Published online Dec 15, 2013. doi: 10.4239/wjd.v4.i6.310
Revised: March 20, 2013
Accepted: April 10, 2013
Published online: December 15, 2013
By 2050 the prevalence of diabetes will more than triple globally, dramatically increasing the societal and financial burden of this disease worldwide. As a consequence of this growth, it is anticipated that there will be a concurrent rise in the numbers of patients with diabetic macular edema (DME), already among the most common causes of severe vision loss worldwide. Recent available therapies for DME target the secreted cytokine, vascular endothelial growth factor (VEGF). This review focuses on the treatment of DME using the first humanized monoclonal antibody targeting VEGF that has been Food and Drug Administration-approved for the use in the eye, ranibizumab (Lucentis®).
Core tip: This article reviews the use of ranibizumab for diabetic macular edema. The article presents recent data on which the practice of ranibizumab injections for diabetic macular edema is based, and highlights issues regarding efficacy, safety, and other important considerations for any retina provider using ranibizumab in practice.
- Citation: Krispel C, Rodrigues M, Xin X, Sodhi A. Ranibizumab in diabetic macular edema. World J Diabetes 2013; 4(6): 310-318
- URL: https://www.wjgnet.com/1948-9358/full/v4/i6/310.htm
- DOI: https://dx.doi.org/10.4239/wjd.v4.i6.310
Diabetic retinopathy is a leading cause of visual impairment worldwide, and diabetic macular edema (DME) is the most common cause of vision loss in these patients[1]. DME is a consequence of breakdown of the vascular inner blood-retinal barrier (BRB), and may occur in one of two ways[2]: (1) focal leakage arising from microaneurysms; and (2) diffuse leakage arising from the walls of capillaries (Figure 1). Systemic disease plays a role in the development and progression of diabetic retinopathy, and improving control of blood glucose and blood pressure has been shown to slow the progression of diabetic eye disease[3]. However, in many patients, despite adequate diabetic control, if left untreated DME can result in significant vision loss[4].
Since 1985, the mainstay of treatment for clinically significant (diabetic) macular edema had been focal/grid laser photocoagulation based on the Early Treatment Diabetic Retinopathy Study (ETDRS)[4]. In focal/grid laser photocoagulation, focal laser is applied to leaking microaneurysms while a grid pattern of larger burns of light intensity at the level of the retinal pigment epithelium is used to treat diffuse leakage. In the ETDRS, the 754 eyes treated with laser photocoagulation had approximately a 50% decrease in the risk of vision loss (defined as doubling of the initial visual angle or a loss of three or more lines) compared to the 1490 eyes without treatment. However, laser photocoagulation did not significantly improve vision in patients enrolled in the ETDRS.
In 2002, a National Eye Institute-sponsored collaborative network, the Diabetic Retinopathy Clinical Research Network (DRCR.net), compared steroid versus laser treatment for DME[5]. In this study, 26% of DME patients treated with laser did gain 15 or more letters of vision at three years. Nonetheless, despite these promising results, laser photocoagulation for DME leaves the majority of patients with little hope for an improvement in vision. Thus, while many ophthalmologists continue to employ this modality of treatment, other approaches (e.g., intravitreal steroids) have also been explored (alone or in combination with laser photocoagulation) with the goal of improving on these results; the use of intravitreal steroids for the treatment of DME is discussed elsewhere in this review series.
In this regard, recent efforts to unravel the molecular pathogenesis of DME have led to the development of new therapeutic approaches for the treatment of this disease. The concept that ischemic retinopathies are driven by a secreted angiogenic factor was proposed over half a century ago[6]. More recently, appreciation for a single cytokine, vascular endothelial growth factor (VEGF), as the central player in the development of DME has facilitated a paradigm shift in how we treat this disease[7].
VEGF is a sub-family of growth factors (the platelet-derived growth factor family of cysteine-knot growth factors) produced by hypoxic cells that act as signal proteins to stimulate both vasculogenesis and angiogenesis[8]. The VEGF sub-family includes VEGF-A, VEGF-B, VEGF-C, VEGF-D and placental growth factor (PIGF). These proteins act by binding to VEGF receptors (e.g., VEGFRs 1-3), which are tyrosine kinase receptors with an extracellular ligand binding domain and an intracellular tyrosine kinase domain[9]. Upon ligand (VEGF) binding to the receptor, the receptors dimerize, and the tyrosine kinase domain initiates phosphorylation at the C-terminus of the molecule. This initiates an intracellular signaling cascade that ultimately leads to changes in gene transcription[9].
VEGF affects a number of cell types (e.g., monocytes and macrophages, neurons, tumor cells, kidney epithelial cells). However, VEGFR2 is expressed predominantly on vascular endothelial cells, which are predominantly responsible for the pathological effects of VEGF in the eye. In particular, VEGF-A has been shown to promote the growth and survival of vascular endothelial cells (promoting angiogenesis) and to disrupt endothelial cell-endothelial cell tight junctions (promoting vascular permeability), leading to retinal neovascularization and macular edema in diabetic eye disease, respectively[7].
The rationale for using anti-VEGF agents to treat diseases characterized by dysregulated angiogenesis is based largely on studies demonstrating the role of VEGF in the context of cancer, where this angiogenic cytokine has been shown to play a critical role in tumor growth and metastasis[10]. A role for VEGF has subsequently been established in pathological angiogenesis in other diseases, including those affecting the eye[11]. Indeed, increased levels of VEGF have been demonstrated in the eyes of patients with diabetic retinopathy and diabetic macular edema compared to normal controls[12,13]. Collectively, these observations have prompted exploration of therapies targeting VEGF as an approach for patients with DME.
The first attempt to inhibit VEGF in ocular disease was with the pegylated anti-VEGF aptamer, Pegaptanib (Macugen®), a single strand of nucleic acid that binds with specificity to VEGF-165 mRNA[14]. Pegaptanib was originally developed and approved by the United Sates Food and Drug Administration (FDA) in December 2004 as an anti-angiogenic medicine for the treatment of neovascular (wet) age-related macular degeneration (AMD). The use of Pegaptanib for DME was also explored, and although it was not as effective as investigators had hoped, it did confirm the therapeutic potential of anti-VEGF therapy for diabetic eye disease[15].
An alternative approach developed by Genentech to target VEGF was the use of humanized monoclonal antibodies targeting VEGF-A. Since VEGF is a secreted protein, it was vulnerable to targeting in the extracellular environment. The first monoclonal antibody against VEGF developed by Genentech was bevacizumab (Avastin®). Bevacizumab is a recombinant humanized monoclonal immunoglobulin G1κ antibody that was FDA-approved in February 2004 and marketed as a treatment for colon cancer[16]. However, as emerging evidence pointed to VEGF as a central player in ocular disease, ophthalmologist began to use bevacizumab as an “off-label” treatment for wet AMD and later diabetic eye disease[17,18]; the use of intravitreal bevacizumab for the treatment of DME is discussed briefly in this review and extensively elsewhere in this review series.
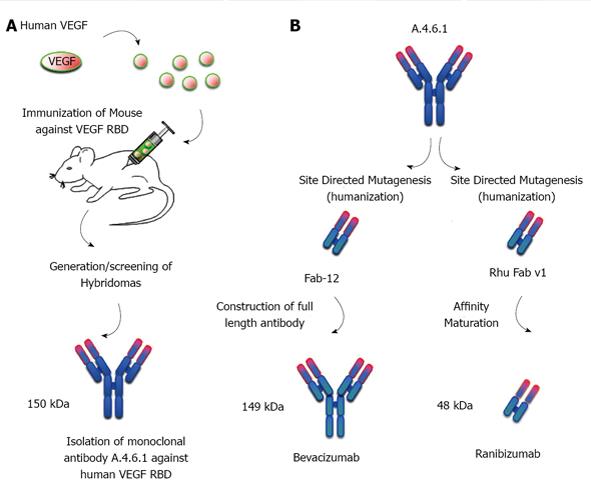
Soon after the release of bevacizumab, Genentech developed a second humanized monoclonal antibodies targeting VEGF-A, ranibizumab (Lucentis®) that was FDA-approved in June 2006 and marketed for use in the eye[19]. Ranibizumab was created from the same parent mouse anti-human VEGF monoclonal antibody (mAb A.4.6.1) as was bevacizumab, and targets the identical epitope of VEGF (AS82-91) from within the receptor-binding domain (AS8-109) of VEGF165 (Figure 2). Ranibizumab underwent affinity purification to improve its binding to VEGF. And like bevacizumab, ranibizumab has neutralizing activity on all VEGF isoforms. However, there are five important differences between bevacizumab and ranibizumab: (1) ranibizumab (48 kDa) contains only the Fab fragment of the parental antibody, while bevacizumab (149 kDa) contains the Fab and Fc fragments (whole antibody); (2) the sequence of ranibizumab differs from the corresponding sequence in bevacizumab by six amino acids; (3) each ranibizumab molecule has one binding site for VEGF (compared to bevacizumab’s two); (4) ranibizumab is produced in prokaryotic Escherichia coli, and therefore it does not carry any glycosylation sites while bevacizumab is produced in a eukaryotic cell line (CHO cells) and is N-glycosylated in its Fc region; and (5) ranibizumab costs approximately 40 fold more than bevacizumab ($2000 per injection compared to approximately $50 per injection, respectively).
Ranibizumab was developed as an Fab fragment because it was thought that enhanced diffusion from the vitreous into the retina and choroid could be achieved with the smaller size molecule relative to full-length antibodies[19]. However, subsequent studies comparing the two suggest that the predicted size advantage may not translate into a therapeutic advantage in patients[20]. Affinity maturation of ranibizumab was also predicted to result in higher affinity binding to VEGF and increased biologic activity compared to bevacizumab[19]. Indeed, initial studies using the monovalent Fab-12 (from which the divalent bevacizumab was derived), suggested that the binding affinity of bevacizumab is markedly lower than what was later demonstrated for ranibizumab[21]. However, in a more recent study using a bivalent antibody (which reflects the two binding sites of bevacizumab), the dissociation constant (KD; inversely proportional to how tightly they bind to a molecule) of ranibizumab and bevacizumab for VEGF-A165 was roughly equivalent[22].
A potential disadvantage of the Fc fragment is that bevacizumab may be more stable systemically than is ranibizumab; studies in animals appear to substantiate this prediction[23]. However, it remains unclear whether the low systemic levels of either bevacizumab or ranibizumab are sufficient to result in unwanted systemic effects. Additionally, reasonable disagreement remains as to whether a recent trial comparing bevacizumab to ranibizumab for the treatment wet AMD demonstrated a difference in the safety of the two therapies[20]. Nonetheless, some authors still argue that ranibizumab has been well studied in many randomized clinical trials with more long-term findings when compared with bevacizumab. Though this remains a hot topic of debate among retina specialists, the superiority of ranibizumab over bevacizumab has not been proven in clinical trials and both drugs are actively in use for treatment of VEGF driven retinopathies. A brief discussion comparing the two medications clinically is found below; extensive discussions on this topic can be found elsewhere[20].
Several large clinical trials have investigated the role of ranibizumab in the treatment of diabetic macular edema (Figure 3). The DRCR.net has conducted (and continues to conduct) large, multicenter, randomized clinical trials evaluating the treatment of diabetic eye disease. In an early study, the DRCR network demonstrated that approximately 30% of patients treated with laser photocoagulation gained two ETDRS lines of vision after 2 years following treatment, but up to 20% of these patients worsened by two ETDRS lines[24]. This led the DRCR to investigate additional treatment modalities. In a hallmark paper published in 2010, the DRCR showed that intravitreal injections of ranibizumab with prompt or deferred laser is more effective than prompt laser treatment alone for center involving DME[25]. In this study, 854 study eyes were randomized to sham injection with prompt laser, 0.5 mg ranibizumab with prompt laser, 0.5 mg ranibizumab with deferred (≥ 24 wk) laser, or 4 mg triamcinolone with prompt laser. Sham or ranibizumab injections were given every four weeks up to week 12 and on a pro re nata (PRN or as needed) basis thereafter. In the two year follow up of this study, 29% of patients receiving ranibizumab plus prompt laser and 28% of patients receiving ranibizumab plus deferred laser had ≥ 15 letters of improvement, compared to 18% of patients in the prompt laser only group and 22% of patients in the triamcinolone plus prompt laser group gaining ≥ 15 letters. The mean change in visual acuity was a gain of 3 ETDRS letters for the prompt laser only group and a gain of 2 ETDRS letters in the triamcinolone plus prompt laser group. In contrast, the ranibizumab plus prompt laser group and the ranibizumab plus deferred laser group had a mean ETDRS letter gain of 7 and 9, respectively. At the three-year follow up[26], the ETDRS letters gained in the ranibizumab plus prompt laser group was 6.8, and in the ranibizumab plus deferred laser group was 9.7. The percentage of patients with ≥ 15 letters of improvement was 26 and 32 in the ranibizumab plus prompt laser and ranibizumab plus deferred laser groups, respectively. Overall, this study clearly demonstrated that ranibizumab therapy (alone or in combination with laser) is superior to laser monotherapy. The suggestion that the ranibizumab plus prompt laser group may have slightly poorer visual outcomes than the ranibizumab plus deferred laser group will be investigated further by the DRCR.net in the longer term follow up of these patients.
The first landmark study to demonstrate the efficacy of ranibizumab monotherapy was the READ-2 Study[27]. This prospective, multicenter trial randomized 126 patients to receive ranibizumab, combination ranibizumab and laser, or laser treatment alone in a 1:1:1 ratio. The ranibizumab group received 0.5 mg ranibizumab at baseline and months 1, 3 and 5. The laser group received laser photocoagulation at baseline and month three if needed, and the combination group received 0.5 mg ranibizumab and laser at baseline and month three. The primary endpoint was 6 mo, but patients were followed to 24 mo. After the primary endpoint of 6 mo, all patients were eligible to receive additional ranibizumab, and patients in the laser or combination group were also eligible to receive additional laser treatments. At the primary endpoint of 6 mo, the mean number of ETDRS letters gained in the ranibizumab group was 7.24 letters, in the combination group was 3.80 letters, and in the laser alone group there was a mean reduction of 0.43 ETDRS letters. In the long term follow up at 24 mo, the laser group had an increase of 5.1 letters, the increase in the ranibizumab group (7.7 letters) and the combination group (6.8 letters) was higher, though not significantly so. At the 24-mo point, 24% of patients in the ranibizumab monotherapy group gained ≥ 15 ETDRS letters compared with 18% of the laser monotherapy patients and 26% of the combination therapy patients. This trial nicely complemented the DRCR trial, as it demonstrated that long-term improvements in visual acuity could be achieved with ranibizumab monotherapy.
The DRCR and READ studies examined the therapeutic potential of ranibizumab for the treatment of DME in the United States. It was anticipated that the efficacy of ranibizumab could be extrapolated to populations outside of the United States; this assumption has been supported by several international clinical trials. The RESOLVE study was a smaller, multi-center, sham controlled trial which randomized 151 patients to receive either sham, ranibizumab 0.3 mg, or ranibizumab 0.5 mg injections, monthly for three months followed by PRN treatment[28]. Rescue treatment with laser was permitted if necessary. This study also allowed for “dose doubling” at the discretion of the investigator; thus, after month one, patients in the 0.3 mg ranibizumab group were eligible to receive 0.6 mg, and patients in the 0.5 mg group were eligible to receive 1.0 mg. At the 12 mo follow up, the pooled ranibizumab group had an average gain of 10.3 ETDRS letters compared to an average decline of 1.4 letters in the sham group. The percentage of patients gaining ≥ 15 ETDRS letters was 33% and 5% for the pooled ranibizumab and sham groups, respectively. These numbers are similar to that found in the READ-2 study. It is unclear how to interpret the variable dosing, and no clear guidelines can be deduced from the dosing scheme.
The RESTORE study group examined patients from 10 European countries[29]. This study randomized 345 patients to one of three treatment groups: ranibizumab 0.5 mg injection monotherapy, laser monotherapy, or ranibizumab plus laser combination therapy. Patients in either the ranibizumab monotherapy or combination group received three initial consecutive monthly injections, followed by PRN monthly injections through month 11. Laser monotherapy or combination patients received initial laser treatment either in one or two sessions, followed by re-treatment every three months if necessary. The investigators found that at the primary endpoint (12 mo), the average gain in ETDRS letters in the ranibizumab monotherapy group was 6.1, in the combination group was 5.9, and in the laser monotherapy group was 0.8. At the 12-mo endpoint, the percentage of patients who gained ≥ 15 letters was 26, 27, and 9 for the ranibizumab monotherapy, combination therapy, or laser monotherapy groups, respectively. These percentages are consistent with those found in the other studies discussed above. The percent of patients gaining ≥ 15 letters with laser monotherapy is lower in this study compared with the DRCR and READ-2 studies, which may reflect the fact that this study only followed patients out to one year. Overall, this study supports the findings of the studies discussed above.
More recently, results from the RISE and RIDE studies were published[30]. These two parallel, phase 3, multicenter, sham controlled studies randomized patients to sham injections, or injections with 0.3 or 0.5 mg ranibizumab, on a monthly basis for 24 mo. Patients were eligible for laser rescue treatment if treatment criteria - as established by the study investigators - were met. The RISE study enrolled 377 patients, whereas the RIDE study enrolled 382 patients. The patients in each study were randomized 1:1:1 to each of the three treatment groups. In the RISE study, at the 24-mo end point, 39.2% of patients receiving monthly ranibizumab at a 0.5 mg dose, and 44.8% of patients receiving monthly ranibizumab at a 0.3 mg dose gained ≥ 15 ETDRS letters, compared to 18.1% of patients receiving sham injections. Similarly, in the RIDE trial, the percentage of patients gaining ≥ 15 ETDRS letters in the 0.5 and 0.3 mg ranibizumab dose was 45.7 and 33.6, respectively, compared with 12.3% in the sham laser group. The studies were not powered to compare the two doses of ranibizumab, but were powered to show significance compared with sham injections.
From the RISE and RIDE studies, it appears that further improvements in visual acuity can be achieved with monthly dosing of ranibizumab rather than PRN dosing. However, it is not clear whether monthly injections will result in a further improvement in vision in an individual who responds well to PRN dosing, or whether a subgroup of patients requires monthly injections to achieve a significant improvement in vision. Ultimately, the additional improvement in visual acuity in an individual patient must be balanced with the theoretical and known safety concerns associated with an increase in the frequency of intravitreal injections of ranibizumab. Though the treatment and dosing varied among the different trials, collectively, these trials demonstrate that ranibizumab therapy (alone or in combination with laser therapy) results in improved visual acuity outcomes than does laser monotherapy.
Each of these trials also reported safety data. In each study, the incidence of ocular adverse events, as well as serious adverse events such as stroke or heart attack were rare. The biggest ocular concern is endophthalmitis. In the RISE and RIDE studies there were four total cases of endophthalmitis out of 500 patients in the two-year follow up of the study (0.8%; 1 in RISE with 0.3 mg ranibizumab, 3 in RIDE, 1 from 0.3 mg group and 2 from 0.5 mg group). The three year follow up of the DRCR study reported a total of 3 cases of endophthalmitis out of 375 (also 0.8%) patients receiving ranibizumab injections, in either the prompt or deferred laser groups. The RESTORE study had no cases of endophthalmitis. RESOLVE had 2 cases of endophthalmitis out of 102 injection patients (2%) over the year of the study. The average number of injections per patient varied in all of these studies, but generally speaking, the rate of endophthalmitis was similar among the trials, and risk of endophthalmitis with an individual injection was extremely low based on these numbers.
The major systemic safety concern with anti-VEGF treatment is thromboembolic events. In the one-year RESTORE study there were 6 arterial thromboembolic events (5.2%) in the ranibizumab (0.5 mg) group, whereas only one such event occurred in the laser group and the laser plus ranibizumab group. The group sizes were similar, and the analysis did not support a statistical difference between ranibizumab treated groups and the laser only group. The one-year RESOLVE study also reported a low incidence of arterial thromboembolic events with no significant difference among treatment groups (3 of 102 in ranibizumab groups, 2 of 49 in sham group). The two-year follow up of the DRCR study also reported no significant difference in thromboembolic events in ranibizumab or sham treated groups. There was a trend, however, for a decreased number of such events in the ranibizumab treated groups. Non-fatal CVAs occurred in 8 out of 130 (6%) sham injection patients, and in 7 out of 375 (2%) ranibizumab treated patients. The total number of cardiovascular events was 17 out of 130 (13%) in the sham injection patients, and 25/375 (7%) in the ranibizumab treated patients.
In the RISE and RIDE studies, thromboembolic events and deaths were similar between sham and treatment groups. These studies did report that the number of deaths and CVAs were numerically higher in the ranibizumab groups compared to sham groups, with the highest incidences of CVA and death being in the ranibizumab 0.5 mg group. The number of CVAs in the RISE and RIDE studies combined were 4 out of 250 (1.6%), 3 out of 250 (1.2%), and 8 out of 250 (3.2%), in the sham, 0.3 mg, and 0.5 mg groups, respectively. The number of deaths in the combined studies was 3 out of 250 (1.2%), 7 out of 250 (2.8%), and 11 out of 250 (4.4%) in the sham, 0.3 mg, and 0.5 mg groups, respectively. At this time, the trend towards increased CVA and death in the higher ranibizumab group should be acknowledged but interpreted with caution. Longer follow up studies will be necessary to substantiate any increased risk of stroke with ranibizumab treatment.
The long term follow up from the DRCR.net clinical trial raises the possibility that combination therapy with ranibizumab and laser treatments may not be as effective as ranibizumab monotherapy alone for the treatment of DME. In clinical practice, many physicians continue to employ focal laser as part of the treatment for these patients. One theoretical advantage of combination treatment is that it may decrease the frequency and total number of required intravitreal ranibizumab injections. In the DRCR studies discussed above, the median number of ranibizumab injections during the first year was 8 and 9, in the ranibizumab plus prompt or deferred laser, respectively, out of a maximum of 13. Between years 1 and 2, the median number of injections was 2 and 3, in the prompt and deferred laser groups, out of a maximum of 13. In year three, the median number of injections decreased to 1 and 2, in the prompt and deferred laser groups, respectively. This study suggests that initiating prompt laser or deferring laser does not significantly affect the number of injections needed, although the trend is towards less total injections in the prompt laser group. However, this decrease in number of injections in the prompt laser groups was speculated to contribute to the slightly worse visual acuity outcomes in three year follow up[26].
The data regarding number of injections in the READ-2 study is difficult to interpret because the frequency of evaluation varied between the two groups (every two months for the injection only group and every three months for the combination group). Perhaps the most straightforward comparison can be made from the RESTORE study in which both ranibizumab monotherapy patients and combination therapy patients were evaluated monthly and eligible for additional ranibizumab injections monthly. At the 12-mo follow up, the ranibizumab monotherapy group and the combination group received a mean of 7 and 6.8 injections, with a median of 7 and 7 injections, respectively. This data from the RESTORE study suggests that combination therapy with laser and ranibizumab does not reduce the number of required injections. However, it is not known what the results would be if these patients were followed to two years and beyond. Based on the current follow up data from clinical trials, the role of focal laser in the treatment of diabetic macular edema in the anti-VEGF era is not well delineated.
The wealth of data from the clinical trials described here strongly suggests that ranibizumab, (and likely other effective anti-VEGF therapies) are beneficial for the treatment of DME. The safety data from these studies suggest that ranibizumab injections, including monthly injections for two years, are well tolerated and safe. It also appears that treatment of DME with ranibizumab may have additional benefits in patients with diabetic eye disease. The progression of diabetic retinopathy in patients treated with monthly injections with ranibizumab is significantly lower than those treated with laser photocoagulation[31]. This was not surprising since VEGF is also known to play a fundamental role in the development and progression of diabetic retinopathy. The use of ranibizumab to treat diabetic retinopathy is discussed elsewhere[31]. The contribution of anti-VEGF therapy to the promotion or inhibition of peripheral retinal and macular ischemia remains unclear; a comprehensive recent review of this topic can be found elsewhere[32].
What is also not clear is whether patients who fail treatment with one anti-VEGF approach would be sensitive to another approach. Certainly there are patients who develop tachyphylaxis after the use of either ranibizumab or bevacizumab; and these patients may respond well to the other anti-VEGF drug. However, given the similarities between these two medications, it would seem unlikely that an individual patient would be sensitive to one treatment but not the other. No large studies directly comparing bevacizumab to ranibizumab have been published in the setting of diabetic macular edema, but a large study directly comparing the two medications for the use of neovascular age-related macular degeneration suggested that the medications are probably equivalent for this disease[20]. Nonetheless, this remains a contentious issue.
The use of bevacizumab for diabetic macular edema was investigated in the BOLT trial[18]. The purpose of this study was to compare bevacizumab therapy to macular laser therapy for diabetic macular edema. The bevacizumab arm patients received three injections, six weeks apart, followed by PRN treatment every six weeks. The results showed that after two years, bevacizumab treatment was superior to focal laser treatment. Patients in the bevacizumab arm gained a mean of 8.6 ETDRS letters, and those in the laser arm lost a mean of 0.5 letters. In the bevacizumab arm, 32% of patients gained at least 15 ETDRS letters, and the median number of injections was 9 during the first year, and 4 during the second year. Although the results cannot be directly compared to the ranibizumab studies due to differences in the specifics of the study design and criteria, the changes seen with bevacizumab are of similar magnitude as the changes in the ranibizumab studies. Given the similarities between bevacizumab and ranibizumab, the introduction of other anti-VEGF therapies (e.g., aflibercept or Eylea®) may be a better alternative for patients who fail treatment with ranibizumab or bevacizumab[33]; the use of intravitreal aflibercept for the treatment of DME is discussed elsewhere in this review series.
A final issue that remains under debate is the cost-effectiveness of ranibizumab compared to bevacizumab. Despite the similarities between these two medications, there is a significant difference in the cost between ranibizumab and bevacizumab. It is not clear whether the cost difference reflects a difference in safety or efficacy of these two medications. Several analyses have been performed exploring this question; a comprehensive recent review of this topic can be found elsewhere[34].
Their remains little doubt that ranibizumab has an important role in the treatment of DME. Current studies are now focused on fine-tuning the recommended treatment regimen to determine the most effective treatment dose, frequency, and duration to optimize visual outcomes and safety. Additional efforts to extend the interval between treatments or to identify populations of patients who require less frequent follow up or injections are also underway. The results from these studies may reduce the risks of monthly injections, and the burden on many patients tethered by their required monthly visits to their ophthalmologists.
We would like to acknowledge the support of our Chairman, Dr Peter J McDonnell.
P- Reviewer: Sourij H S- Editor: Gou SX L- Editor: A E- Editor: Wu HL
| 1. | Klein R, Knudtson MD, Lee KE, Gangnon R, Klein BE. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XXII the twenty-five-year progression of retinopathy in persons with type 1 diabetes. Ophthalmology. 2008;115:1859-1868. [Cited in This Article: ] |
| 2. | Bresnick GH. Diabetic macular edema. A review. Ophthalmology. 1986;93:989-997. [Cited in This Article: ] |
| 3. | Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317:703-713. [Cited in This Article: ] |
| 4. | Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103:1796-1806. [Cited in This Article: ] |
| 5. | Beck RW, Edwards AR, Aiello LP, Bressler NM, Ferris F, Glassman AR, Hartnett E, Ip MS, Kim JE, Kollman C. Three-year follow-up of a randomized trial comparing focal/grid photocoagulation and intravitreal triamcinolone for diabetic macular edema. Arch Ophthalmol. 2009;127:245-251. [Cited in This Article: ] |
| 6. | Michaelson I. The mode of development of the vascular system of the retina with some observations on its significance for certain retinal disorders. UK: Trans Ophthalmol Soc UK 1948; 37-180. [Cited in This Article: ] |
| 7. | Miller JW, Le Couter J, Strauss EC, Ferrara N. Vascular endothelial growth factor a in intraocular vascular disease. Ophthalmology. 2013;120:106-114. [Cited in This Article: ] |
| 8. | Semenza GL. Vascular responses to hypoxia and ischemia. Arterioscler Thromb Vasc Biol. 2010;30:648-652. [Cited in This Article: ] |
| 9. | Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669-676. [Cited in This Article: ] |
| 10. | Ferrara N. Vascular endothelial growth factor as a target for anticancer therapy. Oncologist. 2004;9 Suppl 1:2-10. [Cited in This Article: ] |
| 11. | Campochiaro PA. Molecular targets for retinal vascular diseases. J Cell Physiol. 2007;210:575-581. [Cited in This Article: ] |
| 12. | Funatsu H, Yamashita H, Ikeda T, Nakanishi Y, Kitano S, Hori S. Angiotensin II and vascular endothelial growth factor in the vitreous fluid of patients with diabetic macular edema and other retinal disorders. Am J Ophthalmol. 2002;133:537-543. [Cited in This Article: ] |
| 13. | Aiello LP, Avery RL, Arrigg PG, Keyt BA, Jampel HD, Shah ST, Pasquale LR, Thieme H, Iwamoto MA, Park JE. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331:1480-1487. [Cited in This Article: ] |
| 14. | Keane PA, Sadda SR. Development of Anti-VEGF Therapies for Intraocular Use: A Guide for Clinicians. J Ophthalmol. 2012;2012:483034. [Cited in This Article: ] |
| 15. | Sultan MB, Zhou D, Loftus J, Dombi T, Ice KS. A phase 2/3, multicenter, randomized, double-masked, 2-year trial of pegaptanib sodium for the treatment of diabetic macular edema. Ophthalmology. 2011;118:1107-1118. [Cited in This Article: ] |
| 16. | Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391-400. [Cited in This Article: ] |
| 17. | Tufail A, Patel PJ, Egan C, Hykin P, da Cruz L, Gregor Z, Dowler J, Majid MA, Bailey C, Mohamed Q. Bevacizumab for neovascular age related macular degeneration (ABC Trial): multicentre randomised double masked study. BMJ. 2010;340:c2459. [Cited in This Article: ] |
| 18. | Rajendram R, Fraser-Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD, Peto T, Egan C, Bunce C, Leslie RD. A 2-year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24-month data: report 3. Arch Ophthalmol. 2012;130:972-979. [Cited in This Article: ] |
| 19. | Ferrara N, Damico L, Shams N, Lowman H, Kim R. Development of ranibizumab, an anti-vascular endothelial growth factor antigen binding fragment, as therapy for neovascular age-related macular degeneration. Retina. 2006;26:859-870. [Cited in This Article: ] |
| 20. | Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388-1398. [Cited in This Article: ] |
| 21. | Chen Y, Wiesmann C, Fuh G, Li B, Christinger HW, McKay P, de Vos AM, Lowman HB. Selection and analysis of an optimized anti-VEGF antibody: crystal structure of an affinity-matured Fab in complex with antigen. J Mol Biol. 1999;293:865-881. [Cited in This Article: ] |
| 22. | Papadopoulos N, Martin J, Ruan Q, Rafique A, Rosconi MP, Shi E, Pyles EA, Yancopoulos GD, Stahl N, Wiegand SJ. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012;15:171-185. [Cited in This Article: ] |
| 23. | Miki K, Miki A, Matsuoka M, Muramatsu D, Hackett SF, Campochiaro PA. Effects of intraocular ranibizumab and bevacizumab in transgenic mice expressing human vascular endothelial growth factor. Ophthalmology. 2009;116:1748-1754. [Cited in This Article: ] |
| 24. | Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447-1449. [Cited in This Article: ] |
| 25. | Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, Edwards AR, Ferris FL, Friedman SM, Glassman AR, Miller KM. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064-1077.e35. [Cited in This Article: ] |
| 26. | Elman MJ, Qin H, Aiello LP, Beck RW, Bressler NM, Ferris FL, Glassman AR, Maturi RK, Melia M. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three-year randomized trial results. Ophthalmology. 2012;119:2312-2318. [Cited in This Article: ] |
| 27. | Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, Boyer D, Heier JS, Abraham P, Thach AB. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology. 2010;117:2146-2151. [Cited in This Article: ] |
| 28. | Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, Mitchell P, Sharp D, Wolf-Schnurrbusch UE, Gekkieva M. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399-2405. [Cited in This Article: ] |
| 29. | Mitchell P, Bandello F, Schmidt-Erfurth U, Lang GE, Massin P, Schlingemann RO, Sutter F, Simader C, Burian G, Gerstner O. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615-625. [Cited in This Article: ] |
| 30. | Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, Gibson A, Sy J, Rundle AC, Hopkins JJ. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789-801. [Cited in This Article: ] |
| 31. | Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. Long-term effects of ranibizumab on diabetic retinopathy severity and progression. Arch Ophthalmol. 2012;130:1145-1152. [Cited in This Article: ] |
| 32. | Manousaridis K, Talks J. Macular ischaemia: a contraindication for anti-VEGF treatment in retinal vascular disease? Br J Ophthalmol. 2012;96:179-184. [Cited in This Article: ] |
| 33. | Do DV, Schmidt-Erfurth U, Gonzalez VH, Gordon CM, Tolentino M, Berliner AJ, Vitti R, Rückert R, Sandbrink R, Stein D. The DA VINCI Study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology. 2011;118:1819-1826. [Cited in This Article: ] |
| 34. | Hodge W, Brown A, Kymes S, Cruess A, Blackhouse G, Hopkins R, McGahan L, Sharma S, Pan I, Blair J. Pharmacologic management of neovascular age-related macular degeneration: systematic review of economic evidence and primary economic evaluation. Can J Ophthalmol. 2010;45:223-230. [Cited in This Article: ] |