting; 2014; [citado 10 ago 2016] Available from: http: //www.oblikue.com/bddcostes/. [Cited in This Article: ] |
| 21. | Bot Plus 2. 0 [Internet]. Madrid: Consejo General de Colegios Oficiales de Farmacéuticos; 2014; [citado 10 ago. 2016] Available from: https: //botplusweb.portalfarma.com/. [Cited in This Article: ] |
| 22. | Real Decreto-ley 8/2010, de 20 de mayo, por el que se adoptan medidas extraordinarias para la reducción del déficit público. Available from: http: //www.boe.es/boe/dias/2010/05/24/pdfs/BOE-A-2010-8228.pdf. [Cited in This Article: ] |
| 23. | R Core Team (2015). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available from: https: //www.R-project.org. [Cited in This Article: ] |
| 24. | van Bömmel F, de Man RA, Wedemeyer H, Deterding K, Petersen J, Buggisch P, Erhardt A, Hüppe D, Stein K, Trojan J. Long-term efficacy of tenofovir monotherapy for hepatitis B virus-monoinfected patients after failure of nucleoside/nucleotide analogues. Hepatology. 2010;51:73-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 264] [Cited by in F6Publishing: 285] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 25. | Fung S, Kwan P, Fabri M, Horban A, Pelemis M, Hann HW, Gurel S, Caruntu FA, Flaherty JF, Massetto B. Randomized comparison of tenofovir disoproxil fumarate vs emtricitabine and tenofovir disoproxil fumarate in patients with lamivudine-resistant chronic hepatitis B. Gastroenterology. 2014;146:980-988. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 26. | Fung S, Kwan P, Fabri M, Horban A, Pelemis M, Hann HW, Gurel S, Caruntu FA, Flaherty JF, Massetto B. Tenofovir disoproxil fumarate (TDF) vs. emtricitabine (FTC)/TDF in lamivudine resistant hepatitis B: A 5-year randomised study. J Hepatol. 2017;66:11-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 38] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 27. | Lim YS, Byun KS, Yoo BC, Kwon SY, Kim YJ, An J, Lee HC, Lee YS. Tenofovir monotherapy versus tenofovir and entecavir combination therapy in patients with entecavir-resistant chronic hepatitis B with multiple drug failure: results of a randomised trial. Gut. 2016;65:852-860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 28. | Lim YS, Yoo BC, Byun KS, Kwon SY, Kim YJ, An J, Lee HC, Lee YS. Tenofovir monotherapy versus tenofovir and entecavir combination therapy in adefovir-resistant chronic hepatitis B patients with multiple drug failure: results of a randomised trial. Gut. 2016;65:1042-1051. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 72] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 29. | Huang YH, Yu ML, Peng CY. Y.-H, Chen JJ, Chen CY, Su CW, Lin WY, Lai HC, Wang YJ, Dai CY, Chuang WL, Lin HC. Multicenter randomized controlled trial of switching to tenofovir disoproxil fumarate monotherapy in lamivudine-resistant chronic hepatitis B patients with undetectable HBV viral load under lamivudine/adefovir add-on therapy; interim analysis. J Hepatol. 2016;64:S605. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Yang DH, Xie YJ, Zhao NF, Pan HY, Li MW, Huang HJ. Tenofovir disoproxil fumarate is superior to lamivudine plus adefovir in lamivudine-resistant chronic hepatitis B patients. World J Gastroenterol. 2015;21:2746-2753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Kim LH, Chaung KT, Ha NB, Kin KC, Vu VD, Trinh HN, Nguyen HA, Nguyen MH. Tenofovir monotherapy after achieving complete viral suppression on entecavir plus tenofovir combination therapy. Eur J Gastroenterol Hepatol. 2015;27:871-876. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Mallet V, Schwarzinger M, Vallet-Pichard A, Fontaine H, Corouge M, Sogni P, Pol S. Effect of nucleoside and nucleotide analogues on renal function in patients with chronic hepatitis B virus monoinfection. Clin Gastroenterol Hepatol. 2015;13:1181-8.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 33. | Buti M, Gane E, Seto WK, Chan HL, Chuang WL, Stepanova T, Hui AJ, Lim YS, Mehta R, Janssen HL. Tenofovir alafenamide versus tenofovir disoproxil fumarate for the treatment of patients with HBeAg-negative chronic hepatitis B virus infection: a randomised, double-blind, phase 3, non-inferiority trial. Lancet Gastroenterol Hepatol. 2016;1:196-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 306] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 34. | Dossing A, Tarp S, Furst DE, Gluud C, Beyene J, Hansen BB, Bliddal H, Christensen R. Interpreting trial results following use of different intention-to-treat approaches for preventing attrition bias: a meta-epidemiological study protocol. BMJ Open. 2014;4:e005297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |