Abstract
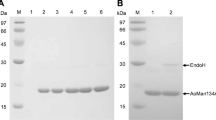
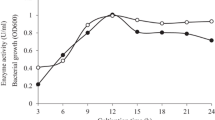
Paenibacillus sp. DZ3, newly isolated from Konjack field, produced β-mannanase (900 U/mL) when grown on glucomannan as a carbon source. β-Mannanase was purified 34-fold to homogeneity resulting in final recovery of 15% and specificity of 169 U/mg protein. The molecular mass was approximately 39 kDa as estimated by sodiumdodecylsulfate-polyacrylamide gel electrophoresis. Active band was observed as clear colourless area on zymogram. The optimal temperature and pH for mannanase activity was 60°C and pH 5.0, respectively. The activity was stable up to 60°C at pH 5.0 and remained stable from pH 5.0-7.0. Mannanase was highly specific towards glucomannan and galactomannan, whereas exhibited low activity towards mushroom powder. The Michaelis constant (K m) and maximum velocity (V max) for glucomannan substrate were 1.05 mg/mL and 714 U/mg, respectively. These results indicate the enzyme is attractive for industrial applications.
Similar content being viewed by others
References
Belitz HD and Grosch W (1987) Enzymes. In Food Chemistry, Hadziev DT (ed.), pp. 73–127, Springer Verlag, Berline, Germany.
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72, 248–254.
Buchert J, Salminen J, Sika-aho M, Ranua M, and Viikari L (1993) The role of Trichoderma reesei xylanase and mannanase in the treatment of softwood kraft pulp prior to bleaching. Holzforschung 47, 473–478.
Cuevas WA, Kantelinen A, Tanner P, Bodie B, and Leskinen S (1996) Purification and characterization of novel mannanases used in pulp bleaching. In Biotechnology in the Pulp and Paper Industry. Srebotnik E and Mesner K (eds.), pp. 123–126, Facultas-Universitätsverlag, Vienna, Austria.
David JL (1991) 16S/23S rRNA sequencing. In Nucleic acid techniques in bacterial systematic, Stackebrandt E and Goodfellow M (eds.), pp. 114–147, Wiley, New York, NY.
El-Naggar MY, El-Aassar SA, Youssef AS, El-Sersy NA, and Beltagy EA (2006) Extracellular β-mannanase production by the immobilization of the locally isolated Aspergillus niger. Int J Agri Biol 8, 57–62.
Ethier HM, Talbot G, and Sygusch J (1998) Gene cloning, DNA sequencing, and expression of thermostable β-mannanase from Bacillus stearothermophilus. Appl Environ Microbiol 64, 4428–4432.
Ferreira HM and Filho EXF (2004) Purification and characterization of a β-mannanase from Trichoderma harzianum strain T4. Carbohyd Polym 57, 23–29.
Francoise M, Ghakis C, Dupont C, Morosoli R, and Kluepfel D (1996) Improved production of mannanase by Streptomyces lividans. Appl Environ Microbiol 62, 4656–4658.
Gubitz GM, Hayn M, Urbanz G, and Steiner W (1996) Purification and properties of an acid β-mannanase from Sclerotium rolfsii. J Biotechnol 45, 165–172.
Hossain MZ, Abe J, and Hizukuri S. (1996) Multiple forms of β-mannanase from Bacillus sp. KK01. Enzyme Microb Technol 18, 95–98.
Jiang Z, Wei Y, Li D, Li L, Chai P, and Kusakabe I (2006) High-level production, purification and characterization of a thermostable β-mannanase from the newly isolated Bacillus subtilis WY34. Carbohydrate Polymers 66, 88–96.
Khanongnuch C, Asada K, Tsuruga H, Ooi T, Kinoshita S, and Lumyong S (1998) β-Mannanase and xylanase of Bacillus subtilis 5H active for bleaching of crude pulp. J Ferment Bioeng 86, 461–466.
Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, Bertero MG, Bessieres P, Bolotin A, Borchert S, Borriss R, Boursier L, Brans A, Braun M, Brignell SC, Bron S, Brouillet S, Bruschi CV, Cladwell B, Capuano V, Carter NM, Choi SK, Codani JJ, Connerton IF, and Canchin A (1997) The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature 390, 249–256.
Kurakake M and Komaki T (2001) Production of β-mannanase and β-mannosidase from Aspergillus awamori K4 and their properties. Curr Microbiol 42, 377–380.
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685.
Lee JT, Bailey CA, and Cartwright AL (2003) Beta mannanase ameliorates viscosity-associated depression of growth in broiler chickens fed guar germ and hull fractions. Poult Sci 82, 1925–1931.
Lee JT, Connor-Appleton S, Bailey CA, and Cartwright AL (2005) Effects of guar meal by-product with and without β-mannanase hemicell on broiler performance. Poult Sci 84, 1261–1267.
Li Y, Yang P, Meng K, Wang Y, Luo H, Wu N, Fan Y, and Yao B (2008) Gene cloning, expression, and characterization of a novel β-mannanase from Bacillus circulans CGMCC 1416. J Microbiol Biotechnol 18, 160–166.
McCleary BV (1988) β-D-Mannanase. In Methods Enzymol, Wood WA and Kellog ST (eds.), 160, pp. 596–610, Academic Press, California, USA.
McCoy M (2001) Soaps and detergents: An update on the latest developments within the detergent industry also introducing the latest new enzyme, a mannanase. Chem Eng News 20, 19–32.
McCutchen MC, Duffaud DG, Leduc P, Peterson HAR, Tayal A, Khan AS, and Kelly MR (1996) Characterization of extremely thermostable enzymatic breakers (α-1,6-galactosidase and β-1,4 mannanase) from the hyperthermophilic bacterium Thermotoga neapolitana 5068 for hydrolysis of guar gum. Biotechnol Bioeng 52, 332–339.
Mendoza NS, Arai M, Kawaguchi T, Cubol FS, Panerio EG, and Yoshida T (1994a) Isolation of mannan-utilizing bacteria and the culture conditions for mannanase production. World J Microbiol Biotechnol 10, 51–54.
Mendoza NS, Arai M, Kawaguchi T, Yoshida T, and Joson LM (1994b) Purification and properties of mannanase from Bacillus subtilis. World J Microbiol Biotechnol 10, 551–555.
Mendoza NS, Arai M, Sugimoto K, Kawaguchi T, and Joson LM (1995) Cloning and sequencing of β-mannanase gene from Bacillus subtilis NM-39. Biochem Biophys Acta 1243, 552–554.
Miller L (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31, 208–218.
Ooi T and Kikuchi D (1995) Purification and some properties of β-mannanase from Bacillus sp. World J Microbiol Biotechnol 11, 310–314.
Puchart V, Vrsanska M, Svoboda P, Pohl J, Ogel ZB, and Biely P (2004) Purification and characterization of two forms of endo-β-1,4-mannanase from a thermotolerant fungus, Aspergillus fumigatus IMI 385708 (formerly Thermomyces lanuginosus IMI 158749). Biochim Biophys Acta 1674, 239–250.
Sachslehner A and Haltrich D (1999) Purification and some properties of a thermostable acidic endo-β-1,4-D-mannanase from Sclerotium (Athelia) rolfsii. FEMS Microbiol Lett 177, 47–55.
Sachslehner A, Foildl G, Foidl N, Gübitz G, and Haltrich D (2000) Hydrolysis of isolated coffee mannan and coffee extract by mannanases of Sclerotium rolfsii. J Biotechnol 80, 127–134.
Sachslehner A, Nidetzky B, Kulbe KD, and Haltrich D (1998) Induction of mannanase, xylanase and endoglucanase activities in Sclerotium rolfsii. Appl Environ Microbiol 64, 594–600.
Schäfer T, Kirk O, Borchert TV, Fuglsang CC, Pedersen S, Salmon S, Olsen HS, Deinhammer R, and Lund H (2002) Enzymes for technical applications. In Biopolymers, Fahnestock SR and Teinbüchel SR (eds.), pp. 377–437, Wiley VCH, Weinheim, Germany.
Stalbrand H, Siika-aho M, Tenkanen M, and Viikari L (1993) Purification and characterization of two β-mannanases from Trichoderma reesei. J Biotechnol 29, 229–242.
Suurnakki A, Tenkanen M, Buchert J, and Viikari L (1997) Hemicellulases in the bleaching of chemical pulps. Adv Biochem Eng Biotechnol 57, 261–287.
Takahashi R, Kusakabe I, Kobayashi H, Murakami K, Maekawa A, and Suzuki T (1984) Purification and some properties of mannanase from Streptomyces sp. Agri Biol Chem 48, 2189–2195.
Takeda N, Hirasawa K, Uchimura K, Nogi Y, Hatada Y, Usami R, Yoshida Y, and Grant WD (2004) Purification and enzymatic properties of a highly alkaline mannanase from alkaliphilic Bacillus sp. strain JAMB-750. J Biol Macromol 4, 67–74.
Talbot G and Sygusch J (1990) Purification and characterization of thermostable β-mannanase and α-galactosidase from Bacillus stearothermophilus. Appl Environ Microbiol 56, 3505–3510.
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, and Higgins DG (1997) The ClustalX windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acid Res 24, 4876–4882.
Wong KKY and Saddler JN (1993) Applications of hemicellulases in the food, feed, pulp and paper industries. In Hemicellulose and Hemicellulases, Coughlan MP and Hazlewood GP (eds.), pp. 127–143, Portland Press, London, UK.
Wu G, Bryant MM, Voitle RA, and Roland DA (2005) Effects of mannanase in corn-soy diets on commercial leghorns in second cycle hens. Poult Sci 84, 894–897.
Zakaria MM, Yamamoto S, and Yagi T (1998) Purification and characterization of an endo-1,4-β-mannanase from Bacillus subtilis KU-1. FEMS Microbiol Lett 158, 25–31.
Zhang J, He ZM, and Hu K (2000) Purification and characterization of β-mannanase from Bacillus licheniformis for industrial use. Biotechnol Lett 22, 1375–1378.
Author information
Authors and Affiliations
Corresponding author
Additional information
M. R. S. Chandra and Y. S. Lee contributed equally.
Rights and permissions
About this article
Cite this article
Chandra, M.R.S., Lee, YS., Park, IH. et al. Isolation, purification and characterization of a thermostable β-mannanase from Paenibacillus sp. DZ3. J. Korean Soc. Appl. Biol. Chem. 54, 325–331 (2011). https://doi.org/10.3839/jksabc.2011.052
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.3839/jksabc.2011.052