Validation Study of UPLC Method for Determination of Morphine, Ropivacaïne and Ziconotide in Combination for Intrathecal Analgesia
Received: 10-Mar-2016 / Accepted Date: 21-Mar-2016 / Published Date: 28-Mar-2016 DOI: 10.4172/2155-9872.1000310
Abstract
Pain is often considered as the most feared symptom amongst individuals living with cancer. It can arrive at any stage during the course of the illness. In 15% to 20% of patients, conventional analgesic therapy either fails to relieve pain or induces adverse effects. Use of analgesic admixture has been recommended by the most recent consensus conferences. Several studies found evidence of synergistic effects of intrathecal analgesic admixtures, most notably these containing ziconotide, morphine and ropivacaïne, administering by a fully implantable pumps. The refills were prepared under a laminar airflow hood under strictly aseptic conditions, by the hospital pharmacist. This group of drugs that commonly used can be at the origin of errors inducing adverse effects in patients. In order to evaluate the accuracy of compounding of intrathecal admixtures, a new analytical method by simple liquid chromatography ultraviolet spectrometry method was developed and validated for the simultaneous quantification of three analgesic drugs (morphine, ropivacaïne and ziconotide). The method was validated according to the recommendation of the US Food and Drug Administration (FDA). The method was linear, between 0.1 to 4 μg/ml for ziconotide, 0.1 to 10 mg/ ml for ropivacaïne and 0.1 to 32 mg/ml for morphine. Forced degradation of ziconotide by acidic conditions allowed formation and detection of degradation products by the analytical method. This method can be considered as a stability indicating method. It is also part of a continuing quality process designed to improve accuracy of preparation.
Keywords: Intrathecal analgesia; Morphine; Ropivacaïne; Ziconotide; UPLC-UV
Introduction
The management of cancer is not limited to the treatment of the disease but must also consider patient pain. In 15% to 20% of cancer patients, conventional analgesic therapy recommended by WHO fails to relieve pain [1]. In France, Intrathecal Analgesia (IA) is recommended by French Society of Anesthesia and Intensive Care Medicine (SFAR) in case of refractory chronic cancer pain, as well as in patients with treatment-related adverse effects impairing patient’s quality of life [2]. The administration of anesthetic drugs in the central nervous system can achieve similar or better analgesic results and can be easier to use for clinicians [3-5].
The administration of these analgesic drugs by intrathecal route is done by using a pump (an internal implantable or external intrathecal pump), connected with a tunneled catheter implanted in the intrathecal area [6,7]. The implantable pump has a reservoir which has to be refilled. External pumps have a bag containing the anesthetic substances that needs to be regularly replaced. This refill is prepared in a controlled atmosphere area under strict aseptic conditions in a vertical laminar flow cabinet by manipulators trained on these preparations to be used internally. Commercial pharmaceutical products are used for preparation.
Over the years, various molecules have been used in combination for IA. Morphine remains the reference in case of strong to intense pain. Ropivacaïne or bupivacaine are used to strengthen morphine effects by blocking nerve conduction in the sodium channels. Ziconotide is a small ω-conotoxine peptide isolated from a marine cone snail, Conus magus and acts as a selective blocker of voltage-sensitive calcium channels of N-type [8-11]. Due to its narrow therapeutic range and its potentially serious adverse effects (psychiatric and nervous system disorders), this drug is only indicated for the treatment of severe, chronic pain in adults who require intrathecal (IT) analgesia [12,13]. Finally, clonidine, sufentanil, ketamine can also be used, although these molecules are not present in the last International recommendations.
The Cancerology Center Paul Papin protocol includes an association of morphine, ropivacaïne and ziconotide in Intrathecal preparations (PIT). Concentrations of each analgesic agent vary from one preparation to another because they are adapted for each patient’s pain evaluated by the physician before the prescription [14]. Concentrations mean and range are listed in the Table 1.
| Mean concentrations [Min-Max] | |
| Morphine | 3.34 [0.05-23.9] mg/mL |
| Ropivacaïne | 7.54 [0.4-9.8] mg/mL |
| Ziconotide | 0.98 [0.04-5.8] μg/mL |
Table 1: Mean concentrations of morphine, ropivacaïne and ziconotide in the PIT.
Dosage errors most likely explain a significant percentage of the side effects [15,16] caused by ziconotide. Ziconotide is a particularly powerful drug. Its therapeutic index is low and adverse effects may occur at low doses (1 μg/day) [8,14,17]. The accuracy of the injected dose is therefore vital and no other clinical study has checked concentrations of ziconotide really infused.
To secure the preparations, a systematic analytical control must be performed before release. Over time, several authors have studied the ziconotide dosage in combination with morphine and ropivacaïne by using methods of liquid chromatography combined with ultra-violet detection, although ziconotide concentrations were higher than those that we are actually using (25 μg/mL) [18-20].
The aim of this study was to develop and validate a new method of determination of ziconotide, morphine and ropivacaïne using ultra-performance liquid chromatography (UPLC) combined with an ultraviolet detector in order to control our preparation according to national and international recommendations [21-23].
Materials and Methods
Analytes and reagents
For development and validation of the method the commercial pharmaceutical products were used. Morphine (CDM Lavoisier, La Chaussée-Saint Victor, France) is available under a ready-to-use intravenous and intrathecal formulation of morphine sulfate 500 mg/10 mL including the excipients sodium chloride, hydrochloric acid and water for injection.
Ropivacaïne (Fresenius Kabi, Sèvres, France) is supplied in 20 mL bottles containing 200 mg and the excipients sodium chloride, hydrochloric acid, sodium hydroxide and water for injection. Ziconotide (Prialt®, Eisai SAS, Paris La Défense, France) is provided in 100 μg/1 mL bottles, including the excipients methionine, sodium chloride, hydrochloric acid, sodium hydroxide and water for injection. The three drugs are mixed together, alone or with a saline solution of 0.9% as excipient (Fresenius Kabi, Sèvres, France), before refilling the intrathecal pump of 40 mL.
Acetonitrile and trifluoroacetic acid were supplied by VWR (Fontenay aux Roses, France) and ultrapure water was obtained from Elga Purelab DV25 system. Solvents are HPLC-MS/MS grade to avoid impurities like metal ions in the mobile phase.
Chromatographic and UV detection conditions
Analyses were conducted using an UPLC Acquity® H-Class® interfaced to a photodiode array detector (PDA) (Waters, Guyancourt, France). The column used was an Acquity UPLC® BEH C18 1.7 μm 2.1 × 50 mm heated at 50°C and the mobile phase was a gradient of ultrapure water with 0.1% trifluoroacetic acid and acetonitrile with 0.1% trifluoroacetic acid. Two liquid chromatography (LC) methods were developed due to concentration range difference between analytes. Respective gradients are presented in Tables 2 and 3. For morphine and ropivacaïne analysis, 0.3 μL were injected into the column whereas 10 μL were injected for ziconotide. Detection was monitored at 285 nm for morphine, at 230 nm for ropivacaïne and at 200 nm for ziconotide. In these conditions, the retention times were of 0.62 minutes, 1.99 minutes and 1.62 minutes, respectively.
| Time (min) | Flow Rate (mL/min) | % Solvent A | % Solvent B |
|---|---|---|---|
| 0 | 0.5 | 95 | 5 |
| 0.5 | 0.5 | 90 | 10 |
| 2.5 | 0.5 | 25 | 75 |
| 2.8 | 0.5 | 0 | 100 |
| 3.8 | 0.5 | 0 | 100 |
| 4.5 | 0.5 | 95 | 5 |
| 5.5 | 0.5 | 95 | 5 |
Table 2: Gradient used for morphine and ropivacaïne LC analysis.
| Time (min) | Flow Rate (mL/min) | % Solvent A | % Solvent B |
|---|---|---|---|
| 0 | 0.5 | 95 | 5 |
| 0.5 | 0.5 | 90 | 10 |
| 0.9 | 0.5 | 75 | 25 |
| 2.0 | 0.5 | 75 | 25 |
| 2.5 | 0.5 | 0 | 100 |
| 3.5 | 0.5 | 0 | 100 |
| 3.8 | 0.5 | 95 | 5 |
| 5.5 | 0.5 | 95 | 5 |
Table 3: Gradient used for ziconotide LC analysis.
Standards and quality controls (QC) preparations
Standards of morphine (0.1 to 32 mg/mL) and ropivacaïne (0.312 to 10 mg/mL) were prepared by serial dilution of the pharmaceutical product with Nacl 0.9% in glass vials. Ziconotide standards (0.1 to 4 μg/ mL) were individually prepared by diluting the commercial solution in polypropylene vials to avoid wrong results when using serial dilution.
Quality controls (QC) containing a mean concentration of the three active substances (Morphine 4 mg/mL, Ropivacaïne 8 mg/mL and ziconotide 1 μg/mL) in NaCl 0.9% were prepared and analyzed during each validation run.
Validation procedure
The validation procedure was performed according to the US Food and Drug Administration (FDA) and Groupe d’Evaluation et de Recherche sur la Protection en Atmosphère Contrôlée (GERPAC) recommendations [22,23].
Selectivity: Selectivity with regard to excipients or other constituents of the mixture has been checked by analyzing individually three samples containing only the excipient (NaCl 0.9%) and the two other active substances in a mean concentration.
Calibration curve: The response of the instrument with regard to the concentration was studied. Concentrations were chosen on the basis of the expected range. For each analyte, six calibration standards and one zero standard have been analyzed during 3 runs (N=3), performed on three days by different manipulators.
Calibration curves were using a linear regression of the calibration points excluding the zero standards. Weighting and adequacy of the regression model was determined by statistical analysis (Levene’s test and lack-of fit test) according to the FDA recommendations [22]. The simplest model was used and between-run accuracy and precision of the standards were calculated.
QC within-run and between-run accuracy and precision: The accuracy and precision of the methods were evaluated by analyzing six individually prepared QC during each run. Within-run accuracy and precision were determined without reagent or manipulator variability in a single run (n=6) whereas between-run precision and accuracy were determined on three runs (total: n=18) performed in different days and involving several manipulators.
Accuracy was expressed as relative error (RE %) compared with nominal values and precision (repeatability) was determined by calculating the relative standard deviation (RSD %) of the measured QC concentration. Limits of acceptable were fixed at ±15% deviation from the expected for RSD and RE.
Carryover: Blanks containing 0.9% sodium chloride without analyte were injected after three high concentration samples (QC samples) in order to evaluate carry over for each analyte. This injection sequence was repeated 3 times.
Forced degradation study: As far as it was possible, a degradation of around 20% of the active substance was carried out by treating the analyte diluted in NaCl 0.9% with 0.5% hydrochloric acid followed by neutralization with 0.5% of sodium hydroxide. Each degradation product was identified by its retention time relative to the main analyte and mass balance was determined.
Results and Discussion
Selectivity
No interfering peaks were detected at the specific retention times of the three analytes of interest in the tested samples. The absence of interference by 0.9% NaCl and the two other active substances of the mixture have been validated for the three analytes. Thus, the matrix effect was not studied.
Response function analysis
Morphine and ziconotide, equality of variances was rejected and calibration curves were linear over the studied concentration ranges with 1/x regression. For ropivacaïne, homogeneity of variances was accepted according to the Levene’s Test (calculated F=2.932<theoretical F=3.106, IC 95%), so the linear regression was carried out without weighting. Calibrators calculated concentrations and between-run accuracy and precision are presented in Table 4. Acceptance criteria were met with absolute RE and RSD <15% for the three analytes. The regression coefficient (R2) for each calibration curve was >0.996.
| Nominal concentrations | Calculated concentrations | Between-run accuracy and precision | |||||
|---|---|---|---|---|---|---|---|
| Run 1 | Run 2 | Run 3 | mean | RE% | RSD% | ||
| Morphine calibrators (mg/mL) | |||||||
| 0.10 | 0.090 | 0.101 | 0.099 | 0.097 | -3.2% | 6.0% | |
| 0.50 | 0.519 | 0.502 | 0.499 | 0.507 | 1.3% | 2.1% | |
| 1.00 | 1.041 | 1.001 | 1.000 | 1.014 | 1.4% | 2.3% | |
| 4.00 | 4.091 | 3.924 | 4.016 | 4.010 | 0.3% | 2.1% | |
| 16.00 | 16.051 | 16.073 | 16.174 | 16.100 | 0.6% | 0.4% | |
| 32.00 | 31.808 | 31.998 | 31.811 | 31.872 | -0.4% | 0.3% | |
| R2 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.001% | ||
| Slope | 0.9939 | 1.0010 | 0.9961 | 0.9970 | 0.361% | ||
| Ropivacaïne calibrators (mg/mL) | |||||||
| 0.312 | 0.285 | 0.302 | 0.306 | 0.298 | -4.6% | 3.8% | |
| 0.625 | 0.606 | 0.619 | 0.619 | 0.615 | -1.6% | 1.2% | |
| 1.25 | 1.245 | 1.245 | 1.244 | 1.244 | -0.4% | 0.1% | |
| 2.50 | 2.529 | 2.510 | 2.508 | 2.516 | 0.6% | 0.5% | |
| 5.00 | 5.054 | 5.023 | 5.023 | 5.033 | 0.7% | 0.4% | |
| 10.00 | 9.969 | 9.987 | 9.988 | 9.981 | -0.2% | 0.1% | |
| R2 | 0.9999 | 0.9999 | 0.9999 | 0.9999 | 0.002% | ||
| Slope | 1.0000 | 1.0000 | 1.0000 | 1.0000 | 0.0% | ||
| Ziconotide calibrators (μg/mL) | |||||||
| 0.10 | 0.108 | 0.110 | 0.102 | 0.107 | 6.8% | 3.7% | |
| 0.20 | 0.206 | 0.217 | 0.211 | 0.211 | 5.6% | 2.6% | |
| 0.40 | 0.375 | 0.362 | 0.364 | 0.367 | -8.3% | 2.0% | |
| 1.00 | 0.919 | 0.943 | 0.981 | 0.948 | -5.2% | 3.3% | |
| 2.00 | 2.027 | 1.810 | 2.087 | 1.975 | -1.3% | 7.4% | |
| 4.00 | 4.064 | 4.258 | 3.955 | 4.092 | 2.3% | 3.8% | |
| R2 | 0.9997 | 0.9969 | 0.9995 | 0.9987 | 0.2% | ||
| Slope | 1.0195 | 1.0517 | 0.9968 | 1.0227 | 2.7% | ||
Table 4: Calibrators between-run accuracy and precision.
QC within-run and between run accuracy and precision
Within-run and between-run accuracy and precision were determined with QC samples as described in the validation procedure section. Within-run precision ranged between -7.0% and 2.4% for ziconotide, -0.7% and -1.6% for ropivacaïne, -0.2% and -0.1% for morphine. Within-run and between accuracy and precision met the acceptance criteria with RSD <15% and inaccuracy ranged from -15% to +15% for the three studied molecules (Table 5).
| Nominal Concentration | Calculated Concentrations | Between-run accuracy and Precision | |||||
|---|---|---|---|---|---|---|---|
| Run 1 | Run 2 | Run 3 | mean | RE% | RSD% | ||
| Morphine | 3.994 | -0.1% | 0.7% | ||||
| QC1 | 4.0 mg/mL | 3.966 | 3.997 | 3.999 | |||
| QC2 | 4.012 | 3.976 | 4.015 | ||||
| QC3 | 4.026 | 3.973 | 3.973 | ||||
| QC4 | 3.954 | 4.002 | 3.940 | ||||
| QC5 | 3.993 | 4.033 | 4.028 | ||||
| QC6 | 4.035 | 3.985 | 3.986 | ||||
| Within-run mean | 3.998 | 3.994 | 3.990 | ||||
| Within-run RE% mean | -0.1% | -0.1% | -0.2% | ||||
| Within-run CV% | 0.8% | 0.6% | 0.8% | ||||
| Ropivacaïne | |||||||
| QC1 | 8.0 mg/mL | 7.905 | 7.844 | 7.935 | 7.937 | -0.8% | 0.9% |
| QC2 | 7.971 | 7.859 | 8.037 | ||||
| QC3 | 7.965 | 7.858 | 7.978 | ||||
| QC4 | 7.859 | 7.834 | 7.994 | ||||
| QC5 | 7.994 | 7.946 | 7.962 | ||||
| QC6 | 7.988 | 7.874 | 8.063 | ||||
| Within-run mean | 7.947 | 7.869 | 7.995 | ||||
| Within-run RE% mean | -0.7% | -1.6% | -0.1% | ||||
| Within-run RSD% | 0.7% | 0.5% | 0.6% | ||||
| Ziconotide | |||||||
| QC1 | 1.0 μg/mL | 1.021 | 0.987 | 0.879 | 0.985 | -1.5% | 5.0% |
| QC2 | 1.065 | 1.003 | 0.921 | ||||
| QC3 | 1.073 | 0.993 | 0.941 | ||||
| QC4 | 1.026 | 1.012 | 0.948 | ||||
| QC5 | 0.981 | 0.999 | 0.949 | ||||
| QC6 | 0.978 | 1.010 | 0.943 | ||||
| Within-run mean | 1.024 | 1.001 | 0.930 | ||||
| Within-run RE% mean | 2.4% | 0.1% | -7.0% | ||||
| Within-run RSD% | 3.9% | 1.0% | 2.9% | ||||
Table 5: Within-run and between-run accuracy and precision obtained for QC samples.
Carryover
No peak was detected in the blank series, meaning that no intersample contamination occurred for ziconotide, morphine and ropivacaïne.
Degradation study
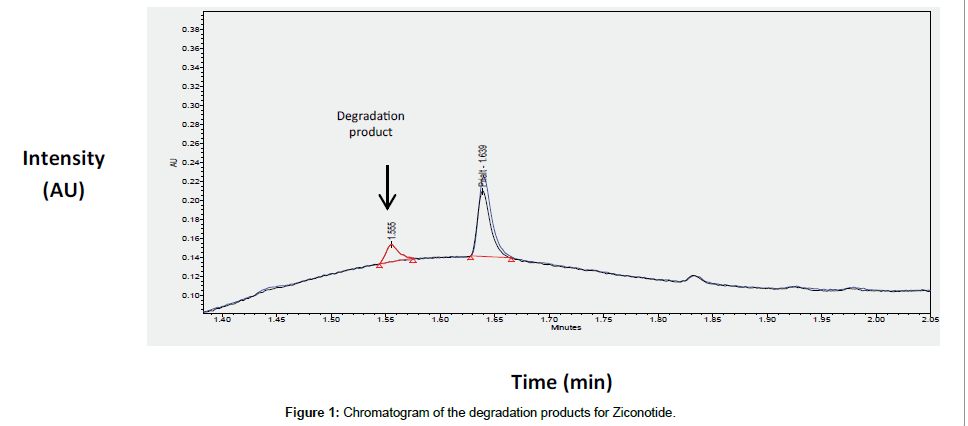
Ziconotide (1.3 μg/mL) was degraded by adding 0.5% HCl to the final concentration. The vial was opened during 3 hours in order to mimic a prolonged exposition to oxygen and allow oxidation reactions representing 20.54% of the initial compound was observed at a relative retention time of 0.95 minutes (Figure 1). The mass balance obtained was 100.1%, which proves that no other degradation product was found (Table 6). This degradation product has a specific retention time of 1.555 minutes and will be therefore visible in samples containing morphine and ropivacaïne.
| Response (Peak area) |
Retention time (RT in min) | Relative retention time (RRT in min) | % Area | Mass balance (%) | |
|---|---|---|---|---|---|
| Peak area analyte non degraded | 71436 | 1.640 | 1.00 | 100.00 | - |
| Peak area analyte - degraded | 56834 | 1.639 | 1.00 | 79.559 | 100.1% |
| Peak area of product of degradation 1 | 14692 | 1.555 | 0.95 | 20.567 |
Table 6: Degradation products of ziconotide.
No degradation product was observed for morphine or ropivacaïne despite treatments with hydrochloric acid, hydrogen peroxide, sodium hydroxide or UV.
Application
After validation, the method has been routinely used for PIT preparation analysis. During the first month 248 PIT mixtures (including 224 with ziconotide) have been prepared and analyzed before release. Samples with concentrations out of range were diluted. The acceptance criteria were ± 10% for morphine and ropivacaïne and ±15% for ziconotide. The absolute RE means were between 2.1% and 3.2% for morphine, between 0.9% and 3.1% for ropivacaïne and between 5.6% and 9.3% for ziconotide (Table 7). One preparation has been rejected (absolute RE -22.80%).
| Concentration range | RE % Min / Max | Absolute RE mean (%) | No of preparations |
|---|---|---|---|
| Morphine (mg/mL) | |||
| 0.1-2.5 | -4.60% / 9.95% | 2.0% | 122 |
| 2.5-5.0 | -5.55% / 8.93% | 2.1% | 50 |
| 5.0-10.0 | -6.03% / 3.77% | 2.4% | 43 |
| >10.0 | -7.09% / 0.43% | 3.2% | 31 |
| Total Range | -7.09% / 9.95% | 2.3% | 246 |
| Ropivacaïne (mg/mL) | |||
| 0.316-2.5 | -7.00% / 2.25% | 3.1% | 14 |
| 2.5-5.0 | -2.40% / 3.07% | 1.3% | 13 |
| 5,0-7.5 | -3.56% / 4.28% | 1.4% | 35 |
| >7.5 | -7.78% / 2.95% | 0.9% | 181 |
| Total Range | -7.78% / 4.28% | 1.1% | 243 |
| Ziconotide (µg/mL) | |||
| 0.1-0.25 | -14.00% / 14.00% | 8.7% | 23 |
| 0.25-0.5 | -10.59% / 13.33% | 6.1% | 40 |
| 0.5-0.75 | -22.80% / 13.15% | 6.9% | 73 |
| >0.75 | -14.75% / 11.13% | 5.6% | 86 |
| Total Range | -22.80% / 14.00% | 6.4% | 222 |
Table 7: Absolute RE mean obtained for routinely analyzed PIT preparations.
Conclusion
Intrathecal Analgesia is in progression and our activity is increasing with a progression of 54% between 2012 and 2013. A method based on UPLC-UV to quantify admixture with morphine, ropivacaïne and ziconotide was developed and fully validated according to FDA recommendations. The calibration range was from 0.1 to 4 μg/mL for ziconotide, 0.316 to 10 mg/mL for ropivacaïne and 0.1 to 32 mg/mL for morphine. This method offers an interesting compromise in terms of analysis time and detection level compared with previously published methods [18,20]. This quantitative analysis limits dosage errors and overdosing, particularly for ziconotide. The method for ziconotide, morphine and ropivacaïne determination that we developed was validated, used in routine and can be consider as a stability indicating method. In order to determine the expiration date, a stability study will be carried out. Results may allow us to develop subcontracting activity which would consist in preparations shipment in other centers.
References
- Meuser T, Pietruck C, Radbruch L, Stute P, Lehmann KA, et al. (2001) Symptoms during cancer pain treatment following WHO-guidelines: a longitudinal follow-up study of symptom prevalence, severity and etiology. Pain 93: 247-257.
- AFSOS (2014) Référentiels en soins oncologiques de support - Prise en charge de la douleur? antalgie intrathécale.
- Deer TR, Smith HS, Burton AW, Pope JE, Doleys DM, et al. (2011) Comprehensive consensus based guidelines on intrathecal drug delivery systems in the treatment of pain caused by cancer pain. Pain Physician 14: E283-312.
- Deer TR, Prager J, Levy R, Rathmell J, Buchser E, et al. (2012) Polyanalgesic Consensus Conference 2012: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation 15: 436-464.
- Deer TR (2012) Polyanalgesic Consensus Conference 2012. Neuromodulation 15: 418-419.
- Bhatia G, Lau ME, Koury KM, Gulur P (1000) Intrathecal Drug Delivery (ITDD) systems for cancer pain. Version 4.
- Bottros MM, Christo PJ (2014) Current perspectives on intrathecal drug delivery. J Pain Res 7: 615-626.
- Dupoiron D, Bore F, Lefebvre-Kuntz D, Brenet O, Debourmont S, et al. (2012) Ziconotide adverse events in patients with cancer pain: a multicenter observational study of a slow titration, multidrug protocol. Pain Physician 15: 395-403.
- Schmidtko A, Lötsch J, Freynhagen R, Geisslinger G (2010) Ziconotide for treatment of severe chronic pain. Lancet 375: 1569-1577.
- Mathur VS (2000) Ziconotide: A new pharmacological class of drug for the management of pain. Journal of Critical Care 19: 67-75.
- Vink S, Alewood PF (2012) Targeting voltage-gated calcium channels: developments in peptide and small-molecule inhibitors for the treatment of neuropathic pain: VGCC ligands and pain. Br J Pharmacol 167: 970-989.
- Alicino I, Giglio M, Manca F, Bruno F, Puntillo F (2012) Intrathecal combination of ziconotide and morphine for refractory cancer pain: A rapidly acting and effective choice. Pain 153: 245-249.
- Dupoiron D, Devys C, Bazin C, Folliard C, Lebrec N, et al. (2015) Rationale for Prospective Assays of Intrathecal Mixtures Including Morphine, Ropivacaine and Ziconotide: Prevention of Adverse Events and Feasibility in Clinical Practice. Pain Physician 18: 349-357.
- Rauck RL, Wallace MS, Leong MS, Minehart M, Webster LR, et al. (2006) A Randomized, Double-Blind, Placebo-Controlled Study of Intrathecal Ziconotide in Adults with Severe Chronic Pain. J Pain Symptom Manage 31: 393-406.
- Staats PS, Yearwood T, Charapata SG, Presley RW, Wallace MS, et al. (2004) Intrathecal Ziconotide in the Treatment of Refractory Pain in Patients with Cancer or AIDS: A Randomized Controlled Trial. JAMA 291: 63-70.
- Maier C, Gockel HH, Gruhn K, Krumova EK, Edel MA (2011) Increased risk of suicide under intrathecal ziconotide treatment?- a warning. Pain 152: 235-237.
- Shields D, Montenegro R (2007) Chemical stability of ziconotide-clonidine hydrochloride admixtures with and without morphine sulfate during simulated intrathecal administration. Neuromodulation 10 Suppl 1: 6-11.
- Shields DE, Liu W, Gunning K, Montenegro R (2008) Statistical evaluation of the chemical stability of ziconotide solutions during simulated intrathecal administration. J Pain Symptom Manage 36: e4-6.
- Shields D, Montenegro R, Ragusa M (2005) Chemical stability of admixtures combining ziconotide with morphine or hydromorphone during simulated intrathecal administration. Neuromodulation 8: 257-263.
- Sautou V, Brossard D, Chedru-Legros V, Crauste-Manciet S, Fleury-Souverain S, et al. (2013) Methodological guidelines for stability studies of hospital pharmaceutical preparations. GERPAC.
Citation: Rossignol E, Sorrieul J, Beaussart H, Kieffer H, Folliard C, et al. (2016) Validation Study of UPLC Method for Determination of Morphine, Ropivacaïne and Ziconotide in Combination for Intrathecal Analgesia. J Anal Bioanal Tech 7:310. Doi: 10.4172/2155-9872.1000310
Copyright: © 2016 Rossignol E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 11581
- [From(publication date): 6-2016 - May 07, 2024]
- Breakdown by view type
- HTML page views: 10833
- PDF downloads: 748