Abstract
The study was designed to assess whether repeated administration of diazepam (Valium®, Roche)—a benzodiazepine exerting an agonist action on GABAA receptors—may alleviate both the short (1 week, 1W) and long-term (6 weeks, 6W) deleterious effects of alcohol withdrawal occurring after chronic alcohol consumption (6 months; 12% v/v) in C57/BL6 male mice. More pointedly, we first evidenced that 1W and 6W alcohol-withdrawn mice exhibited working memory deficits in a sequential alternation task, associated with sustained exaggerated corticosterone rise and decreased pCREB levels in the prefrontal cortex (PFC). In a subsequent experiment, diazepam was administered i.p. for 9 consecutive days (1 injection/day) during the alcohol withdrawal period at decreasing doses ranging from 1.0 mg/kg to 0.25 mg/kg. Diazepam was not detected in the blood of withdrawn mice at the time of memory testing, occurring 24 hours after the last diazepam injection. Repeated diazepam administration significantly improved alternation rates and normalized levels of glucocorticoids and pCREB activity in the PFC in 1W but not in 6W withdrawn mice. Thus, repeated diazepam administration during the alcohol-withdrawal period only transitorily canceled out the working memory impairments and glucocorticoid alterations in the PFC of alcohol-withdrawn animals.
Similar content being viewed by others
Introduction
There is substantial evidence that memory deficits are either dramatically enhanced or gradually developed after alcohol withdrawal (Farr, Scherrer, Banks, Flood, & Morley, 2005; Lukoyanov, Madeira, & Paula-Barbosa, 1999; Schandler, Clegg, Thomas, & Cohen, 1996). Congruently, we recently demonstrated that alcohol withdrawal in mice produced working memory (WM) impairments up to 6 weeks after withdrawal. Conversely, such impairments were not observed in mice still consuming alcohol. Enduring WM disorders were related to long-lasting changes of neural activity and glucocorticoid alterations in the prefrontal cortex (PFC) and the hippocampus (HPC) of withdrawn mice, as well as epigenetic alterations in these brain regions (Dominguez, Belzung, et al., 2016, Dominguez, Dagnas, et al., 2016; Mons & Beracochea, 2016).
One of the main disturbances associated with alcohol withdrawal involves a dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis, which accounts for excessive glucocorticoid (GCs) release (Adinoff et al., 1990; Errico et al., 2002; Little et al., 2008). Interestingly, several studies have shown that the GABAergic system regulates the HPA axis response to stress (Calogero et al., 1988a; Arvat et al., 2002; Cullinan et al., 2008). Indeed, GABA is an inhibitory neurotransmitter that reduces the release of ACTH (Makara and Stark, 1974), via a central action on CRH neurons of the paraventricular nucleus of the hypothalamus (Cullinan et al., 2008). Diazepam—a benzodiazepine having an agonist action on the GABAA receptor—is delivered transiently among alcoholics during and shortly after alcohol withdrawal, mainly to reduce anxiety and decrease neural excitability induced by the cessation of alcohol intake (Adinoff, 1994). However, the efficacy of diazepam to reverse glucocorticoids and cognitive disorders, particularly after long withdrawal periods, remains largely unknown.
The purpose of the present study was to determine if repeated administration of diazepam during alcohol withdrawal could alleviate the long-lasting neurobiological and cognitive alterations that progressively develop in withdrawn mice, as shown in earlier studies (Dominguez, Belzung, et al., 2016, Dominguez, Dagnas, et al., 2016). Three experiments were designed as follows: In Experiment 1, we studied the impact of two different protocols of repeated diazepam administration on emotional reactivity in the elevated plus-maze in 1W alcohol withdrawn mice. The diazepam administration protocol that had the greater corrective effect on anxiety-like reactivity in withdrawn animal was hence elected for the subsequent experiments. In Experiment 2, we studied the effects of the selected repeated diazepam procedure in 1W or 6W withdrawn mice on WM disorders and neural alterations in the PFC and dHPC—two main structures involved in WM. Hence, we used CREB phosphorylation (pCREB) as a biological marker of neural plasticity (Dominguez, Belzung, et al., 2016). Because Experiment 2 showed pCREB alterations in withdrawn mice’s PFC, and insofar as earlier data from our group evidenced that pCREB alterations are associated with abnormal corticosterone levels in the PFC of withdrawn animals (Dominguez, Belzung, et al., 2016), we investigated in Experiment 3 the impact of repeated diazepam injections on the test-induced corticosterone rise in the PFC in 1W and 6W alcohol withdrawn mice using intracerebral microdialysis in relation to working memory performance and pCREB activity.
Material and methods
Animals
Animals were mice of the C57/BL6 strains obtained from Charles River (L’Arbresle, France) and were 6 weeks old upon arrival in the laboratory. They were housed by groups of 20 until they were 10 months old in a temperature-controlled colony room (22 ± 1 °C), under a 12:12 light-dark cycle (lights on at 7:00 a.m.). They were provided with food and water or alcohol ad libitum. All procedures were performed during the light phase of the cycle. Two weeks before the experiments, they were housed individually. All experimental procedures were performed between 8:00 and 12:00 a.m. to prevent any circadian rhythm side effect on GC levels (Rodriguez, Terron, Duran, Ortega, & Barriga, 2001).
All experimental procedures were conducted in accordance with the EU Directive 2010/63/EU for animal experiments and local ethical committee (#5012089).
Alcohol administration and withdrawal procedures
These procedures have been described previously (Dominguez, Belzung, et al., 2016, Dominguez, Dagnas, et al., 2016). At 4 months of age, mice were given as their sole liquid source water that contained increasing concentrations of ethanol (Prochilab, France) as follows: 4% (v/v) the first week, 8% (v/v) the second week, and 12% (v/v) for the 6 consecutive months. At the end of this period, alcohol-treated mice were withdrawn from the alcohol regimen. To perform withdrawal, ethanol was progressively replaced by water, as follows: 8% (v/v) for 3 days, 4% (v/v) for the next 3 days then water for 7 days (start of behavioral experiments) to the end of testing (Dominguez, Belzung, et al., 2016). Thus, behavioral testing began either after 1 week (on the 7th day of water supply, Withdrawn 1W) or 6 weeks (Withdrawn 6W) of water supply. The control groups received permanent water supplies (Control 1W and Control 6W groups). During the week before behavioral experiments, all mice were handled 5 min/day during 6 consecutive days to reduce fear reactivity toward the experimenter. Alcohol consumption was measured during the 6 months of treatment by scoring the decrease of liquid consumption on graduated bottles. The mean alcohol intake per mouse was calculated over the 6 months of alcohol exposure.
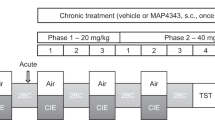
Repeated diazepam administration (Fig. 1)
Diazepam (Valium®, Roche) was diluted in saline (0.9% NaCl). The solutions were injected intraperitoneally (10 ml/kg, 1 injection/day). In all experiments, diazepam administration started the two final days of the alcohol-withdrawal phase when mice were still under a 4% ethanol v/v regimen and extended over 9 consecutive days. In Experiment 1, diazepam was administered by daily i.p. injection in withdrawn mice according to two different protocols varying only by the concentrations of the doses of diazepam (Fig. 1). Both procedures allowed to elicit the most potent protocol prone to counteract the increase of anxiety-like reactivity induced by alcohol withdrawal.
Diazepam was administered by i.p. injection (1/day) both in Control (upper part) or in Withdrawn mice (lower part). In the first (1st) protocol, all mice received on the first 6 days of treatment a 0.5-mg/kg diazepam dose, followed on two consecutive days by a 0.25-mg/kg dose, and finally by a single 0.12-mg/kg dose on the last day of treatment. In the second (2nd) protocol, mice were subjected to the same injection procedure, except that they first received a 1.0-mg/kg solution on the first 6 days of treatment, followed by 2 days at 0.5 mg/kg, and finally by a 0.25-mg/kg injection. Both protocols begin the last 2 days of ethanol consumption (4% v/v; grey rectangles) and lasted during the 7 days of alcohol withdrawal (Water, white rectangles). Behavioral testing (Elevated plus maze in Experiment 1 or sequential alternation in Experiments 2 and 3) occurred 24 hours the last day of the withdrawal period (7th day)
In both procedures, diazepam doses were progressively decreased from Day1 to Day 9. Indeed, chronic diazepam intake can lead to the development of dependence to this compound (Brett & Munion, 2015). Thus, to avoid negative effects of the cessation of diazepam administration, we progressively reduced the administered dose from Day 1 to Day 9 and started behavioral and neurobiological testing 24 hours after the last diazepam administration—a time point at which diazepam was not detected in the blood. The repeated administration of diazepam in water controls allows to verify that the cessation of diazepam administration per se induces no negative cognitive nor neurobiological effects.
Experiment 1: Evaluation of anxiety-like behavior in the elevated plus-maze
This experiment was performed on four independent groups of mice: control-vehicles (N = 10), withdrawn 1W-vehicles (N = 10), withdrawn 1W-1st protocol (starting dose: 0.5 mg/kg: n = 8) and withdrawn 1W-2nd protocol (starting dose: 1.0 mg/kg; N = 10).
Anxiety-like behavior was evaluated using an elevated plus-maze made of grey Plexiglas with four arms arranged in the shape of a plus sign. Each arm was 30-cm long, 7-cm wide, and elevated 40-cm above the ground. The four arms were joined at the center by a 7-cm square platform. Two opposite arms were “closed” by 17-cm high side walls, whereas the other arms did not have side walls. Mice were allowed to explore all arms freely for 5 minutes, and their behavior was recorded through an automated tracking system, allowing measurements of the time and distance (m) spent by area. The % “time ratio” and “distance ratio” spent in the open arms was used to measure anxiety-like behavior. Thus, the smaller are these ratios, the more “anxious-like” is the mouse.
Experiment 2: Working memory and Immunohistochemistry
Drug administration
The injections of diazepam were the same as in Experiment 1, according to the second protocol only. Diazepam was administered in controls and withdrawn mice. Experiment 2 was conducted on eight independent groups of mice assigned to WM testing: control-vehicles 1W and 6W (N = 7 and N = 6 respectively); control–diazepam 1W and 6W (N = 7 and N = 6 respectively); withdrawn 1W-vehicles and 6W-vehicles (N = 6 in both groups); and withdrawn 1W-diazepam and 6W-diazepam (N = 8 and N = 7 respectively). After WM testing, mice were sacrificed for the immunohistochemical study.
Working memory task
Spontaneous alternation was tested in a T-maze. After two habituation sessions, mice were subjected to a training phase consisting of seven successive trials separated by 30 seconds intertrial interval (ITI) to familiarize them with the experimental procedure. At the beginning of each trial, the mouse was placed in the start box for 30 seconds before the door to the stem was opened. When the subject entered one of the goal-arms, the door to that arm was closed and the choice was recorded. After a 30-second confinement period into the chosen arm, the mouse was placed back in the start-box for a new trial.
Test session
Because no sequential alternation deficits were observed among withdrawn groups in the training phase, mice were submitted 24 hours later to the same procedure but with a 90-second ITI. Lengthening the ITI increases delay-dependent interference over the series (Beracochea & Jaffard, 1987; Vandesquille et al., 2013). An alternation response was scored each time the subject entered the arm opposite the one visited on the immediate preceding trial. To avoid olfactory cues in the apparatus, the entire surface of the maze was washed with water between the 90-second intertrial interval to remove visible traces of urine and feces.
A mean alternation rate was calculated over the seven consecutive trials and expressed in percentage. Running latencies were registered, allowing calculation of the mean choice latency over the 7 trials. To dissociate memory deficits from a potential gradual loss of motivation to alternate over the series, an eighth trial was added, separated by a shorter 5-second ITI from the seventh one.
Immunohistochemistry
The procedure has been described in full elsewhere (Dominguez, Belzung, et al., 2016, Dominguez, Dagnas, et al., 2016). Thirty minutes after the end of behavioral testing, mice were sacrificed with an overdose of pentobarbital (200 mg/ml) and transcardially perfused with 4% paraformaldehyde dissolved in phosphate buffer (0.1M; pH 7.4). The brains were removed, postfixed overnight, and then sectioned (50-μm thin) using a Vibratome (Leica). Immunostainings of pCREB were performed using rabbit polyclonal, anti phospho(ser133)-CREB antibodies (1:6000, Millipore, USA). In addition to mice subjected to behavioral testing, control-1W and -6W as well as withdrawn 1W- and 6W-naïve mice were subjected to the same pharmacological protocol but remained in their home cage until sacrifice (3/4/groups). This naïve (basal) condition allows measuring the specific effect of WM testing on pCREB levels and on the variations of plasma corticosterone concentrations according to each specific experimental group.
Phosphorylated CREB (pCREB) immunostainings were performed using rabbit polyclonal, anti-phospho(ser133)-CREB (1:6000, Millipore, USA). Given their involvement in WM and emotional processes, counts were made in the following brain regions, according to Paxinos and Franklin atlas (Paxinos & Franklin, 2001): PFC: (prelimbic cortex, PL; from bregma: +1.98 to +1.50 mm) and the dorsal hippocampus (dCA1; from bregma: −1.70 to −2.30 mm). The choice of the PL and dCA1 rests on previous data having shown pCREB alterations in withdrawn groups in these two brain regions (Dominguez, Belzung, et al., 2016, Dominguez, Dagnas, et al., 2016). Digital images were captured at 10X magnification using an Olympus (BX50) and an imaging analysis system (ImageJ®). For each region, three to four consecutive sections (every first section in each set of four 50-μm sections) were examined and mean number of positive nuclei/mm2 was determined.
Plasma samples
Blood samples were collected between 08:00 a.m. and 12:00 a.m. by submandibular procedure with 25-gauge needles after anesthesia (Isoflurane®) in independent groups of mice, either in naïve condition or 30 minutes after the beginning of WM testing, to be in accordance with the delay of sacrifice used for the immunohistochemistry study. The blood was collected in tubes containing 10% EDTA. After 10 min of centrifugation at 3,000 rpm, plasma samples were stored at −80 °C.
Measurement of diazepam concentration in blood
The concentrations of diazepam in blood and of its active metabolites oxazepam and nordiazepam were determined in independent naïve 1W withdrawn mice (N = 3) at three time points (1 hr, 24 hr, and 48 hr) for each mouse after the last diazepam injection. For that purpose, blood samples were collected by retro-orbital punction and sent to the Laboratory of Pharmacology and Toxicology (Bordeaux, France) for analyses by the LC-MS-MS technique.
Experiment 3: Time-course evolution of PFC corticosterone concentrations during and after working memory testing
Experiment 3 was conducted on eight independent groups of mice assigned to WM testing under microdialysis collection. All groups (control-vehicles 1W and 6W; control–diazepam 1W and 6W; withdrawn 1W-vehicles and withdrawn 6W-vehicles; withdrawn 1W-diazepam and withdrawn 6W-diazepam) involved seven mice. After WM testing, all mice were sacrificed for the immunohistochemical study.
Intracerebral microdialysis
The procedure has been described in full elsewhere (Dorey, Pierard, Chauveau, David, & Beracochea, 2012; Dominguez, Belzung, et al., 2016, Dominguez, Dagnas, et al., 2016). Twenty hours before microdialysate collection, a dialysis probe was implanted into the PFC or dHPC (CMA/7; CMA Microdialysis AB, Sweden; length: 1 mm; molecular cutoff 6 kDa). Mice were immediately tied at a swivel bracket allowing the animals to move freely with the dialysis probe (CMA/120; CMA Microdialysis AB, Sweden) and were placed in the dialysis bowl (with food and water or alcohol ad libitum) during 20 hours (habituation phase). During the habituation phase, the probe was perfused with a flow rate of 0.1 μl/min with a sterile-filtered saline solution (Dulbecco's phosphate buffered saline; SIGMA; in g/l: CaCl2, 0.133; MgCl2, 0.1; KCl, 0.2; KH2PO4, 0.2; NaCl 8.0; Na2HPO4, 1.15; pH between 7.1 and 7.5). Two hours before microdialysate collection, the stabilization phase was performed at a 1 μl/min flow rate using a microinfusion pump, which allows the equilibration of the extracellular metabolites. Subsequently, the baseline dialysates were collected every 15 minutes before (60 minutes), during (30 minutes), and after (90 minutes) WM testing. During behavioral testing, the removable swivel bracket was placed above the maze allowing the mouse to move freely; then, the swivel bracket was replaced above the dialysis bowl at the end of behavioral testing. All dialysates were collected between 08:00 and 12:00 a.m. and were stored at −80 °C until corticosterone measurements.
Plasma samples
Blood samples were collected by submandibular procedure with 25-gauge needles after anesthesia (Isoflurane®) in mice being either in naïve condition or 30-min after the beginning of WM testing. The blood was collected in tubes containing 10% EDTA, centrifuged for 10-min, and the plasma samples were stored at −80 °C until further analysis.
Plasma and dialysate samples corticosterone assays
A commercially prepared Enzyme Immunoassay kit was used to measure plasma and dialysate samples corticosterone concentrations (Correlate-EIATM, Assay Designs, Ann Arbor, MI). The sensitivity of the assay was 0.08 nmol/L. Therefore, the baseline sample concentration was more than tenfold superior than the sensibility threshold.
Statistical analyses
Statistical analyses were performed using the Statview 5.0 software. Data were expressed as mean ± SEM. Behavioral performance, corticosterone assays, and immunohistochemical data were analyzed using one- or two-way analyses of variance (ANOVAs). Comparisons of WM performances with chance level were calculated with one-sample Student’s t test (with hypothesized mean-chance level = 50%). Microdialysis data were analyzed using one- or two-way repeated-measures ANOVA. Post-hoc Bonferroni/Dunnett’s multiple comparisons analyses were performed when adequate. For correlation analyses, the Spearman’s correlation coefficient, R, was determined. For all tests, p < 0.05 was considered statistically significant, whereas p > 0.05 were considered nonsignificant (NS).
Results
Measurement of alcohol concentration in blood
In the present study, the mean daily alcohol intake was 3.08 ± 0.8 mL/mouse, representing 14.34 ± 3.3 g/kg of alcohol per day. Ethanol was under level of quantification in Water controls (Elisa kit; 0 ± 0 g/L) and Withdrawn mice (0 ± 0 g/L) at the time of testing.
Measurement of diazepam concentration in blood
The blood concentrations of diazepam and its active metabolites, oxazepam and nordiazepam, were measured 1 hour, 24 hours, and 48 hours after the last diazepam injection (0.25 mg/kg) within the second protocol. Concentrations of these compounds were found below the limit of quantification at the 24-hour and 48-hour points. In contrast, small concentrations of diazepam and nordiazepam were detected only at the 1-hour time point. Thus, concentrations of diazepam and its metabolites oxazepam and nordiazepam were below the limit of quantification at the time of behavioral and biological studies (Table 1).
Experiment 1: repeated diazepam injections alleviate the increase of anxiety-like reactivity in the elevated plus-maze in alcohol-withdrawn mice
The total number of entries was similar among the four groups (F(3,34) = 2.09; p = 0.11) as well as the total time spent visiting the open and closed arms of the maze (F(3,34) < 1.0) (data not shown). ANOVA performed on the entry ratio revealed a significant between-group difference (F(3,34) = 11.19; p < 0.001). Post-hoc analyses showed a small significant decrease of entry ratio in 1W-vehicle mice (21.09 ± 2.05%) compared with control-vehicle mice (33.7 ± 2.6%, p < 0.05); in contrast, a significant increase of entry ratio was observed in the withdrawn 1W-2nd protocol group (50.3 ± 33.3%) compared with both 1W-vehicle and control-vehicle groups (p < 0.01 in both comparisons). A weaker increase of entry ratio also was observed in withdrawn 1W-1st protocol group (35.54 ± 4.3%), which was significant only compared with 1W-vehicle mice (p < 0.05; NS in all other comparisons; Fig. 2a).
1W-vehicle withdrawn mice showed a small significant decrease of entry ratio as compared to control-vehicles. In contrast, a significant increase of entry ratio was observed in withdrawn 1W-1st protocol group compared with 1W-vehicles, whereas the 2nd protocol of diazepam administration produced a greater enhancement of entry ratio compared with both 1W withdrawn and control-vehicle mice (a). No significant difference was observed on the % time ratio. (*p <0.05 and **p < 0.01 vs. control-vehicle; #p < 0.05 and ##p < 0.01 vs. Withdrawal 1W-vehicle)
ANOVA performed on the time ratio revealed a nonsignificant between-groups difference (F(3,34) = 2.14; p < 0.11; control-vehicle mice: 21.7 ± 5.7%; withdrawn 1W-vehicle mice: 15.82 ± 3.2%; withdrawn 1W-1st protocol group: 19.16 ± 4.25; withdrawn 1W-2nd protocol group: 32.13 ± 5.5%; Fig. 2b).
Experiment 2: Repeated diazepam injections transiently improve WM and restore CREB phosphorylation in the PFC of withdrawn mice
Given the data obtained in the first experiment, diazepam administration was performed according to the second protocol, which has been found to be the most effective in reversing the anxiety-like disorders of withdrawn mice.
Working memory
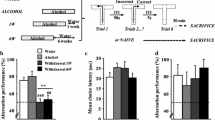
ANOVA analyses showed a significant “treatment” effect (vehicle vs. diazepam; F(1,45) = 9.42; p = 0.003) and the interaction “Condition (control versus withdrawn) X Treatment” was also significant (F(3,45) = 3.90; p = 0.014). Withdrawn 1W-vehicles (50.00±4.30%) exhibited lower alternation rates compared with Control-1W vehicles (69.05±2.38%p<0.02). Withdrawn 6W vehicles (55.55±5.55%) also exhibited a significant decrease of alternation rates as compared to control 6W vehicles (72.22 ± 3.51%; p < 0.04; Fig. 3a). Diazepam improves significantly the alternation rate of the withdrawn 1W group (77.08 ± 3.05%; p < 0.001 vs withdrawn-1W vehicles) but failed to improve it in withdrawn-6W mice (65.3 ± 7.1%; p = 0.065 vs withdrawn-6W vehicles). In water groups, diazepam does not significantly modify the alternation rates (Control-1W diazepam: 64.28 ± 4.34%; NS vs vehicles; Control-6W diazepam: 76.19 ± 8.80%; NS vs. respective vehicles).
Effects of repeated diazepam on alternation performance. a In vehicles (white squares) Withdrawn 1W- and 6W mice exhibited a significant reduction of alternation rates compared with respective controls. In diazepam-treated mice (black squares), the alternation performance was normalized compared with vehicle-controls. Diazepam did not modify performance compared with respective vehicle-controls. b Withdrawal and/or diazepam did not modify alternation rates at the eighth trial with 5-s ITI compared with vehicle-controls. Dashed lines for A and B represent chance level. Results are expressed as mean ± SEM. (*p < 0.05 compared with respective Water groups; ###p < 0.001 compared with 1W-withdrawn mice). c Plasma corticosterone (expressed in ng/mL). Diazepam did not significantly modified plasma corticosterone concentrations both in basal (naïve) and after WM testing (p > 0.05 for all comparisons)
Choice latency was not significantly different among the groups, whatever the treatments and conditions considered (p > 0.05 in all comparisons). Moreover, all groups exhibited high alternation rates (above 70% in all groups, (F(3,45) = 0.5; NS; Fig. 3b) on the last trial (8th) of the series.
Immunohistochemistry
Naïve Condition
Data, expressed as number of immunostained cells/mm2, are summarized in Table 2. Differences between groups and treatments were analyzed in the PFC and in the dCA1. ANOVA analyses showed no significant between-group nor treatment difference (p > 0.10 in all analyses); likewise, the interaction between Groups X Treatments did not prove significant regardless the brain structure considered (NS for all comparisons).
Test Condition
Table 3 describes the number of immunostained cells/mm2 (mean ± SEM) in the PFC and dCA1 of vehicle and diazepam-treated groups. In the PFC, a global ANOVA performed on the raw number of immunostained cells in vehicle and diazepam-treated groups showed a significant group effect (F(3,45) = 5.17, p = 0.01), a significant treatment effect (F(1,45) = 4.56; p < 0.05), and a significant interaction between groups and treatments (F(3,45) = 4.69; p < 0.05). More specifically, both 1W and 6W withdrawn groups exhibited a significant decrease in the number of immunostained cells compared with their respective controls (p < 0.05 in both analyses). Diazepam administration induced a significant increase of cell labeling in withdrawn-1W mice compared with withdrawn vehicle-ones (p < 0.05). Thus, they did not significantly differ from Control-1W mice (p > 0.10). In contrast, the increase of pCREB labelling induced by diazepam in withdrawn-6W mice was not statistically significant compared with both vehicle withdrawn-6W and Control-6W mice (p > 0.05 in both analyses).
In the dCA1, a global ANOVA performed on the raw number of immunostained cells in vehicle and diazepam-treated groups showed a nonsignificant group effects (F(3,45) = 0.50, p = 0.68), a nonsignificant treatment effect (F(1,45) = 3.55; p = 0.065), and a nonsignificant interaction between groups and treatments (F(3,45) = 0.77; p = 0.51).
Plasma corticosterone (expressed in ng/mL)
Naïve (basal) condition (Fig. 3c left)
No significant between-groups difference was observed (F(3,19) = 2.78, NS) and the “treatment” (vehicle vs. diazepam) effect was not significant (F(1,19) = 0.63, NS). The interaction Groups X Treatments also was not significant (F(3,19) = 1.68; p = 0.20). Diazepam did not alter significantly corticosterone levels in all groups (Control-1W: 23.55 ± 10.3, Withdrawn-1W: 21.2 ± 6.5, Control-6W: 18.7 ± 1.9 and Withdrawn-6W: 26.2 ± 9.5) compared with respective vehicle groups (Control-1W: 6.32 ± 2.3, Withdrawn-1W: 18.2 ± 8.8, Control-6W: 11.9 ± 3.2 and Withdrawn-6W: 36.9 ± 8.2; p > 0.05 for all comparisons).
Test conditions (Fig. 3c, right)
No significant between-groups difference was observed (F(3,45) = 0.46, NS) as well as no significant “treatment” (vehicle vs. diazepam) effect (F(1,45) = 1.09, NS) and the interaction between groups and treatments also was not significant (F(3,45) = 1.13; p = 0.34). Diazepam did not significantly modify plasma corticosterone concentrations after WM testing (Control-1W: 123.9 ± 22.9, Withdrawn-1W: 124.0 ± 7.4, Control-6W: 130.6 ± 15.5 and Withdrawn-6W: 124.5 ± 9.6) compared with respective vehicle groups (Control-1W: 128.3 ± 20.4, Withdrawn-1W: 162.3±24.7, Control-6W: 119.9 ± 13.2 and Withdrawn-6W: 145.1 ± 21.6; p > 0.05 for all comparisons).
Interestingly, alternation performance correlated positively with pCREB levels in the PFC in Withdrawn-1W (R = −0.31: p = 0.04; Fig. 4a) and Withdrawn-6W mice (R = −0.49, p = 0.01; Fig. 4b). No significant correlations between WM scores and pCREB levels in the PFC or dHPC were found in the other groups (data not shown).
Regression analysis between individual changes in pCREB levels and percentage of spontaneous alternation rates during WM testing in the PFC for the 1W (a) and the 6W (b) groups in vehicle (white circle) and diazepam (black circles) conditions. As observed in 1W mice, diazepam–treated animals exhibit the higher pCREB levels, which are positively correlated with the higher alternation performance. A positive correlation also was observed in 6W mice between WM performance and % variation and pCREB levels even though diazepam–treated animals are less homogeneously distributed compared with 1W-treated mice
Experiment 3. Repeated diazepam injections reduce the exaggerated test-induced increase of corticosterone in the PFC and normalized pCREB levels in 1W but not 6W withdrawn mice
Behavior
The percentage of alternation observed in mice during the microdialysis experiment are shown in Fig. 5a.
a Results are expressed as mean ± SEM percentage of alternation. During WM testing, both 1W and 6W-withdrawn mice showed reduced spontaneous alternation rates on Trials 2-7 compared with the respective water-controls (p < 0.05 in both analyses). Diazepam increase the alternation rate in 1W withdrawn mice but not in 6W withdrawn. b No between-groups difference was observed at the eighth trial of the series. c Microphotograph illustrating the positioning of the cannula-tip in the PFC. d Basal corticosterone levels (expressed in ng/mL) in dialysates (i.e., mean ± SEM from 4 points measured before behavioral testing) evidenced no significant difference in diazepam-treated mice compared with respective controls. e–f Corticosterone responses measured in the four experimental groups in the PFC before (−60 to −15-min points), during (0 and 15-min points) and after (30 to 105-min points) behavioral testing (grey rectangle). Values are expressed as percentage of respective baseline values. Compared with respective Water groups, significant greater corticosterone concentrations were observed at the 30-min point (during memory testing) in withdrawn 1W and 6W mice (p < 0.05 in both analyses); the exaggerated increase of corticosterone lasted up to the 45-min point in both withdrawn groups (p < 0.01 versus respective controls) before returning to baseline. f Diazepam significantly reduced corticosterone concentrations at the time of memory testing (+15 min and +30 min) in both withdrawn 1W and 6W groups and normalized the time-course evolution of corticosterone after test. Bars represent mean ± SEM. *p < 0.05 and **p < 0.01 for 1W–withdrawn mice versus respective controls. #p < 0.05 and ##p < 0.01 for 6W–withdrawn mice versus respective controls
In mice receiving the vehicle solution, alternation rates were above chance level (50%) in Control-1W (69.05 ± 4.34%; t(6) = 4.38, p < 0.004) and Control-6W (76.2 ± 6.15%; t(10) = 6.48, p < 0.001) mice but not in Withdrawn-1W and -6W groups (1W: 52.38 ± 5.67%; t(6) = 0.42, p = NS, 6W: 52.38% ± 5.67; t(6) = 0.42, p = NS).
In groups treated with diazepam, control mice alternated significantly above chance level (50%) (diazepam-Control-1W: 85.71 ± 6.73%; t(6) = 4.38, p < 0.004; diazepam-Control-6W: 83.3 ± 5.14%; t(10) = 6.48, p < 0.001). In contrast, in alcohol withdrawn groups, only the 1W group alternated significantly above chance level (76.19 ± 6.48%; t(6) = 7.81, p = 0.001), whereas 6W mice did not (61.42% ± 3.07; t(6) = 0.42, p = NS).
Overall, in 1W and 6W withdrawn mice, animals receiving the vehicle solution responded at chance level whereas diazepam significantly improved alternation rates only in 1W but not in 6W mice.
ANOVA analyses showed a significant between-groups difference (F(7, 48) = 5.33; p = 0.0001). More pointedly, post-hoc analyses evidenced that diazepam improves significantly alternation rates in 1W but not 6W withdrawn groups relative to their respective vehicles (1W: p = 0.014; 6W: p = 0.065). In contrast, diazepam did not significantly modify performance in both 1W and 6W Control groups as compared to their respective vehicle groups (NS vs respective vehicle groups in both comparisons).
The mean choice latency did not differ among all groups (p > 0.05 in all comparisons; data not shown). Moreover, all groups exhibited a similar alternation rates at the eighth trial of the series, separated from the seventh one by a short (5-sec) ITI (F(7,48) = 1.00; p = 0.44; Fig. 5b).
Intracerebral corticosterone assay
Figure 5c displays a typical site of guide-canulae localization in the PFC. The analysis of absolute concentrations (expressed in ng/mL) of baseline corticosterone levels in dialysates (i.e., mean ± SEM from 4 points measured before behavioral testing) are displayed in Fig. 5d. A two-way ANOVA evidenced a significant group effect (F(3,48) = 4.99; p = 0.004) but no significant treatment effect (F(1,48) = 3.04; NS) and the interaction between factors also was not significant (F(3,48) = 0.27; NS). More precisely, regarding diazepam-treated mice, corticosterone concentrations were higher in Withdrawn-1W (256.01 ± 44.9) compared with Withdrawn-6W mice (135.19 ± 13.3; p = 0.005).
A two-way repeated measures ANOVA performed on the time-course evolution of corticosterone shows a significant interaction of the factors “groups” and “treatments” (F(3,528) = 2.64; p = 0.05). In vehicle groups (Fig. 5e), repeated measures ANOVAs evidence a different time-course evolution of corticosterone levels among the groups (F(33,264) = 1.55; p = 0.03). Indeed, compared with baseline, WM testing induces a significant and progressive increase of corticosterone concentrations from 15 to 60 minutes after test in Water-1W and Withdrawn-1W and -6W groups (p ≤ 0.05 in both analyses). In contrast, the time-course increase of corticosterone concentrations after behavioral testing was slightly delayed in Water-6W mice (from 30 to 60 minutes post-test; p ≤ 0.05). Finally, the test-induced corticosterone increase was greater in Withdrawn-1W at the 45-minute timepoint compared with Water-1W (p = 0.01) and in Withdrawn-6W at the 30-minute timepoint (p = 0.03 vs. Water-1W and p = 0.01 vs. Water-6W).
In diazepam-treated groups (Fig. 5f), analyses did not evidence a significant between-group difference in the time-course evolutions of corticosterone (F(33,264) = 1.18; p = NS). Such a result evidences that repeated diazepam administration normalizes corticosterone levels. Indeed, compared with their respective vehicle groups, diazepam reduced significantly corticosterone levels only in Withdrawn-1W mice (F(1,132) = 7.40; p = 0.018); however, not significantly in Water-1W mice (F(1,132) = 0.17; NS), Water-6W subjects (F(1,132) = 1.12; NS) as well as in Withdrawn-6W mice (F(1,110) = 1.30; NS). Specifically, diazepam decreased corticosterone levels at 30 minutes (F(1,12) = 4.82; p = 0.04) and at 45 minutes timepoints (F(1,12) = 7.3; p = 0.019) in Withdrawn-1W compared with respective vehicle groups.
Discussion
Our study shows that both a short (1-week) and a long (6-week) periods of alcohol withdrawal induced WM impairments in a sequential alternation task. The WM impairments were associated with significant exaggerated test-induced corticosterone rises and reduced levels of pCREB in the PFC in withdrawn animals as compared with water controls. Repeated administration of diazepam (9 consecutive days) during the alcohol withdrawal period counteracted in 1W but not 6W withdrawn animals both the WM and biological (corticosterone and pCREB) alterations previously observed in the PFC.
Alcohol withdrawal triggered significant WM impairments. Interestingly, we previously showed that these impairments were due to withdrawal itself, because mice still submitted to alcohol did not exhibit any deficit (Dagnas, Guillou, Prévôt, & Mons, 2013; Dominguez, Belzung, et al., 2016, Dominguez, Dagnas, et al., 2016). The low alternation rates observed in withdrawn mice were not attributable to a decreased motivation to alternate during trial series, because alternation performances were improved when the ITI was shortened from 90-s to 5-s at the eighth trial of the test series. Moreover, no deficit was observed in withdrawn groups during the training trials performed with an ITI of 30-sec, suggesting that attentional mechanisms are not likely impaired in withdrawn animals. Thus, the increase of the delay between trials (from 30 sec to 90 sec) at the test session emerges as a key component of memory failure in withdrawn animals.
Effects of alcohol withdrawal on anxiety-like reactivity and working memory
Human and rodent studies have reported enhanced anxiety-like behaviors in various tests during ethanol withdrawal (Kliethermes, 2005). In our experimental conditions, an increase of anxiety-like reactivity in an elevated plus-maze also was observed. This finding is in agreement with other animal studies showing depression and stress-related behavior in withdrawn rodents (Brady & Sonne, 1999; Fukushiro et al., 2012; Pnag, Renoir, Lawrence, & Hannan, 2013). Indeed, withdrawal is accompanied by a dysregulation of the balance between inhibitory and excitatory neurotransmission (mainly GABA and Glutamate respectively) leading to increased cell’s excitability and multiple brain neurotransmitters alterations, as well as of the HPA axis (Ludlow et al., 2009; Ostroumov et al., 2016).
Interestingly, previous findings from our team showed that 6W withdrawn mice exhibited a similar level of fear reactivity in the elevated plus-maze compared with Water controls in contrast to 1W animals that exhibited a significant increase of fear reactivity in this task (Dominguez, Dagnas, et al., 2016). These findings suggested that the persistent WM deficits seen in 6W withdrawn mice are not likely due to an anxiety increase but must be considered as resulting more specifically from long-lasting neurobiological impairments in the brain areas sustaining WM. Indeed, as shown in the present study and earlier papers (Dominguez, Belzung, et al., 2016), withdrawn mice displayed an exaggerated rise of intra-prefrontal corticosterone response both during and after memory testing, which lasted at least 6 weeks after withdrawal compared with Water animals. These findings are congruent with the finding that glucocorticoids-induced alterations of WM are mediated primarily through influences within the PFC (Dalley, Cardinal, & Robbins, 2004; Runyan & Dash, 2005; Roozendaal, McReynolds, & McGaugh, 2004) and with studies in detoxified alcoholic patients exhibiting selective disruption of frontal cortical functions during withdrawal (Moselhy, Georgiou, & Kahn, 2001; O’Daly et al., 2012; Pfefferbaum et al., 2001). Extensive evidence indicates that excessive levels of GCs lead to PFC dysfunction and WM impairments as observed in depression (Sakai, 2003) or Cushing's syndrome (Patil, Lad, Katznelson, & Laws Jr., 2007; Starkman, Giordani, Berent, Schork, & Schteingart, 2001). Similar memory disruptions have been observed in both humans and rodents after increased levels of GCs (Moisan, Seckl, & Edwards, 1990; Seckl & Walker, 2001; Dominguez, Dagnas, et al., 2016). Thus, the increase of corticosterone in the PFC emerges as a key factor of WM impairments in withdrawn animals. This conclusion is further strengthened by our earlier studies that provide two-fold evidence as follows: (a) the blockade of corticosterone synthesis by metyrapone canceled out both the exaggerated increase of corticosterone in the PFC and WM deficits in withdrawn mice; (b) a pretest administration of a glucocorticoid receptor (GR) antagonist into the PFC restored normal WM performance in withdrawn animals (Dominguez, Belzung, et al., 2016). It is noteworthy that there is existing evidence to date that the medial prefrontal cortex is a target site for the negative-feedback effects of glucocorticoids on stress-induced HPA activity (Diorio, Viau, & Meaney, 1993).
GCs effects on WM could crucially depend on noradrenergic activity within the PFC to activate cAMP/PKA/CREB cascade (Arnsten, 2009; Barsegyan, Mackenzie, Kurose, McGaugh, & Roozendaal, 2010; Roozendaal, Quirarte, & McGaugh, 2002). Indeed, there is existing evidence about the relationships between CREB phosphorylation and GCs. Thus, Focking, Holker, and Trapp (2003) evidenced an alteration of CREB transcriptional activity in neurons repeatedly treated with GCs. It has been shown that both MR (Grossmann, Ruhs, Seiferth, & Gekle, 2010; Grossmann et al., 2010) and GR receptors (Arnsten, 2009; Barsegyan, Mackenzie, Kurose, McGaugh, & Roozendaal, 2010; Roozendaal et al., 2010) are involved in such an interaction. Interestingly, chronic ethanol treatment significantly decreased GR protein expression and GR response element (GRE)-DNA binding both in the rat cortex and the dorsal hippocampus and these changes persisted in the cortex only during alcohol withdrawal (Roy, Mittal, Zhang, & Pandey, 2002). Congruently, we found in earlier studies that the bidirectional modulation of plasma corticosterone levels (decrease or increase) by systemic administration of metyrapone or corticosterone injections in withdrawn or water mice respectively, also produced a bidirectional modulation of pCREB levels in the PFC associated with either a WM improvement (metyrapone in withdrawn animals) or impairment (corticosterone in Water controls) (Dominguez, Dagnas, et al., 2016). Thus, the regional level of corticosterone concentration is a key determinant of pCREB activity in the PFC and accordingly of WM performance.
Repeated diazepam administration alleviated transiently only the cognitive and biological alterations induced by alcohol-withdrawal
During alcohol withdrawal, GABA receptors are desensitized and down-regulated, inducing a reduction of the inhibitory action of GABAergic receptors and neuronal hyper-excitability (in Beracochea, 2006), leading to emotional, cognitive, and psychomotor dysfunction. The benzodiazepine diazepam is known to reduce neuronal excitability by its agonist action on GABAA receptors and by increasing the opening of the post-synaptic chloride channel at the membrane level (Cartmell & Mitchell, 1994; Deeb, Nakamura, Frost, Davies, & Moss, 2013). Given its anxiolytic action, diazepam is widely used during withdrawal in alcoholics. Indeed, beneficial effects of benzodiazepines over cognitive functions have been observed in alcoholics in the wake of an 8-day treatment (Ritson & Chick, 1986) or on locomotor activity and social behaviors in withdrawn rats (Knapp et al., 2005). Similarly, our data, which show a significant reduction of corticosterone concentration in the PFC of 1W withdrawn mice after repeated diazepam administration, are congruent with previous clinical findings in alcoholics (Nava et al., 2007). Interestingly, it is known that diazepam reduces the HPA axis response to stress via its inhibitory action on CRH cells of the paraventricular nucleus of the hypothalamus or other areas of the HPA axis (Calogero et al., 1988b; Arvat et al., 2002; Cullinan et al., 2008). Accordingly, we previously reported that an acute systemic diazepam injection in aged mice reduced significantly the exaggerated stress-induced increase of corticosterone concentrations both in plasma and into the hippocampus (Beracochea et al., 2011).
In keeping with earlier findings, we hypothesized that the sustained increase of the GABAergic neurotransmission by diazepam could alleviate the cognitive and neural alterations resulting from an excessive release of corticosterone during behavioral testing in withdrawn animals. Such a hypothesis is buttressed in the present study, because we showed that repeated diazepam administration (a) significantly reduced the test-induced increase of corticosterone, (b) normalized pCREB levels in the PFC, and (c) induced an improvement of memory scores in withdrawn mice. Memory enhancement in diazepam-treated mice may indeed seem unexpected given the known amnestic effects of this compound (Venault et al., 1986). Our own earlier studies also reported that an acute administration of diazepam impaired spatial and working memory (Borde, Jaffard, & Béracochéa, 1998; Borde & Beracochea, 1999; Krazem, Borde, & Béracochéa, 2001). In contrast, we reported that an acute diazepam administration induced a memory-enhancing effect through the alleviation of the excessive intra-hippocampus corticosterone release among aged-mice under behavioral testing conditions (Beracochea et al., 2011). In contrast to these previous studies, diazepam is repeatedly administered throughout the present study and is not detected in the blood of animals at the time of memory testing. Thus, the beneficial effects of diazepam on memory in 1W withdrawn mice stem more likely from a sustained attenuation of the HPA axis response during the withdrawal period rather than to an acute effect of the drug at the time of behavioral testing.
We reported that the beneficial effects of repeated diazepam administration are only transient, because they are not statistically significant in 6W withdrawn animals. The failure of repeated diazepam to counteract significantly the cognitive and neurobiological disorders in 6W withdrawn mice may stem either from persistent alterations of GABAA receptors (Liang et al., 2014; Cagetti, Liang, Spigelman, & Olsen, 2003) or other alcohol-induced neuroadaptations that may progressively develop over time after withdrawal. Indeed, these alcohol-induced adaptations involve the dysregulation of numerous signaling cascades, leading to persistent long-term changes in transcriptional profiles of genes, through the actions of transcription factors such as cAMP response element-binding protein (CREB) and chromatin remodeling, as reported earlier (Dagnas et al., 2013; Mons & Beracochea, 2016; Palmisano & Pandey, 2017). It then highlights the role of alterations of cAMP-PKA-CREB signaling cascade and potentially of histone acetylation within cortical and sub-cortical structures in the long-term persistence of withdrawal-induced cognitive impairments (Arora et al., 2013; Dominguez, Belzung, et al., 2016; Mons & Beracochea, 2016).
Conclusions
Our study evidenced that the repeated administration of diazepam counteracts only transitorily the exaggerated test-induced increase of corticosterone concentrations and pCREB alterations into the PFC as well as WM deficits in alcohol withdrawn mice. In view of preventing the reinstatement of alcohol-seeking behavior and relapse often observed in abstinent alcoholics, our present findings emphasize the need to study the relative efficacy of pharmacological compounds to counteract the long-lasting cognitive and biological alterations resulting from alcohol withdrawal.
References
Adinoff B. Double-blind study of alprazolam, diazepam, clonidine, and placebo in the alcohol withdrawal syndrome: Preliminary findings.Alcoholism, Clinical and Experimental Research 1994;18(4):873-8.
Adinoff B, Martin PR, Bone GH, Eckardt MJ, Roehrich L, George DT, Moss HB, Eskay R, Linnoila M, Gold PW (1990) Hypothalamic-pituitary-adrenal axis functioning and cerebrospinal fluid corticotropin releasing hormone and corticotropin levels in alcoholics after recent and long-term abstinence. Archives of General Psychiatry 47:325-330.
Arnsten AF (2009) Stress signalling pathways that impair prefrontal cortex structure and function. Nature Reviews. Neuroscience 10:410-422.
Arora DS, Nimitvilai S, Teppen TL , McElvain MA, Sakharkar AJ, You C, Pandey SC, Brodie MS (2013) Hyposensitivity to gamma-aminobutyric acid in the ventral tegmental area during alcohol withdrawal: Reversal by histone deacetylase inhibitors. Neuropsychopharmacology 38:1674–1684.
Bachmanov AA, Tordoff MG, Beauchamp GK (1996) Ethanol consumption and taste preferences in C57BL/6ByJ and 129/J mice. Alcoholism, Clinical and Experimental Research 20:201-206.
Barsegyan A, Mackenzie SM, Kurose BD, McGaugh JL, Roozendaal B (2010) Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. Proceedings of the National Academy of Sciences of the United States of America 107:16655-16660.
Belknap JK, Crabbe JC, Young ER (1993) Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology 112:503-510.
Beracochea D (2006) Anterograde and retrograde effects of benzodiazepines on memory. Scientific World Journal 16:1460-5.
Beracochea DJ and Jaffard R (1987) Impairment of spontaneous alternation behavior in sequential test procedures following mammillary body lesions in mice: Evidence for time-dependent interference-related memory deficits. Behavioral Neuroscience 101:187-197.
Borde N, Beracochea DJ (1999) Effects of diazepam or chronic alcohol treatment on spatial reversal learning in mice. Pharmacology, Biochemistry, and Behavior 62:719-25.
Borde N, Jaffard R, Béracochéa D (1998) Effects of chronic alcohol consumption or Diazepam administration on item recognition and temporal ordering in a spatial working memory task in mice. The European Journal of Neuroscience 10:2380-7.
Brady KT and Sonne SC (1999) The role of stress in alcohol use, alcoholism traetment and relapse. Alcohol Research & Health 23:263-275.
Brett J, Munion B (2015) Management of benzodiazepine misuse and dependence. Australian Prescriber 38:152-155
Cagetti E, Liang J, Spigelman I, Olsen RW (2003)Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABAA receptors. Molecular Pharmacology 63(1):53-64.
Cartmell SM and Mitchell D (1994) Diazepam attenuates hyperexcitability and mechanical hypersensitivity of dorsal horn convergent neurons during reperfusion of the rat's tail following ischaemia. Brain Research. 659(1-2):82-90.
Chauveau F, Tronche C, Pierard C, Liscia P, Drouet I, Coutan M, Beracochea D (2010) Rapid stress-induced corticosterone rise in the hippocampus reverses serial memory retrieval pattern. Hippocampus 20:196-207.
Dagnas M, Guillou JL, Prévôt T, Mons N (2013) HDAC inhibition facilitates the switch between memory systems in young but not aged mice. The Journal of Neuroscience 33(5):1954-63
Dalley JW, Cardinal RN, Robbins TW (2004) Prefrontal executive and cognitive functions in rodents: Neural and neurochemical substrates. Neuroscience and Biobehavioral Reviews 28:771-784.
Deeb TZ, Nakamura Y, Frost GD, Davies PA, Moss SJ. (2013) Disrupted Cl(-) homeostasis contributes to reductions in the inhibitory efficacy of diazepam during hyperexcited states. The European Journal of Neuroscience 38(3):2453-67.
Diorio D, Viau V, Meaney MJ (1993) The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. The Journal of Neuroscience 13:3839-47
Dominguez G, Belzung C, Pierard C, David V, Henkous N, Decorte L, Mons N, Beracochea D (2016). Alcohol withdrawal induces long-lasting spatial working memory impairments: Relationship with changes in corticosterone response in the prefrontal cortex. Addiction Biology 22:898-910.
Dominguez G, Dagnas M, Decorte L, Vandesquille M, Belzung C, Beracochea D, Mons N (2016) Rescuing prefrontal cAMP-CREB pathway reverses working memory deficits during withdrawal from prolonged alcohol exposure. Brain Structure & Function 221:865-77.
Dorey R, Pierard C, Chauveau F, David V, Beracochea D (2012) Stress-induced memory retrieval impairments: Different time-course involvement of corticosterone and glucocorticoid receptors in dorsal and ventral hippocampus. Neuropsychopharmacology 37:2870-2880.
Errico AL, King AC, Lovallo WR, Parsons OA (2002) Cortisol dysregulation and cognitive impairment in abstinent male alcoholics. Alcoholism, Clinical and Experimental Research 26:1198-1204.
Farr SA, Scherrer JF, Banks WA, Flood JF, Morley JE (2005) Chronic ethanol consumption impairs learning and memory after cessation of ethanol. Alcoholism, Clinical and Experimental Research 29:971-982.
Focking M, Holker I, Trapp T (2003) Chronic glucocorticoid receptor activation impairs CREB transcriptional activity in clonal neurons. Biochemical and Biophysical Research Communications 304:720-723.
George O, Sanders C, Freiling J, Grigoryan E, Vu S, Allen CD, Crawford E, Mandyam CD, Fukushiro DF, Josino FS, Saito LP, Berro LF, Morgaddo F and Frussa-Filho R. (2012) Acute and chronic ethanol differentially modify the emotional significance of a novel environment: Implications for addiction. International Journal of Neuropsychopharmacology 15: 1109-1120.
Grossmann C, Ruhs S, Seiferth A, Gekle M (2010) Interaction between mineralocorticoid receptor and cAMP/CREB signaling. Steroids 75:539-543.
Grossmann C, Wuttke M, Ruhs S, Seiferth A, Mildenberger S, Rabe S, Schwerdt G, Gekle M (2010) Mineralocorticoid receptor inhibits CREB signaling by calcineurin activation. The FASEB Journal 24:2010-2019.
Jacquot C, Croft AP, Prendergast MA, Mulholland P, Shaw SG, Little HJ (2008) Effects of the glucocorticoid antagonist, mifepristone, on the consequences of withdrawal from long term alcohol consumption. Alcoholism, Clinical and Experimental Research 32:2107-2116.
Keedwell PA, Poon L, Papadopoulos AS, Marshall EJ, Checkley SA (2001) Salivary cortisol measurements during a medically assisted alcohol withdrawal. Addiction Biology 6:247-256.
Kesner RP, Churchwell JC (2011) An analysis of rat prefrontal cortex in mediating executive function. Neurobiology of Learning and Memory 96:417-431.
Kliethermes CL (2005) Anxiety-like behaviors following repeated ethanol exposure. Neuroscience and Biobehavioral Reviews 28:837-850.
Koob GF (2012) Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proceedings of the National Academy of Sciences of the United States of America 109:18156-18161.
Krazem A, Borde N, Béracochéa D (2001).Effects of diazepam and beta-CCM on working memory in mice: Relationships with emotional reactivity. Pharmacology, Biochemistry, and Behavior 68:235-44.
Lee I, Kesner RP (2003) Time-dependent relationship between the dorsal hippocampus and the prefrontal cortex in spatial memory. The Journal of Neuroscience 23:1517-1523.
Liang J, Lindemeyer KA, Suryanarayanan A, Meyer EM, Marty VNS, Ahmad O, Xuesi Shao XM, Olsen RW, Spigelman I (2014) Plasticity of GABAA receptor-mediated neurotransmission in the nucleus accumbens of alcohol-dependent rats. Journal of Neurophysiology 112(1): 39–50.
Little HJ, Croft AP, O'Callaghan MJ, Brooks SP, Wang G, Shaw SG (2008) Selective increases in regional brain glucocorticoid: A novel effect of chronic alcohol. Neuroscience 156:1017-1027.
Lukoyanov NV, Madeira MD, Paula-Barbosa MM (1999) Behavioral and neuroanatomical consequences of chronic ethanol intake and withdrawal. Physiology & Behavior 66:337-346.
McEwen BS (2000) Effects of adverse experiences for brain structure and function. Biological Psychiatry 48:721-731.
Misra K, Pandey SC (2003) Differences in basal levels of CREB and NPY in nucleus accumbens regions between C57BL/6 and DBA/2 mice differing in inborn alcohol drinking behavior. Journal of Neuroscience Research 74:967-975.
Moisan MP, Seckl JR, Edwards CR (1990) 11 beta-hydroxysteroid dehydrogenase bioactivity and messenger RNA expression in rat forebrain: Localization in hypothalamus, hippocampus, and cortex. Endocrinology 127:1450-1455.
Mons N and Beracochea D (2016). Behavioral neuroadaptation to alcohol: From glucocorticoids to histone acetylation. Frontiers in Psychiatry 7:165.
Moselhy HF, Georgiou G, Kahn A (2001) Frontal lobe changes in alcoholism: A review of the literature. Alcohol and Alcoholism 36:357-368.
Newcomer JW, Selke G, Melson AK, Hershey T, Craft S, Richards K, Alderson AL (1999) Decreased memory performance in healthy humans induced by stress-level cortisol treatment. Archives of General Psychiatry 56:527-533.
O'Daly OG, Trick L, Scaife J, Marshall J, Ball D, Phillips ML, Williams SS, Stephens DN, Duka T (2012) Withdrawal-associated increases and decreases in functional neural connectivity associated with altered emotional regulation in alcoholism. Neuropsychopharmacology 37:2267-2276.
Palmisano M, Pandey SC (2017). Epigenetic mechanisms of alcoholism and stress-related disorders. Alcohol 60:7-18.
Patil CG, Lad SP, Katznelson L, Laws ER, Jr. (2007) Brain atrophy and cognitive deficits in Cushing's disease. Neurosurgical Focus 23:E11.
Paxinos, G, Franklin, KBJ (2001) The mouse brain in stereotaxic coordinates. 2nd ed. San Diego: Academic Press.
Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV (2001) Reorganization of frontal systems used by alcoholics for spatial working memory: An fMRI study. NeuroImage 14:7-20.
Pnag TY, Renoir T, Lawrence AJ, and Hannan AJ. (2013) Depression-related behviours displayed by female C57BL/6J mice during abstinence from chronic ethanol consumption are rescued by wheel-running . The European Journal of Neuroscience.37: 1803-1810
Rodriguez AB, Terron MP, Duran J, Ortega E, Barriga C (2001) Physiological concentrations of melatonin and corticosterone affect phagocytosis and oxidative metabolism of ring dove heterophils. Journal of Pineal Research 31:31-38.
Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, Haettig J, Wood MA (2010) Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. The Journal of Neuroscience 30:5037-5046.
Roozendaal B, McReynolds JR, McGaugh JL (2004) The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. The Journal of Neuroscience 24:1385-1392.
Roozendaal B, Quirarte GL, McGaugh JL (2002) Glucocorticoids interact with the basolateral amygdala beta-adrenoceptor--cAMP/cAMP/PKA system in influencing memory consolidation. The European Journal of Neuroscience 15:553-560.
Roy A, Mittal N, Zhang H, Pandey SC (2002) Modulation of cellular expression of glucocorticoid receptor and glucocorticoid response element-DNA binding in rat brain during alcohol drinking and withdrawal. The Journal of Pharmacology and Experimental Therapeutics 301:774-784.
Runyan JD, Dash PK (2005) Distinct prefrontal molecular mechanisms for information storage lasting seconds versus minutes. Learning & Memory 12:232-238.
Sakai K (2003) Reactivation of memory: role of medial temporal lobe and prefrontal cortex. Rev Neurosci. 14(3):241-52.
Sapolsky RM (2000) Stress hormones: Good and bad. Neurobiology of Disease 7:540-542.
Schandler SL, Clegg AD, Thomas CS, Cohen MJ (1996) Visuospatial information processing in intoxicated, recently detoxified, and long-term abstinent alcoholics. Journal of Substance Abuse 8:321-333.
Seckl JR, Walker BR (2001) Minireview: 11beta-hydroxysteroid dehydrogenase type 1- a tissue-specific amplifier of glucocorticoid action. Endocrinology 142:1371-1376.
Starkman MN, Giordani B, Berent S, Schork MA, Schteingart DE (2001) Elevated cortisol levels in Cushing's disease are associated with cognitive decrements. Psychosomatic Medicine 63:985-993
Terfehr K, Wolf OT, Schlosser N, Fernando SC, Otte C, Muhtz C, Beblo T, Driessen M, Spitzer C, Lowe B, Wingenfeld K (2011) Hydrocortisone impairs working memory in healthy humans, but not in patients with major depressive disorder. Psychopharmacology 215:71-79.
Vandesquille M, Baudonnat M, Decorte L, Louis C, Lestage P, Beracochea D (2013) Working memory deficits and related disinhibition of the cAMP/PKA/CREB are alleviated by prefrontal alpha4beta2*-nAChRs stimulation in aged mice. Neurobiology of Aging 34(6):1599-609
Vaz LJ, Pradella-Hallinan M, Bueno OF, Pompeia S (2011) Acute glucocorticoid effects on the multicomponent model of working memory. Human Psychopharmacology 26:477-487.
Venault P, Chapouthier G, de Carvalho LP, Simiand J, Morre M, Dodd RH, Rossier J (1986). Benzodiazepine impairs and beta-carboline enhances performance in learning and memory tasks. Nature 321(6073):864-6.
Voltaire-Carlsson A, Hiltunen AJ, Koechling UM, Borg S (1996) Effects of long-term abstinence on psychological functioning: A prospective longitudinal analysis comparing alcohol-dependent patients and healthy volunteers. Alcohol 13:415-421
Wan RQ, Pang K, Olton DS (1994) Hippocampal and amygdaloid involvement in nonspatial and spatial working memory in rats: Effects of delay and interference. Behavioral Neuroscience 108:866-882.
Watanabe KI, Ogihara-Hashizume A, Kobayashi Y, Mitsushio H, Komiyama T (2001) Impaired sleep during the post-alcohol withdrawal period in alcoholic patients. Addiction Biology 6:163-169.
Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z (2009) Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proceedings of the National Academy of Sciences of the United States of America 106:14075-14079.
Acknowledgments
This research was supported by grants from FRA (Fondation pour la Recherche en Alcoologie, Paris France) attributed to NM and DB. This work has benefited from the facilities and expertise of the imagery platform Imag’In (www.incia.u-bordeaux1.fr), which is supported by CNRS and REGION AQUITAINE. The authors thank Dr. Karine TITIER-DEBEAUPUIS (Laboratoire de Pharmacology et Toxicology, Bordeaux, France) for the quantification of diazepam by the LC-MS-MS technique, Dr. Mathieu COURTAND (INCIA, UMR CNRS 5287, Bordeaux France) for the creation of plugin ImageJ®, and Dr. Frances ASH for language proofreading (frances.ash@orange.fr).
Author information
Authors and Affiliations
Contributions
GD and NH were involved in experimentation and neurobiological studies. GD, NM, CB, CP and DB were involved in experimental designs, data analyses, writing of the paper and critical comments.
Corresponding author
Ethics declarations
Financial disclosure
Except for income received from primary employers, no financial support or any compensation has been received from either any individual or corporate entity over the past 3 years for either research or professional service in relation with this study. Further, no single personal financial holding may exist nor be perceived as constituting a potential conflict of interest.
Rights and permissions
About this article
Cite this article
Dominguez, G., Henkous, N., Pierard, C. et al. Repeated diazepam administration reversed working memory impairments and glucocorticoid alterations in the prefrontal cortex after short but not long alcohol-withdrawal periods. Cogn Affect Behav Neurosci 18, 665–679 (2018). https://doi.org/10.3758/s13415-018-0595-3
Published:
Issue Date:
DOI: https://doi.org/10.3758/s13415-018-0595-3