Published online Mar 16, 2019. doi: 10.4253/wjge.v11.i3.193
Peer-review started: January 17, 2019
First decision: January 26, 2019
Revised: January 29, 2019
Accepted: February 13, 2019
Article in press: February 13, 2019
Published online: March 16, 2019
According to the American Cancer Society and Colorectal Cancer Statistics 2017, colorectal cancer (CRC) is one of the most common malignancies in the United States and the second leading cause of cancer death in the world in 2018. Previous studies demonstrated that 8%-29% of patients with primary CRC present malignant colonic obstruction (MCO). In the past, emergency surgery has been the primary treatment for MCO, although morbidity and surgical mortality rates are higher in these settings than in elective procedures. In the 1990s, self-expanding metal stents appeared and was a watershed in the treatment of patients in gastrointestinal surgical emergencies. The studies led to high expectations because the use of stents could prevent surgical intervention, such as colostomy, leading to lower morbidity and mortality, possibly resulting in higher quality of life. This review was designed to provide present evidence of the indication, technique, outcomes, benefits, and risks of these treatments in acute MCO through the analysis of previously published studies and current guidelines.
Core tip: This review was designed to provide present evidence of the indication, technique, outcomes, benefits, and risks of colon stenting and emergency surgery in acute malignant colonic obstruction through the analysis of previously published studies with 1A evidence and current guidelines.
- Citation: Ribeiro IB, de Moura DTH, Thompson CC, de Moura EGH. Acute abdominal obstruction: Colon stent or emergency surgery? An evidence-based review. World J Gastrointest Endosc 2019; 11(3): 193-208
- URL: https://www.wjgnet.com/1948-5190/full/v11/i3/193.htm
- DOI: https://dx.doi.org/10.4253/wjge.v11.i3.193
According to the American Cancer Society[1] and Colorectal Cancer Statistics 2017[2], colorectal cancer (CRC) is one of the most common malignancies in the United States and the second leading cause of cancer death in the world in 2018. Acute abdomen obstructive (AAO) due to CRC occurs in 8%-29% of patients and is a gastrointestinal (GI) emergency requiring urgent decompression considering the risk of necrosis and perforation as a result of massive distension of the loop. Bacterial translocation and imbalance of intracorporal electrolytes also contribute to the high mortality rate[3-5].
In the 1990s, self-expanding metal stents (SEMS) appeared, which were a watershed in the treatment of patients in GI surgical emergencies[6-9]. The initial studies had encouraging results since the use of the stent could remove the patient from a surgical emergency, improve their performance status, reducing not only morbidity but mortality and preventing a colostomy giving them a better quality of life (QOL)[10-13]. Palliative patients were major beneficiaries of this development since they often can not tolerate more invasive surgical procedures, and up to 94% of emergency surgeries can be avoided with this strategy[14].
For patients presenting with acute left-sided colonic obstruction secondary to an operable malignancy, SEMS placement allows colonic decompression, preoperative bowel preparation, and preoperative colonoscopy to assess for synchronous cancers. Patients may then undergo a one-stage surgical procedure, possibly laparoscopically, with a primary anastomosis[6,15].
In the past, emergency surgery (ES) has been the primary treatment for AAO, although morbidity and surgical mortality rates are higher in these settings than in elective procedures[16]. ES in this setting is associated with a morbidity rate of 32%-64%[17] and mortality rate of 15%-34%.
There are currently 13 randomized controlled trials (RCTs)[18-30] and 20 meta-analyses[7,14,15,30-47] reporting results of colonic SEMS as a bridge to surgery. There are also 4 RCTs[12,48-50] and 4 meta-analyses[42,51-53] evaluating SEMS for palliative indications. Nevertheless, there are still doubts about the management of AAO by CRC.
This review was designed to provide present evidence for the indications, techniques, outcomes, benefits and risks of these treatments in the management of acute malignant colonic obstruction (MCO) through the analysis of previously published studies and current guidelines.
A systematic search was performed, with no restriction regarding the idiom or the year of publication, since the inception of database till October 01, 2018 using PubMed, MEDLINE, Web of Science, EMBASE, and Cochran Central Register of Controlled Trials databases. Both MeSH and non-MeSH terms were included in the search.
All studies comparing colonic stent vs surgery for acute malignant large bowel obstruction were included. Relevant studies about colonoscopy, acute obstructive abdomen due to neoplasia were also included.
Studies were excluded from this review according to the following criteria: use of the stent for benign treatment, stents placed by an interventional radiologist; unclear or missing data for the outcomes variables.
Randomized trials (Evidence 1A) were prioritized as well as previous reviews on the topic.
Bowel obstruction is defined as the absence of gas or bowel movements for ≥ 24 h, and it is associated with abdominal pain, abdominal bloating or distension and the visualization of dilated colon on an abdominal imaging[16,54].
Computed tomography (CT) is recommended when MCO is suspected and[55] can confirm obstruction and clarify the level of the stricture, as well as identify the etiology of obstruction[41,56] .
Currently, indications for stent placement in patients with MCO are: Stent as a “bridge to surgery” to avoid ES[57]; Palliative CRC patients[58]; Extra colonic tumors causing acute abdominal obstruction (e.g., advanced gastric cancer, ovarian cancer)[59-61].
Signs of systemic toxicity or septic shock as these are signs of colonic ischemia or perforation[62]. Intra-abdominal abscess. Excessively dilated cecum (> 9 cm) as endoscopic insufflation may precipitate colonic perforation. Distal rectal lesions that would require the stent to cross the dentate line as this can induce severe pain, tenesmus, and rectal bleeding[34,63]. Persistent coagulopathy (relative)[64].
Rarely, extrinsic lesions can compress the colon causing MCO. The most frequent causes of extrinsic obstruction are primary pelvic malignancies (ovarian, uterine, and bladder cancer), advanced gastric cancer or metastatic lesions to the pelvis[65]. Extrinsic obstruction occurs more frequently in the left colon, especially in the distal region, and in these cases endoscopic tissue biopsy is not technically possible and the exact etiology and extent of obstruction is often not clear[60,61].
Patients with extrinsic colonic obsltruction, in the vast majority of cases, have advanced disease with reduced life expectancy and no potential curative surgical ressection. .Nevertheless, the technical and clinical success of stenting in these cases, is less effective then when apllyied to primary colorrectal tumors, and there no ideal option[59,65].
Whether it is in palliative patients or in those who will use the stent as a bridge for elective surgery, there is a high chance of colonic perforation if chemotherapy is associated, especially with angiogenesis inhibitors such as bevacizumab[66,67]. The European Society of Gastrointestinal Endoscopy does not recommend the combination therapy of stent with antiangiogenic drugs[62].
Chemotherapy for metastatic CRC (CRCM) has evolved in the last decade from cytotoxic agents to molecular targeting agents[68]. Currently, four cytotoxic agents [5-fluorouracil (5-FU), capecitabine, irinotecan, oxaliplatin][69,70] and five molecular targeting agents [bevacizumab (BV), cetuximab, panitumumab, aflibercept, and regorafenib] are used as chemotherapeutics for CRCM, given in combination or alone[71-74].
New chemotherapy schedules can take approximately 24 mo[75-77], twelve months longer than that used in the classic 5-FU + leucovorin scheme[78]. Chemotherapy decreases the risk of tumor ingrowth compared to the use of SEMS alone; however, chemotherapy is considered a significant risk factor for long-term complications, including perforation and stent migration[79]. Regarding the use of bevacizumab, studies have reported that it is an independent risk factor for late complications and even without a stent increased the risk of perforation by 19.6 times[80,81].
Considering the benefits of increased survival and QOL achieved by new chemotherapies, there still is a role for stents in palliation, however, with parsimony[66,82].
SEMS may be either covered or uncovered; however only uncovered are available in the United States. All colorectal stents work very similarly[83].
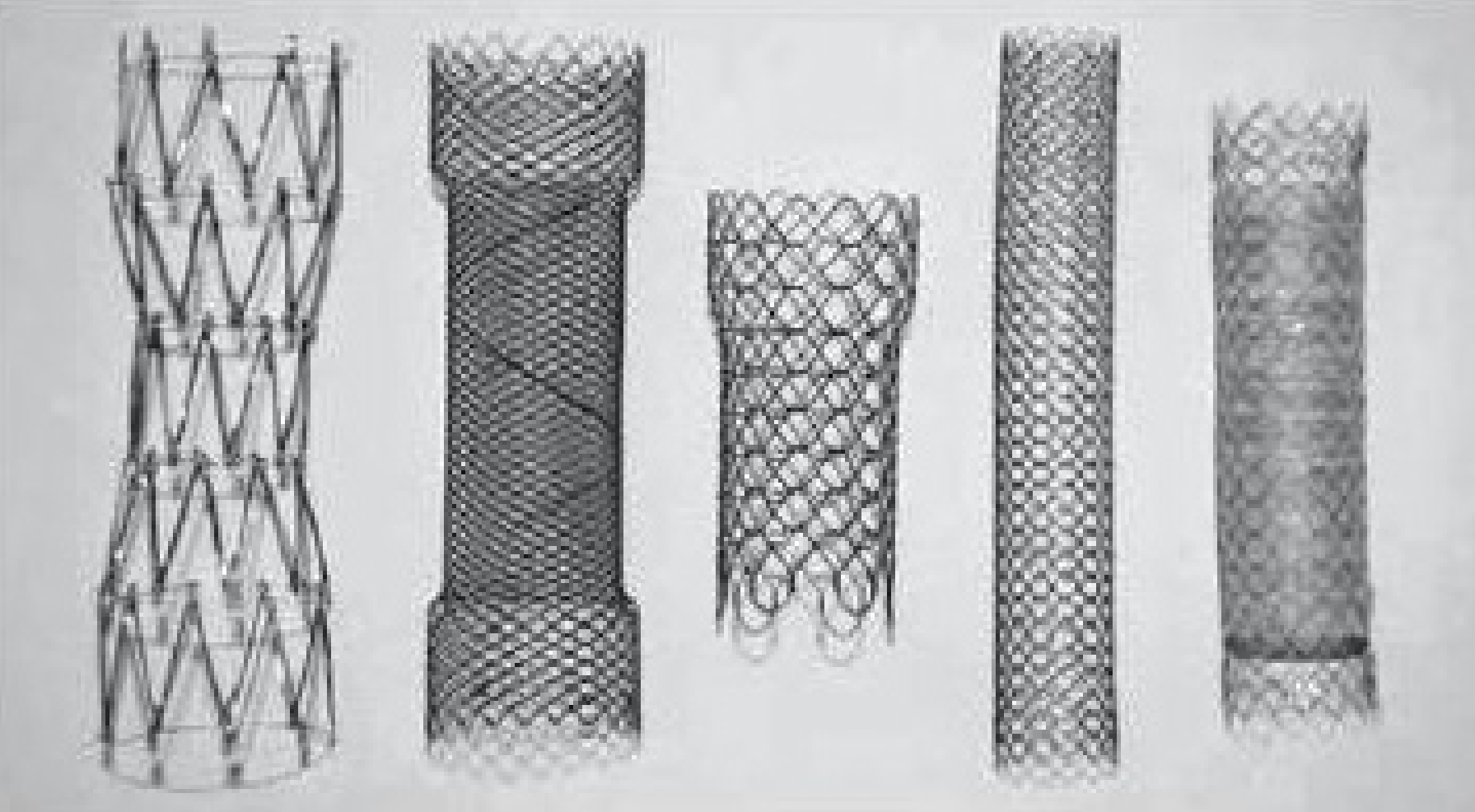
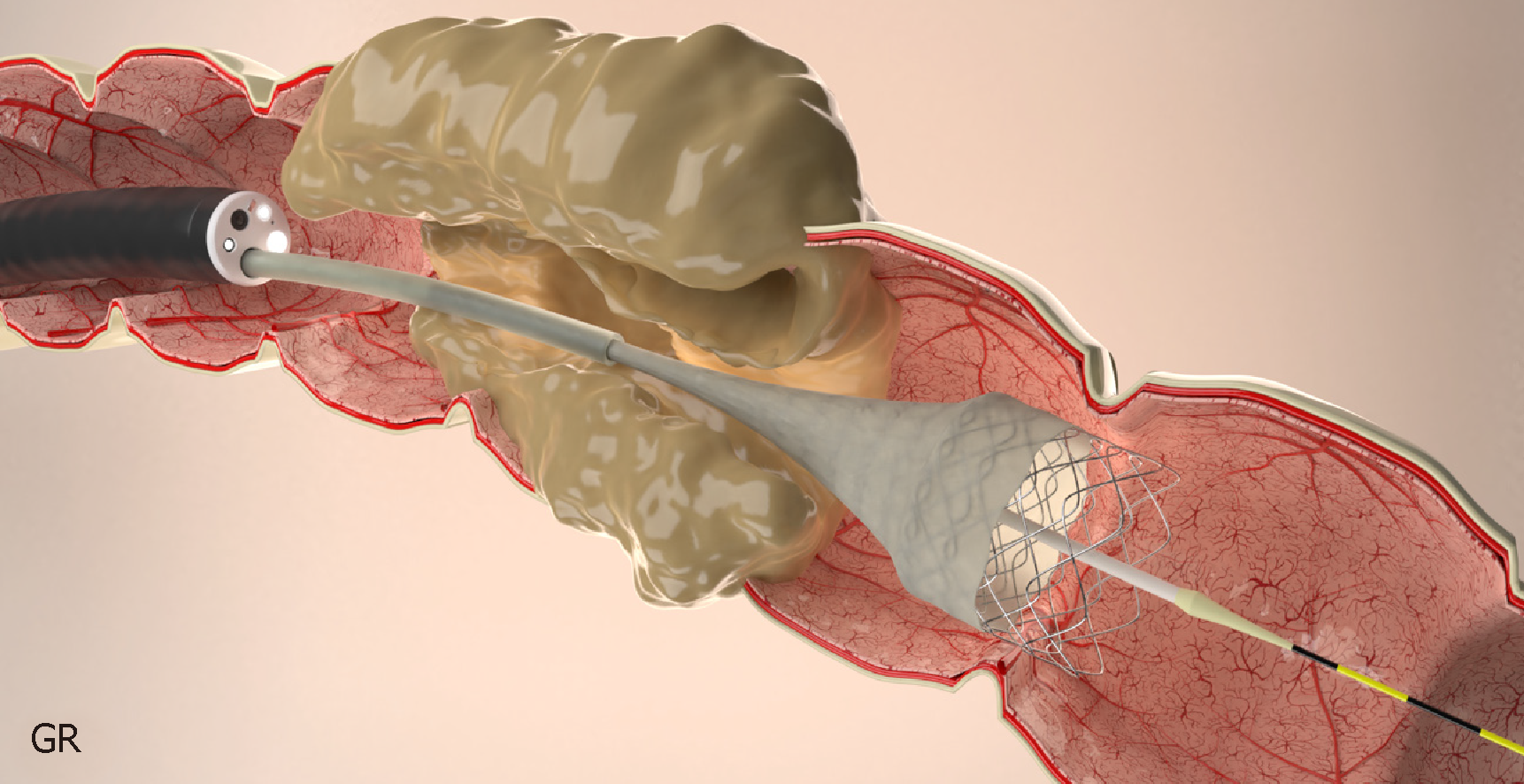
The available stents are mostly made of a nickel-titanium metal alloy (nitinol) (Figure 1). An important characteristic of this material is that it is malleable at low temperatures and has strong radial force at body temperature without losing flexibility[84].
Delivery systems can be introduced into the colon parallel to the endoscope, over the wire (OTW) or through the scope (TTS). TTS is typically preferred and facilitates the treatment of right colon lesions[3,85]. Commercially available stents are reported in Table 1.
| Manufacturer and model | Material | Delivery system | Diameter (mm) | Flare | Flare diameter (mm) | Length (mm) | Covered/uncovered |
| Boston Scientific | |||||||
| Wallstent Colonic1 | Nitinol | TTS | 22, 25 | 1 | 27, 30 | 60, 90, 120 | Uncovered |
| Wallstent Endoprothesis1 | Stainless steel | TTS | 20, 22 | 0 | – | 60, 90, 120 | Uncovered |
| Ultraflex Precision Colonic1 | Nitinol | OTW | 25 | 1 | 30 | 57, 87, 117 | Uncovered |
| Micro-Tech Europe | OTW | 30 | 0 | – | 75, 88, 112, 123, 136 | Uncovered/fully covered | |
| Micro-Tech Europe Colon and Rectum stent | Nitinol | OTW | 20, 30 | 2 | 26, 36 | 60, 80, 100 | Uncovered/partially covered |
| Leufen Medical GmbH | TTS | 25 | 2 | 30 | 80, 100, 120 | Uncovered | |
| Colon Rectum Stent | Nitinol | OTW | 25, 30 | 2 | 30, 36 | 80, 100 | Uncovered/partially covered |
| TTS | 25 | 2 | 30 | 80, 100 | Uncovered | ||
| Cook | |||||||
| Evolution Colonic1 | Nitinol | TTS | 25 | 2 | 30 | 60, 80,100 | Uncovered |
| MI Tech | |||||||
| Hanarostent Colon/Rectum1 | Nitinol | TTS | 22, 24 | 2 | 26, 28 | 80, 110, 140, 170 | Uncovered |
| TTS/OTW | 20, 24 | 2 | 26, 32 | 60, 90, 100, 120, 130, 160 | Fully covered | ||
| OTW | 24 | 2 | 32 | 50,80,110,150 | Fully Covered | ||
| Choostent Colon/Rectum | Nitinol | OTW | 22,24 | 2 | 30, 32 | 100, 180 | Fully covered |
| EndoChoice | OTW | 22,24 | 2 | 30, 32 | 80, 120 | Fully covered | |
| Bonastent | Nitinol | TTS | 22, 24, 26 | 0 | – | 60, 80, 100 | Uncovered/partially covered |
| Taewoong Medical | |||||||
| Niti-S Enteral Colonic D-type1 | Nitinol | TTS | 18, 20, 22, 24, 26, 28 | 0 | – | 60, 80, 100, 120 | Uncovered |
| Niti-S Enteral Colonic S-type1 | Nitinol | TTS | 20, 22 | 2 | 28, 30 | 60, 80, 100, 120 | Fully/partially covered |
| OTW | 22, 24, 26, 28 | 2 | 30, 32, 34 | 60, 80, 100, 120 | Fully/partially covered | ||
| Self expandable Stent | |||||||
| Braile Endomédica | Nitinol | TTS | 26 | 0 | - | 70, 100, 130 | Partially covered |
Covered stents are mainly used in the establishment of colonvesical fistulas, coloenteric and cervicovaginal malignancies[86]. Although the theoretical advantage of covered stents is that they have a lower risk of tumor ingrowth, they are also more likely to migrate compared to uncovered stents.
In a randomized trial[84] including 151 patients with AAO by CRC, there was no difference in the clinical success rate for the placement of covered stents compared with uncovered stents (96% vs 92%). There was a higher rate of migration (21% vs 2%) and a trend towards less tumor ingrowth in covered stents (4% vs 15%). There were no differences in relation to adverse events or obstruction by debris.
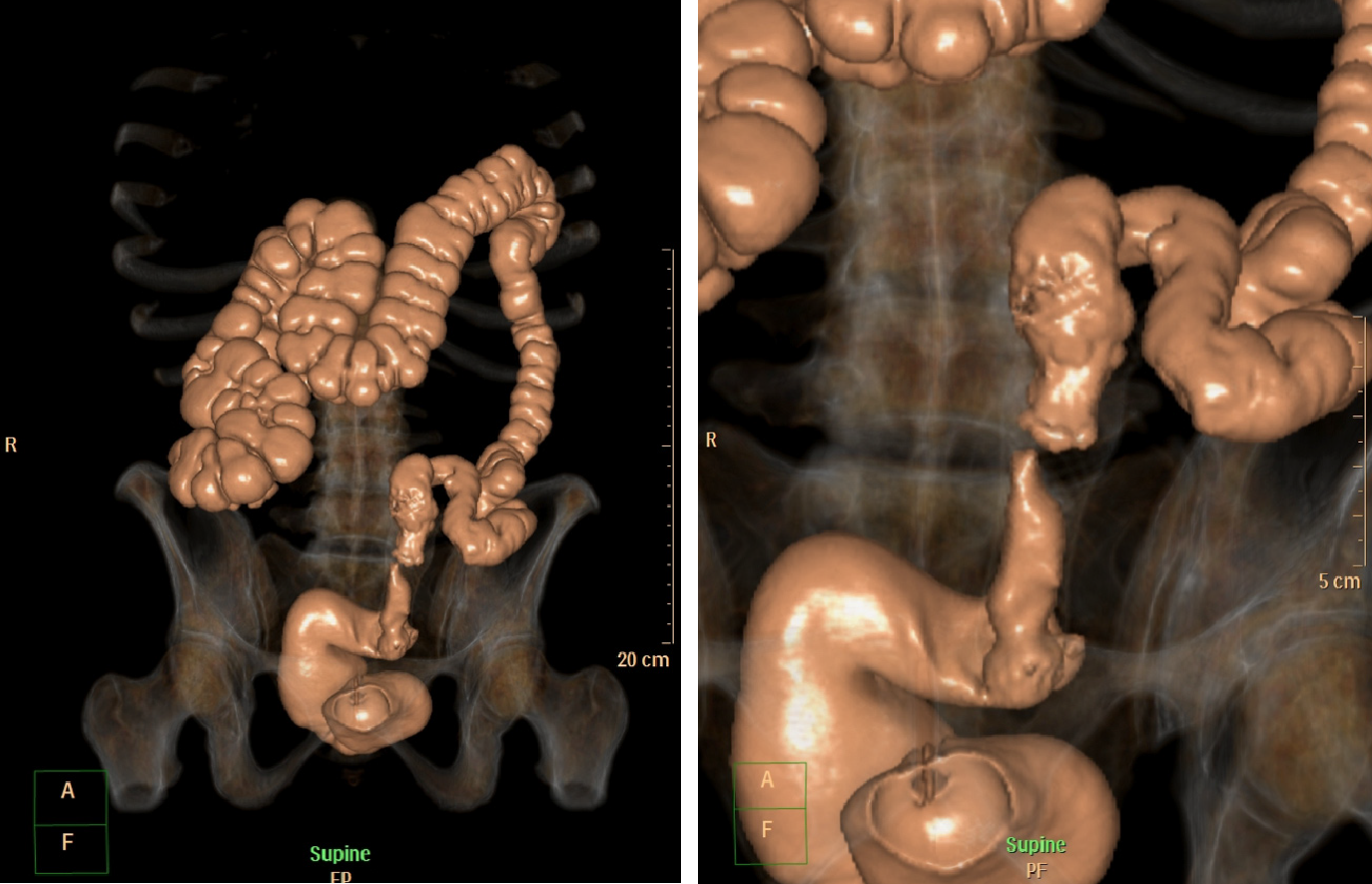
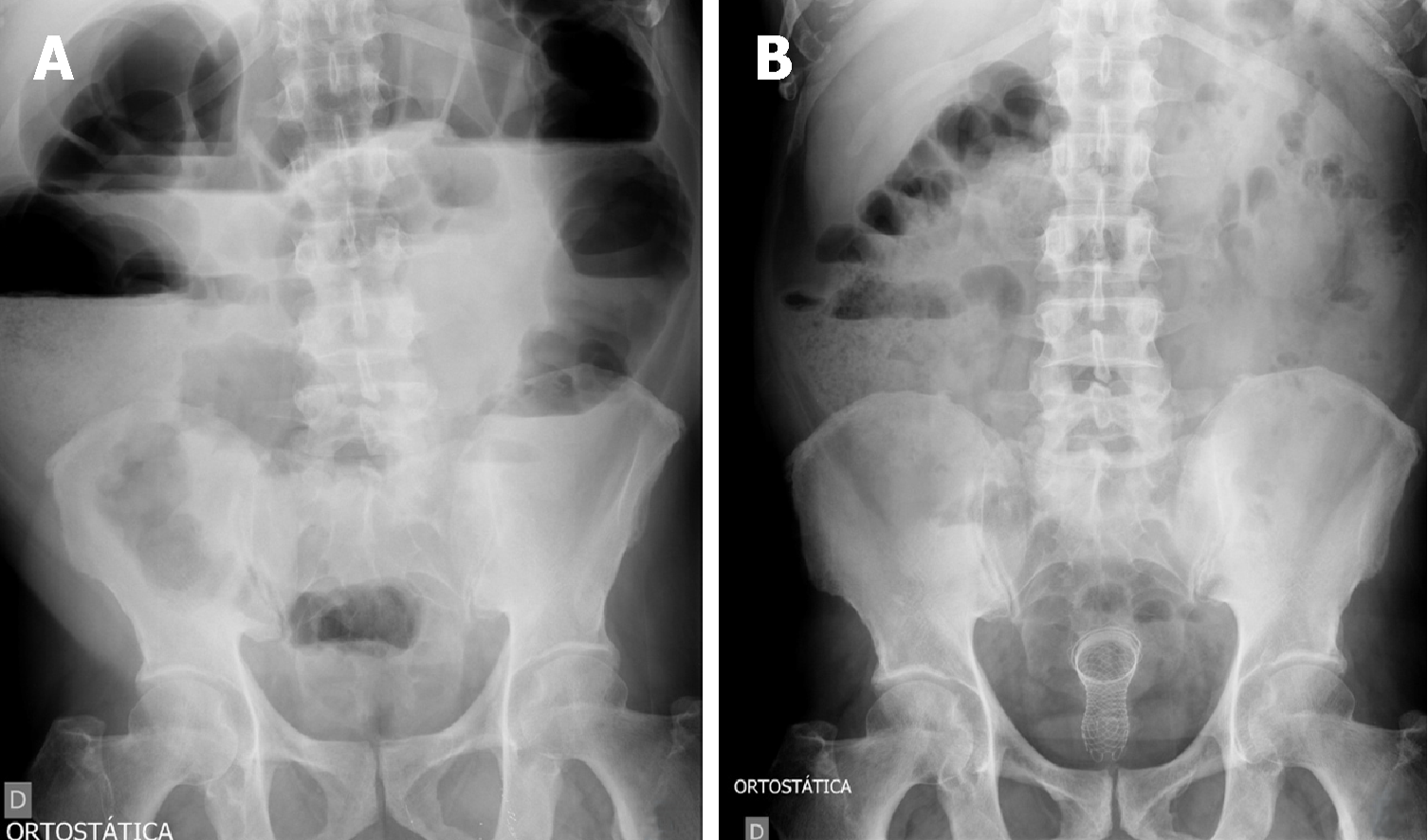
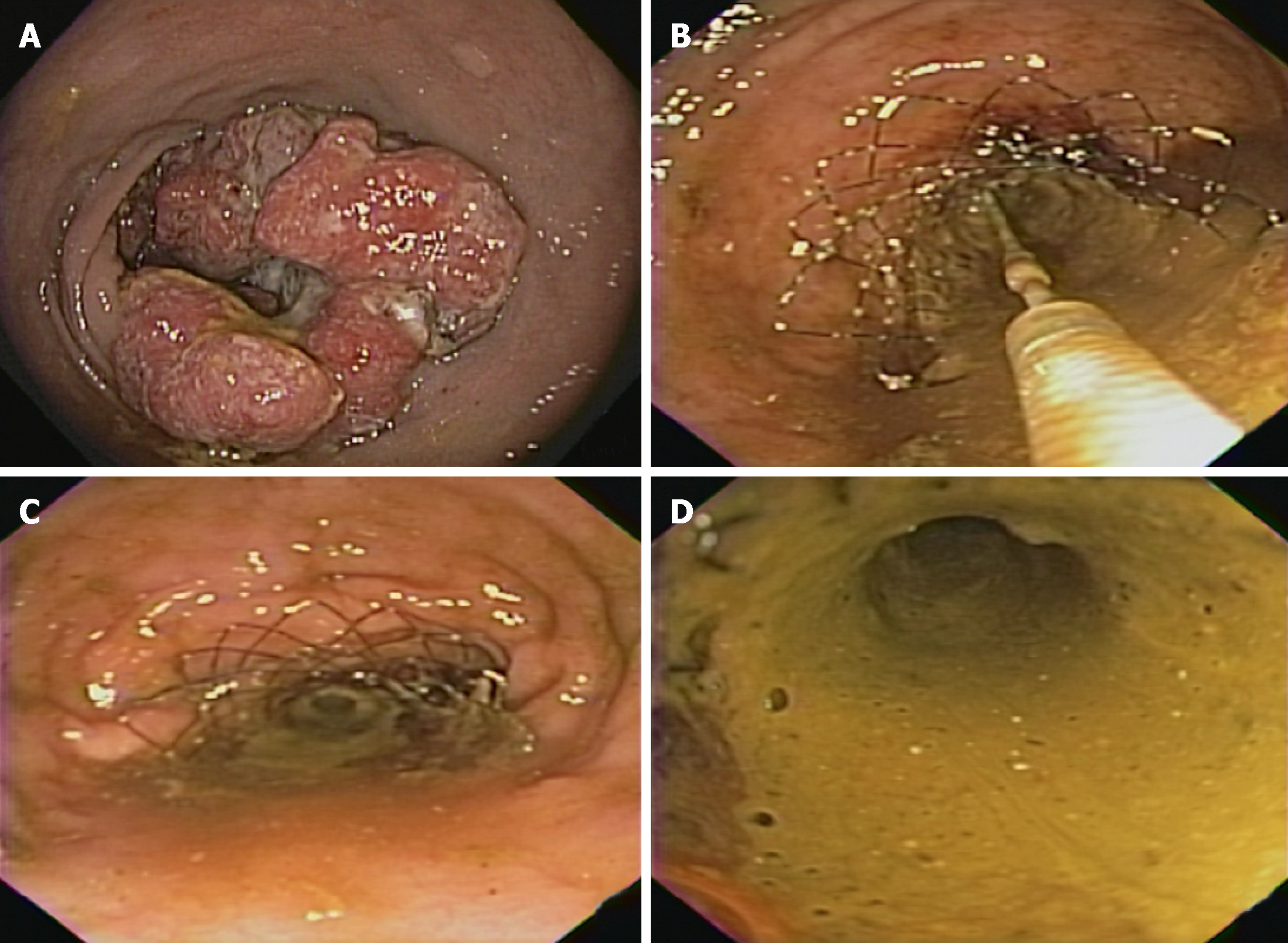
The diagnosis of colonic obstruction is made through symptoms and complemented by imaging tests (for example, simple radiography and/or CT (Figures 2 and 3). Additional exams such as colonoscopy may be performed prior to stent placement procedure. Pre-procedure colonoscopy may provide direct endoscopic visualization of the site of obstruction, and tissue biopsies can be performed for histological diagnosis if needed. Important tumors characteristics can also be ascertained , such as precise location, length of stenosis, topography (extrinsic or intrinsic), and adjacent anatomic considerations (angulation, mucosa inflammation, ischemia or diverticulae)[87,88].
The degree of obstruction should be assessed by attempting to navigate the stenosis with the endoscope; however, it is not necessary to advance the endoscope through the tumor to perform stent placement. Examination with a water-soluble enema or rectal CT may be useful, but not absolutely necessary, to obtain a map of the colonic anatomy, length of stenosis, and degree of obstruction. This radiographic evaluation may also identify additional sites of obstruction that may prevent successful stent placement[33,56].
Although patients may have AAO, bowel preparation should be attempted and preparation depends on location and degree of obstruction: For partial obstruction in the distal colon, two water-soluble enemas (250-500 mL) are sufficient; For partial obstruction of a proximal lesion, oral colon preparation may be attempted and discontinued if symptoms such as abdominal pain or emesis occur[89]; For complete colonic obstruction, oral preparations are contraindicated due to high risk of perforation. Rectal water-soluble enemas should be considered[90].
Prophylaxis is not mandatory for patients undergoing stenting. However, in patients with complete obstruction, we suggest prophylaxis considering the risk of micro perforation and bacteremia during insufflation[62,91].
Lower endoscopic procedures can typically be performed anywhere on the sedation spectrum, from sedation to general anesthesia. However, this is not applied to patients who need to use a stent because they are in an obstructive emergency. General anesthesia with active airway management should be mainly performed to prevent bronchoaspiration with feculent emesis for example and also does not move at the time of the procedure increasing the chance of perforation[92,93].
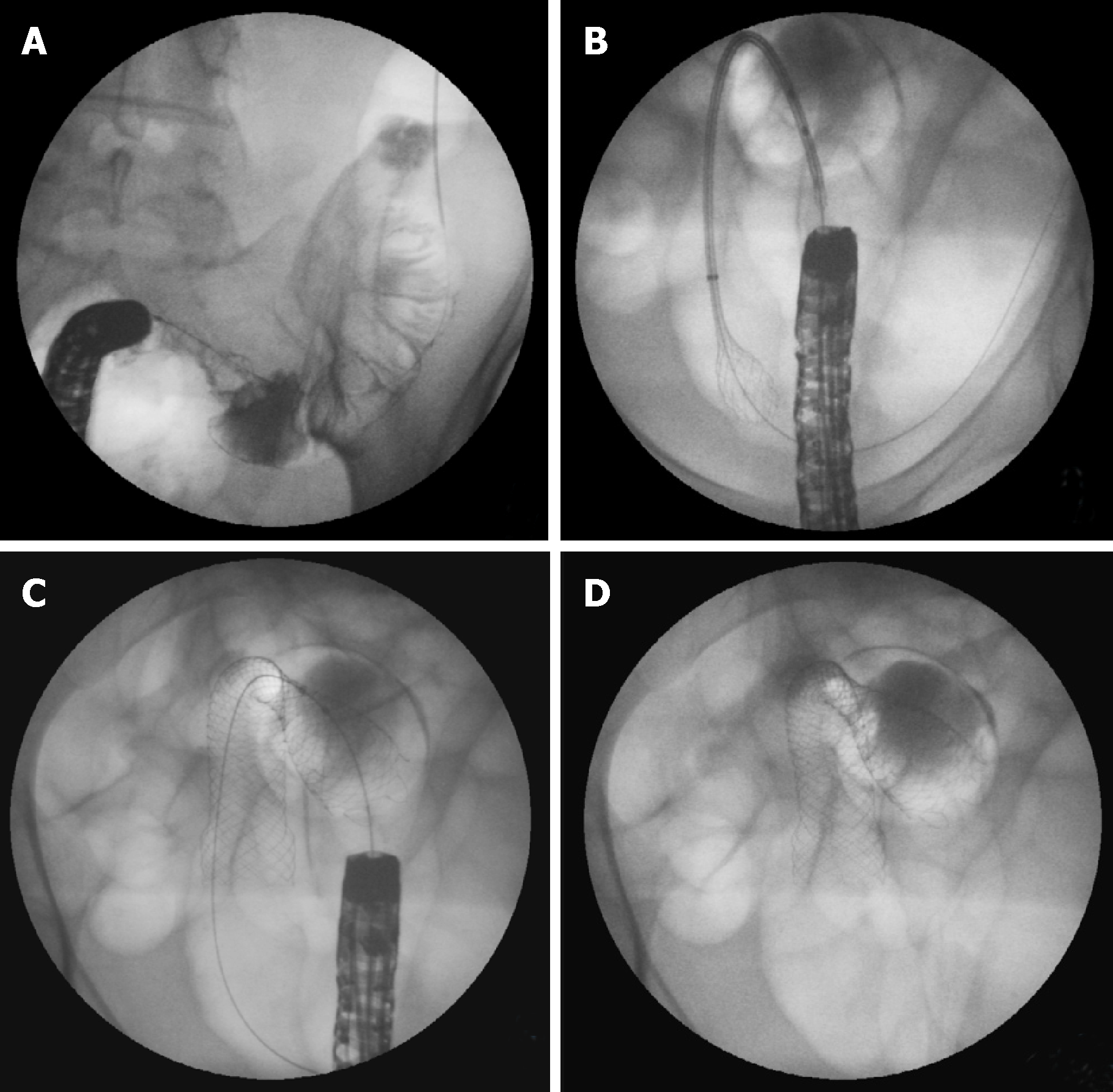
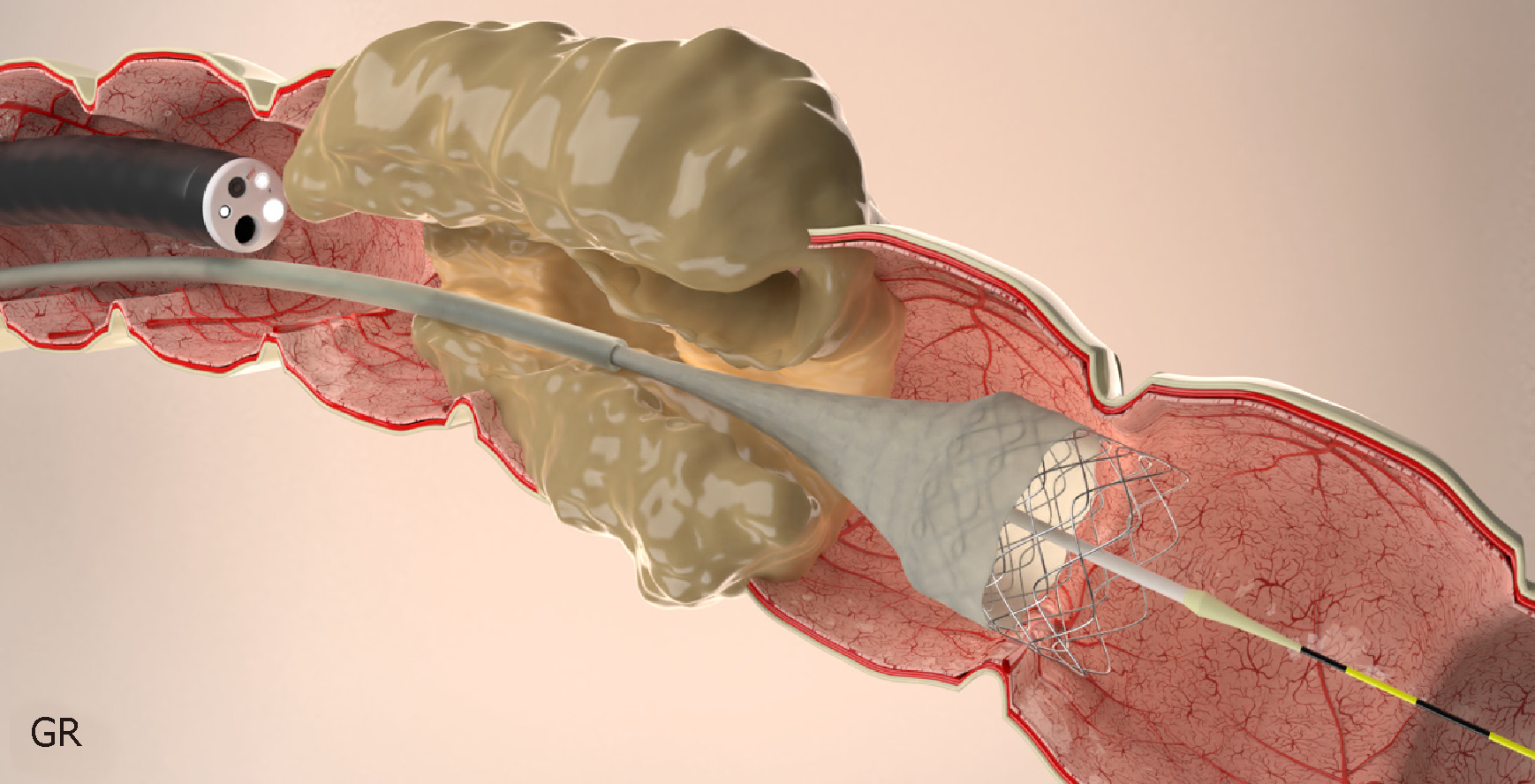
Stents are always placed under endoscopic guidance with the aid of fluoroscopy[62,94] (Figure 4). During colonoscopy, limited insufflation should be used to minimize the risk of perforation due to the risk of a closed loop between the obstructive lesion and the ileocecal valve. The use of carbon dioxide has largely supplanted air for this procedure, and most complex therapeutic cases[95]. A water-immersed colonoscopy is another technique that can be used to minimize bowel distention[96].
Upon reaching the lesion, an attempt can be made to cross the stenosis with the endoscope. However, if the endoscope does not traverse through the obstruction easily, a 0.035-inch guidewire may be passed through stenosis under fluoroscopic guidance.
The first RCT[48] comparing stenting versus ES in palliative patients included balloon dilation of the stenosis prior to stent placement, which is no longer considered and acceptable practice. If endoscope passage through the stenosis is not possible, fluoroscopic guidance is preferred to balloon dilation as the latter is associated with increased risk of perforation[84].
After confirming the length of stenosis, either through the passage of the endoscope or with contrast injection under fluoroscopic guidance, technique of stent placement depends on the type of stent being used.
For this method, a therapeutic endoscope is needed to introduce the stent through the working channel (Figure 5). If the stenosis cannot be traversed, contrast injection helps to delineate the stenosis and to confirm guidewire placement trough the stenosis under fluoroscopy.
The stent is then passed over the guidewire to the proximal margin of the tumor and then implanted under fluoroscopic guidance and endoscopic visualization of the distal portion of the stent. Each end of the stent must be at least 2 cm longer than the stenosis (4 cm of safety margin), as these stents typically shorten after deployment and expansion[62,85,97] (Figure 6).
To prevent migration, it is not recommended that the endoscope be passed through the stent once the stent is placed, although endoscopic/fluoroscopic visualization should be used to rule out early complications.
After the guidewire placement, endoscopic visualization is still preferred, however, not absolutely essential[64]. This technique may be helpful when there is an acute angulation or others factors limiting endoscopic visualization. The stent is inserted over the guidewire and implanted under fluoroscopic guidance (Figure 7).
The correct position of the stent reveals a waist in the center of the stent that crosses the tumor with a widening of the proximal and distal ends. If either end of the stent is not adequately expanded to produce a waist, it may be too short to cross the stenosis. In such cases, a second or third overlapping stent can be used without removing the first to completely cross the stenosis[98].
After stent placement in the left colon, stool softeners should be used to prevent fecal impaction within the stent[62]. Low-residue diets added to the use of polyethylene glycol should be followed. Laxative dose titration may be required. Patients should be instructed to avoid high-fiber foods, such as many fruits, vegetables, and whole grains[98]. Patients with stents in the transverse or right colon may resume normal diets, as the feces in these locations is typically liquid[98].
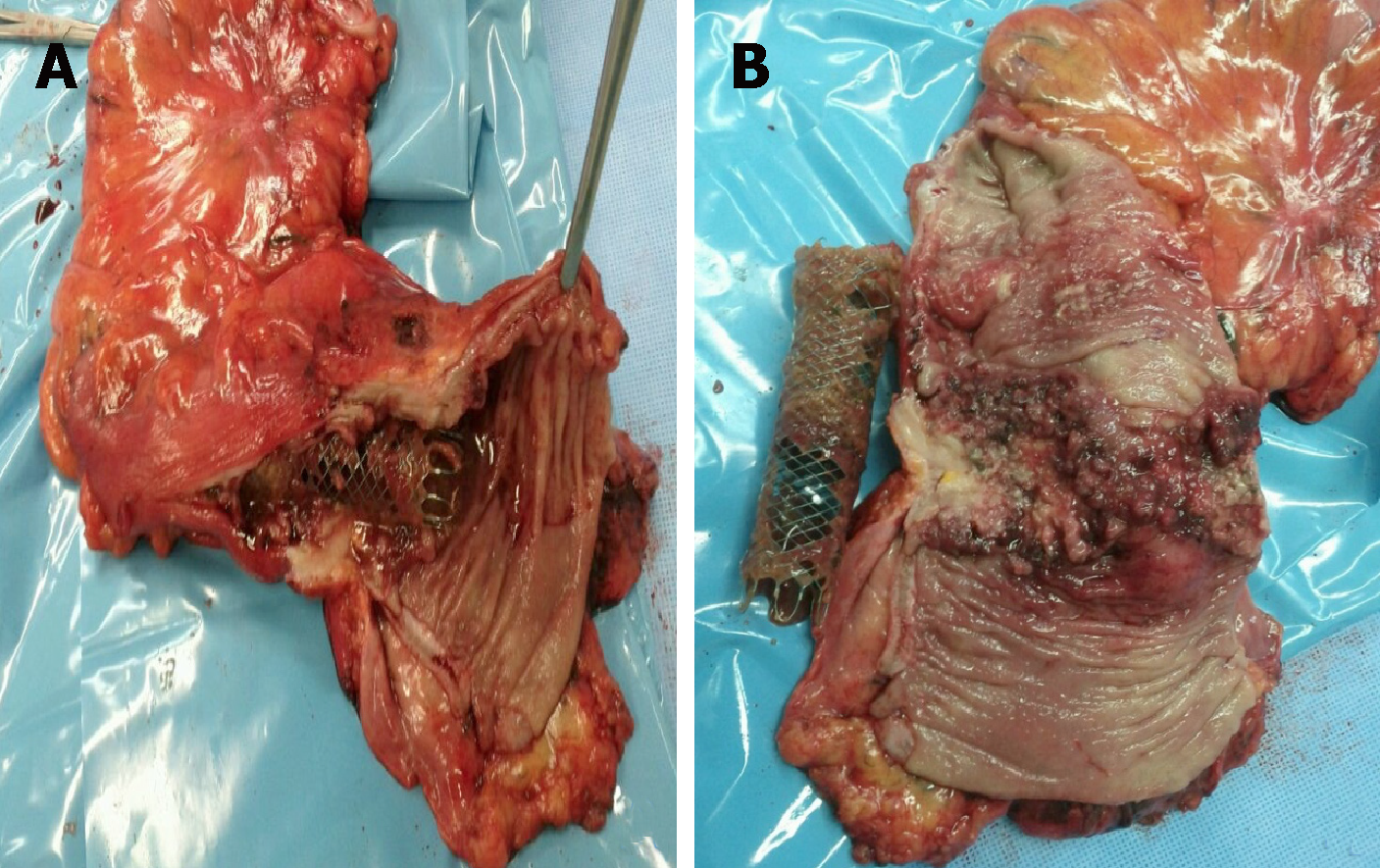
Colonic SEMS placement seems to be relatively safe and effective and has some advantages over surgery, but is associated with an overall complication rate of up to 25%[99-101]. Clinical and technical failures are greater in strictures longer than 4 cm and more adverse events, especially perforation, are reported in complete obstruction[80].
Adverse events may be categorized into minor and major or early (≤ 30 d) and late (> 30 d). Example of major adverse events includes intestinal perforation, obstruction requiring new procedures, bleeding, migration, aspiration during sedation, and death. Typical minor adverse events includes abdominal pain, colic and tenesmus[3].
This may occur late or immediately after the procedure and is associated with poor outcomes[9]. Several factors can increase the risk of perforation including radiotherapy and chemotherapy as well as colonic anatomy[3,66,67]. A meta-analysis in 2018, including palliative patients revealed a perforation rate of 9.5%[53].
Occurs in approximately 10% when used as a bridge to surgery and in 1% of palliative patients, usually one week after insertion. The main causes of migration include incorrect stent selection, stent dimensions being too narrow, small, or short, mild stenosis that is not obstructive, and improvement of stenosis due to radiotherapy or chemotherapy[7,32,53]. Other less common factors that may precipitate stent migration include extrinsic lesion, dilation of stenosis, or use of covered stents.
Occurs in approximately 11.1% of palliative patients. This occurs due to tumor overgrowth at the proximal or distal margins of the stent or through tumor in growth through the cells of the stent[53]. Possible endoscopic treatments for stent obstruction include laser ablation of the tumor, argon plasma coagulation or placement of new stent[48,102].
Immediate post-procedure bleeding may occur due to irritation of the colon mucosa by stent flanges, tumor friability, trauma due to either stent passage or guidewire placement, or endoscope trauma. Late bleeding may be due to stent related ulcerations or erosions in the colonic mucosa[64].
Mild abdominal pain is common and may be prolonged up to five days after stenting. For this, the use of simple analgesics can be helpful. Opioid analgesics may be required within 48 to 72 h of stenting due to expansion of the stent with consequent worsening of pain[34,85]. For low rectal lesions, stent-induced irritation of the nerve endings near the squamous-columnar junction should be avoided.
Despite the high technical success of stent implantation, failure of colonic decompression can occur. This often results in urgent surgery and is considered a serious adverse event[9,103]. The most common reasons are described below[91]: Other additional sites of intestinal obstruction; Stent shorter than stenosis length; Incomplete stent expansion; Stent migration; Underlying motility disorder; Fecal impaction.
In a recent systematic review and meta-analysis performed by Arezzo et al[15], used only RCTs with a total of eight articles: Mortality rate of 9.9% in ES group and 9,6% was demonstrated in the SEMS group; Adverse events rate of 51.2% was demonstrated in the ES group versus 33.9% in the patients with stent; Temporary colostomy rate of 33.9% was demonstrated in the stent group vs 51.4% of patients undergoing ES; The definitive colostomy rate of 22.2% was demonstrated in the stent group versus 35.2% demonstrated in patients of ES group; Regarding the success of the primary anastomosis, there was a 70% rate for the stent group versus 54.1% for the ES group; The need for surgical intervention due to adverse events was 10.9% in the patient with SEMS versus 8.7% in the ES group; The operating time: 172 min in patients submitted ES and 146 minutes in the SEMS group; The hospitalization time was, on average, 14.5 d for patients submitted to ES and 15.5 d for those submitted to STENT; Tumor recurrence was 40.5% in patients in the stent group and 26.6% in the ES group (Table 2).
| Outcome | Stent as bridge to surgery;Arezzo et al[13], 2017 | Stent as bridge to surgery;Arezzo et al[13], 2017 | Palliative patients;Ribeiro et al[51], 2018 | Palliative patients;Ribeiro et al[51], 2018 |
| Stent | Emergency surgery | Stent | Emergency surgery | |
| No. of patients | 251 | 246 | 63 | 62 |
| Mortality | 9.6% | 9.9% | 6.3% | 6.4% |
| Adverse events | 33.9% | 51.2% | 36.5% | 24.1% |
| Survival | NA | NA | 279 d | 244 d |
| Clinical success | NA | NA | 84% | 96% |
| Technical success | NA | NA | 84% | 96% |
| Temporary colostomy | 33.9% | 51.4% | NA | NA |
| Definitive colostomy | 22.2% | 35.2% | 14.3% | 86.1% |
| Primary anastomosis | 70% | 54.1% | NA | NA |
| Hospital stay | 15.5 d | 14.5 d | 17.5 d | 35.5 d |
| ICU hospitalization | NA | NA | 0 | 1 d |
A recent systematic review and meta-analysis performed by Ribeiro et al[53] in 2018, compared the use of stents to surgical intervention, only in palliative patients, as a definitive treatment. Only RCTs were included, with a total of four articles including 125 patients: 30-d mortality rate of 6.4% in patients submitted ES vs 6.3% in the stent group; Analyzed survival was 244 d in the ES group and 279 d in the SEMS group; Clinical success was 84% in the SEMS group and 96% in the ES group; 30-d adverse event rates were 36.5% in the stent group and 24% in the ES group; Technical success was favorable to the surgery group (84% for stent group and 97% for ES group); Rate of permanent colostomy was higher in the surgery group (86.1% versus 14.3%); Length of intensive care stay was not statistically significant between groups; The mean time of hospitalization was 35.5 d for patients undergoing ES and 17.5 for the stent group; Perforation was the most common complication found in the stent group, representing 42.8% of total adverse events, with six of sixty-three patients (9.5%) having perforation, one (1.5%) migration and seven (11.1%) obstruction.
Studies comparing emergent surgery to the use of stents as a bridge to surgery demonstrate a lower rate of a temporary and permanent stoma and a lower short-term morbidity, in a patient undergoing stent placement. This may also positively influence patient’s QOL, however, questions remain regarding longer-term durability. Until more long-term oncological studies are available, stenting cannot be established as the gold standard of treatment. Regarding the use of stents in acute abdominal obstruction in palliative patients, mean survival, early complications, ICU length of stay, and mortality are similar to surgery. Surgery was associated with greater clinical success, while stents demonstrated shorter hospital stay and fewer definitive stomas. Therefore, stenting may be an alternative for patients with incurable obstructive tumors in acute abdomen, with the advantage of early hospital discharge and the potential for improved QOL with avoidance of a permanent stoma.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Wan QQ S- Editor: Ji FF L- Editor: A E- Editor: Wu YXJ
| 1. | American Cancer Society. Colorectal Cancer FactsFigures 2017-2019. Available from: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf. [Cited in This Article: ] |
| 2. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2526] [Cited by in F6Publishing: 2757] [Article Influence: 393.9] [Reference Citation Analysis (2)] |
| 3. | Shimura T, Joh T. Evidence-based Clinical Management of Acute Malignant Colorectal Obstruction. J Clin Gastroenterol. 2016;50:273-285. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Goldenberg BA, Holliday EB, Helewa RM, Singh H. Rectal Cancer in 2018: A Primer for the Gastroenterologist. Am J Gastroenterol. 2018;. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Sanoff HK. Best Evidence Supports Annual Surveillance for Resected Colorectal Cancer. JAMA. 2018;319:2083-2085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Verstockt B, Van Driessche A, De Man M, van der Spek P, Hendrickx K, Casneuf V, Dobbels P, Van Molhem Y, Vandervoort J. Ten-year survival after endoscopic stent placement as a bridge to surgery in obstructing colon cancer. Gastrointest Endosc. 2018;87:705-713.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg. 2002;89:1096-1102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 479] [Cited by in F6Publishing: 511] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 8. | Ribeiro IB, Gestic MA, Utrini MP, Chaim FDM, Chaim EA, Cazzo E. Drain amylase levels may indicate gastrojejunostomy leaks after Roux-En-Y gastric bypass. Arq Gastroenterol. 2018;55:66-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Lee YJ, Yoon JY, Park JJ, Park SJ, Kim JH, Youn YH, Kim TI, Park H, Kim WH, Cheon JH. Clinical outcomes and factors related to colonic perforations in patients receiving self-expandable metal stent insertion for malignant colorectal obstruction. Gastrointest Endosc. 2018;87:1548-1557.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 10. | Di Saverio S, Birindelli A, Segalini E, Novello M, Larocca A, Ferrara F, Binda GA, Bassi M. "To stent or not to stent?": immediate emergency surgery with laparoscopic radical colectomy with CME and primary anastomosis is feasible for obstructing left colon carcinoma. Surg Endosc. 2018;32:2151-2155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Wrenn SM, Cepeda-Benito A, Ramos-Valadez DI, Cataldo PA. Patient Perceptions and Quality of Life After Colon and Rectal Surgery: What Do Patients Really Want? Dis Colon Rectum. 2018;61:971-978. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 52] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 12. | Young CJ, De-Loyde KJ, Young JM, Solomon MJ, Chew EH, Byrne CM, Salkeld G, Faragher IG. Improving Quality of Life for People with Incurable Large-Bowel Obstruction: Randomized Control Trial of Colonic Stent Insertion. Dis Colon Rectum. 2015;58:838-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Furuke H, Komatsu S, Ikeda J, Tanaka S, Kumano T, Imura KI, Shimomura K, Taniguchi F, Ueshima Y, Takashina KI, Lee CJ, Deguchi E, Ikeda E, Otsuji E, Shioaki Y. Self-expandable Metallic Stents Contribute to Reducing Perioperative Complications in Colorectal Cancer Patients with Acute Obstruction. Anticancer Res. 2018;38:1749-1753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Tilney HS, Lovegrove RE, Purkayastha S, Sains PS, Weston-Petrides GK, Darzi AW, Tekkis PP, Heriot AG. Comparison of colonic stenting and open surgery for malignant large bowel obstruction. Surg Endosc. 2007;21:225-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 15. | Arezzo A, Passera R, Lo Secco G, Verra M, Bonino MA, Targarona E, Morino M. Stent as bridge to surgery for left-sided malignant colonic obstruction reduces adverse events and stoma rate compared with emergency surgery: results of a systematic review and meta-analysis of randomized controlled trials. Gastrointest Endosc. 2017;86:416-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 16. | Biondo S, Parés D, Frago R, Martí-Ragué J, Kreisler E, De Oca J, Jaurrieta E. Large bowel obstruction: predictive factors for postoperative mortality. Dis Colon Rectum. 2004;47:1889-1897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 149] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 17. | Smothers L, Hynan L, Fleming J, Turnage R, Simmang C, Anthony T. Emergency surgery for colon carcinoma. Dis Colon Rectum. 2003;46:24-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 193] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 18. | Arezzo A, Balague C, Targarona E, Borghi F, Giraudo G, Ghezzo L, Arroyo A, Sola-Vera J, De Paolis P, Bossotti M, Bannone E, Forcignanò E, Bonino MA, Passera R, Morino M. Colonic stenting as a bridge to surgery versus emergency surgery for malignant colonic obstruction: results of a multicentre randomised controlled trial (ESCO trial). Surg Endosc. 2017;31:3297-3305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 108] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 19. | Öistämö E, Hjern F, Blomqvist L, Falkén Y, Pekkari K, Abraham-Nordling M. Emergency management with resection versus proximal stoma or stent treatment and planned resection in malignant left-sided colon obstruction. World J Surg Oncol. 2016;14:232. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 20. | Sloothaak DA, van den Berg MW, Dijkgraaf MG, Fockens P, Tanis PJ, van Hooft JE, Bemelman WA; collaborative Dutch Stent-In study group. Oncological outcome of malignant colonic obstruction in the Dutch Stent-In 2 trial. Br J Surg. 2014;101:1751-1757. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 146] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 21. | van den Berg MW, Sloothaak DA, Dijkgraaf MG, van der Zaag ES, Bemelman WA, Tanis PJ, Bosker RJ, Fockens P, ter Borg F, van Hooft JE. Bridge-to-surgery stent placement versus emergency surgery for acute malignant colonic obstruction. Br J Surg. 2014;101:867-873. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 22. | Ghazal AH, El-Shazly WG, Bessa SS, El-Riwini MT, Hussein AM. Colonic endolumenal stenting devices and elective surgery versus emergency subtotal/total colectomy in the management of malignant obstructed left colon carcinoma. J Gastrointest Surg. 2013;17:1123-1129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 76] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Cheung DY, Kim JY, Hong SP, Jung MK, Ye BD, Kim SG, Kim JH, Lee KM, Kim KH, Baik GH, Kim HG, Eun CS, Kim TI, Kim SW, Kim CD, Yang CH. Outcome and safety of self-expandable metallic stents for malignant colon obstruction: a Korean multicenter randomized prospective study. Surg Endosc. 2012;26:3106-3113. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Ho KS, Quah HM, Lim JF, Tang CL, Eu KW. Endoscopic stenting and elective surgery versus emergency surgery for left-sided malignant colonic obstruction: a prospective randomized trial. Int J Colorectal Dis. 2012;27:355-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 106] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Alcántara M, Serra-Aracil X, Falcó J, Mora L, Bombardó J, Navarro S. Prospective, controlled, randomized study of intraoperative colonic lavage versus stent placement in obstructive left-sided colonic cancer. World J Surg. 2011;35:1904-1910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 26. | Pirlet IA, Slim K, Kwiatkowski F, Michot F, Millat BL. Emergency preoperative stenting versus surgery for acute left-sided malignant colonic obstruction: a multicenter randomized controlled trial. Surg Endosc. 2011;25:1814-1821. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 27. | van Hooft JE, Bemelman WA, Oldenburg B, Marinelli AW, Lutke Holzik MF, Grubben MJ, Sprangers MA, Dijkgraaf MG, Fockens P; collaborative Dutch Stent-In study group. Colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction: a multicentre randomised trial. Lancet Oncol. 2011;12:344-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 303] [Cited by in F6Publishing: 293] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 28. | Cui J, Zhang JL, Wang S, Sun ZQ, Jiang XL. [A preliminary study of stenting followed by laparoscopic surgery for obstructing left-sided colon cancer]. Zhonghua Weichang Waike Zazhi. 2011;14:40-43. [PubMed] [Cited in This Article: ] |
| 29. | Kim JS, Hur H, Min BS, Sohn SK, Cho CH, Kim NK. Oncologic outcomes of self-expanding metallic stent insertion as a bridge to surgery in the management of left-sided colon cancer obstruction: comparison with nonobstructing elective surgery. World J Surg. 2009;33:1281-1286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 30. | Martinez-Santos C, Lobato RF, Fradejas JM, Pinto I, Ortega-Deballón P, Moreno-Azcoita M. Self-expandable stent before elective surgery vs. emergency surgery for the treatment of malignant colorectal obstructions: comparison of primary anastomosis and morbidity rates. Dis Colon Rectum. 2002;45:401-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 218] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 31. | Repici A, Pagano N, Hervoso CM, Danese S, Nicita R, Preatoni P, Malesci A. Metal stents for malignant colorectal obstruction. Minim Invasive Ther Allied Technol. 2006;15:331-338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Watt AM, Faragher IG, Griffin TT, Rieger NA, Maddern GJ. Self-expanding metallic stents for relieving malignant colorectal obstruction: a systematic review. Ann Surg. 2007;246:24-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 340] [Cited by in F6Publishing: 254] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 33. | Farrell JJ. Preoperative colonic stenting: how, when and why? Curr Opin Gastroenterol. 2007;23:544-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Sagar J. Colorectal stents for the management of malignant colonic obstructions. Cochrane Database Syst Rev. 2011;CD007378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 35. | Zhang Y, Shi J, Shi B, Song CY, Xie WF, Chen YX. Self-expanding metallic stent as a bridge to surgery versus emergency surgery for obstructive colorectal cancer: a meta-analysis. Surg Endosc. 2012;26:110-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 36. | Ye GY, Cui Z, Chen L, Zhong M. Colonic stenting vs emergent surgery for acute left-sided malignant colonic obstruction: a systematic review and meta-analysis. World J Gastroenterol. 2012;18:5608-5615. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 38] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 37. | Zhao RS, Wang H, Wang L, Huang MJ, Chen DK, Wang JP. [Meta-analysis of safety and efficacy of self-expending metallic stents as bridge to surgery versus emergency surgery for left-sided malignant colorectal obstruction]. Zhonghua Weichang Waike Zazhi. 2012;15:697-701. [PubMed] [Cited in This Article: ] |
| 38. | Cirocchi R, Farinella E, Trastulli S, Desiderio J, Listorti C, Boselli C, Parisi A, Noya G, Sagar J. Safety and efficacy of endoscopic colonic stenting as a bridge to surgery in the management of intestinal obstruction due to left colon and rectal cancer: a systematic review and meta-analysis. Surg Oncol. 2013;22:14-21. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 39. | De Ceglie A, Filiberti R, Baron TH, Ceppi M, Conio M. A meta-analysis of endoscopic stenting as bridge to surgery versus emergency surgery for left-sided colorectal cancer obstruction. Crit Rev Oncol Hematol. 2013;88:387-403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 40. | Cennamo V, Luigiano C, Coccolini F, Fabbri C, Bassi M, De Caro G, Ceroni L, Maimone A, Ravelli P, Ansaloni L. Meta-analysis of randomized trials comparing endoscopic stenting and surgical decompression for colorectal cancer obstruction. Int J Colorectal Dis. 2013;28:855-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 41. | Frago R, Ramirez E, Millan M, Kreisler E, del Valle E, Biondo S. Current management of acute malignant large bowel obstruction: a systematic review. Am J Surg. 2014;207:127-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 42. | Liu Z, Kang L, Li C, Huang M, Zhang X, Wang J. Meta-analysis of complications of colonic stenting versus emergency surgery for acute left-sided malignant colonic obstruction. Surg Laparosc Endosc Percutan Tech. 2014;24:73-79. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Huang X, Lv B, Zhang S, Meng L. Preoperative colonic stents versus emergency surgery for acute left-sided malignant colonic obstruction: a meta-analysis. J Gastrointest Surg. 2014;18:584-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 127] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 44. | Matsuda A, Miyashita M, Matsumoto S, Matsutani T, Sakurazawa N, Takahashi G, Kishi T, Uchida E. Comparison of long-term outcomes of colonic stent as "bridge to surgery" and emergency surgery for malignant large-bowel obstruction: a meta-analysis. Ann Surg Oncol. 2015;22:497-504. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 96] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 45. | Atukorale YN, Church JL, Hoggan BL, Lambert RS, Gurgacz SL, Goodall S, Maddern GJ. Self-Expanding Metallic Stents for the Management of Emergency Malignant Large Bowel Obstruction: a Systematic Review. J Gastrointest Surg. 2016;20:455-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Flor-Lorente B, Báguena G, Frasson M, García-Granero A, Cervantes A, Sanchiz V, Peña A, Espí A, Esclapez P, García-Granero E. Self-expanding metallic stent as a bridge to surgery in the treatment of left colon cancer obstruction: Cost-benefit analysis and oncologic results. Cir Esp. 2017;95:143-151. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 47. | Wang X, He J, Chen X, Yang Q. Stenting as a bridge to resection versus emergency surgery for left-sided colorectal cancer with malignant obstruction: A systematic review and meta-analysis. Int J Surg. 2017;48:64-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Xinopoulos D, Dimitroulopoulos D, Theodosopoulos T, Tsamakidis K, Bitsakou G, Plataniotis G, Gontikakis M, Kontis M, Paraskevas I, Vassilobpoulos P, Paraskevas E. Stenting or stoma creation for patients with inoperable malignant colonic obstructions? Results of a study and cost-effectiveness analysis. Surg Endosc. 2004;18:421-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 140] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 49. | van Hooft JE, Fockens P, Marinelli AW, Timmer R, van Berkel AM, Bossuyt PM, Bemelman WA; Dutch Colorectal Stent Group. Early closure of a multicenter randomized clinical trial of endoscopic stenting versus surgery for stage IV left-sided colorectal cancer. Endoscopy. 2008;40:184-191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 221] [Cited by in F6Publishing: 206] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 50. | Fiori E, Lamazza A, Schillaci A, Femia S, Demasi E, Decesare A, Sterpetti AV. Palliative management for patients with subacute obstruction and stage IV unresectable rectosigmoid cancer: colostomy versus endoscopic stenting: final results of a prospective randomized trial. Am J Surg. 2012;204:321-326. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 51. | Zhao XD, Cai BB, Cao RS, Shi RH. Palliative treatment for incurable malignant colorectal obstructions: a meta-analysis. World J Gastroenterol. 2013;19:5565-5574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 94] [Cited by in F6Publishing: 88] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 52. | Takahashi H, Okabayashi K, Tsuruta M, Hasegawa H, Yahagi M, Kitagawa Y. Self-Expanding Metallic Stents Versus Surgical Intervention as Palliative Therapy for Obstructive Colorectal Cancer: A Meta-analysis. World J Surg. 2015;39:2037-2044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 53. | Ribeiro IB, Bernardo WM, Martins BDC, de Moura DTH, Baba ER, Josino IR, Miyahima NT, Coronel Cordero MA, Visconti TAC, Ide E, Sakai P, de Moura EGH. Colonic stent versus emergency surgery as treatment of malignant colonic obstruction in the palliative setting: a systematic review and meta-analysis. Endosc Int Open. 2018;6:E558-E567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 54. | Geng WZM, Fuller M, Osborne B, Thoirs K. The value of the erect abdominal radiograph for the diagnosis of mechanical bowel obstruction and paralytic ileus in adults presenting with acute abdominal pain. J Med Radiat Sci. 2018;65:259-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 55. | Li PH, Tee YS, Fu CY, Liao CH, Wang SY, Hsu YP, Yeh CN, Wu EH. The Role of Noncontrast CT in the Evaluation of Surgical Abdomen Patients. Am Surg. 2018;84:1015-1021. [PubMed] [Cited in This Article: ] |
| 56. | Frager D, Rovno HD, Baer JW, Bashist B, Friedman M. Prospective evaluation of colonic obstruction with computed tomography. Abdom Imaging. 1998;23:141-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 57. | Baron TH, Wong Kee Song LM, Repici A. Role of self-expandable stents for patients with colon cancer (with videos). Gastrointest Endosc. 2012;75:653-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 58. | Ribeiro IB, Bernardo WM, da Costa Martins B, de Moura DT, Moura ET, Miyajima NT, Ide E, Madruga Neto AC, Coronel MA, Martins RK, da Ponte AM, de Moura EG. Self-expanded metal stent versus emergency surgery as treatment of malignant colonic obstruction in the palliative setting: a Systematic review and meta-analysis. Gastrointest Endosc. 2018;87:AB490. [DOI] [Cited in This Article: ] |
| 59. | Lin JL, David D, Lee B. Su1699 Efficacy of Self-Expandable Metallic Stents for Colonic and Extracolonic Malignant Obstruction. Gastrointest Endosc. 2017;85:AB401. [DOI] [Cited in This Article: ] |
| 60. | Faraz S, Salem SB, Schattner M, Mendelsohn R, Markowitz A, Ludwig E, Zheng J, Gerdes H, Shah PM. Predictors of clinical outcome of colonic stents in patients with malignant large-bowel obstruction because of extracolonic malignancy. Gastrointest Endosc. 2018;87:1310-1317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Trompetas V, Saunders M, Gossage J, Anderson H. Shortcomings in colonic stenting to palliate large bowel obstruction from extracolonic malignancies. Int J Colorectal Dis. 2010;25:851-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 62. | van Hooft JE, van Halsema EE, Vanbiervliet G, Beets-Tan RG, DeWitt JM, Donnellan F, Dumonceau JM, Glynne-Jones RG, Hassan C, Jiménez-Perez J, Meisner S, Muthusamy VR, Parker MC, Regimbeau JM, Sabbagh C, Sagar J, Tanis PJ, Vandervoort J, Webster GJ, Manes G, Barthet MA, Repici A; European Society of Gastrointestinal Endoscopy. Self-expandable metal stents for obstructing colonic and extracolonic cancer: European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy. 2014;46:990-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 249] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 63. | Song HY, Kim JH, Kim KR, Shin JH, Kim HC, Yu CS, Kim JC. Malignant rectal obstruction within 5 cm of the anal verge: is there a role for expandable metallic stent placement? Gastrointest Endosc. 2008;68:713-720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 64. | Kaplan J, Strongin A, Adler DG, Siddiqui AA. Enteral stents for the management of malignant colorectal obstruction. World J Gastroenterol. 2014;20:13239-13245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 22] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 65. | Shin SJ, Kim TI, Kim BC, Lee YC, Song SY, Kim WH. Clinical application of self-expandable metallic stent for treatment of colorectal obstruction caused by extrinsic invasive tumors. Dis Colon Rectum. 2008;51:578-583. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 66. | Bong JW, Lee JL, Kim CW, Yoon YS, Park IJ, Lim SB, Yu CS, Kim TW, Kim JC. Risk Factors and Adequate Management for Complications of Bevacizumab Treatment Requiring Surgical Intervention in Patients With Metastatic Colorectal Cancer. Clin Colorectal Cancer. 2018;17:e639-e645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 67. | van Halsema EE, van Hooft JE, Small AJ, Baron TH, García-Cano J, Cheon JH, Lee MS, Kwon SH, Mucci-Hennekinne S, Fockens P, Dijkgraaf MG, Repici A. Perforation in colorectal stenting: a meta-analysis and a search for risk factors. Gastrointest Endosc. 2014;79:970-982.e7; quiz 983.e2, 983.e5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 68. | Grothey A, Sobrero AF, Shields AF, Yoshino T, Paul J, Taieb J, Souglakos J, Shi Q, Kerr R, Labianca R, Meyerhardt JA, Vernerey D, Yamanaka T, Boukovinas I, Meyers JP, Renfro LA, Niedzwiecki D, Watanabe T, Torri V, Saunders M, Sargent DJ, Andre T, Iveson T. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N Engl J Med. 2018;378:1177-1188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 639] [Cited by in F6Publishing: 581] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 69. | Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, Gruia G, Awad L, Rougier P. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-1047. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2407] [Cited by in F6Publishing: 2336] [Article Influence: 97.3] [Reference Citation Analysis (1)] |
| 70. | Cassidy J, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Saltz L. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006-2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 592] [Cited by in F6Publishing: 607] [Article Influence: 37.9] [Reference Citation Analysis (0)] |
| 71. | Jonker DJ, O'Callaghan CJ, Karapetis CS, Zalcberg JR, Tu D, Au HJ, Berry SR, Krahn M, Price T, Simes RJ, Tebbutt NC, van Hazel G, Wierzbicki R, Langer C, Moore MJ. Cetuximab for the treatment of colorectal cancer. N Engl J Med. 2007;357:2040-2048. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1468] [Cited by in F6Publishing: 1437] [Article Influence: 84.5] [Reference Citation Analysis (0)] |
| 72. | Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, Canon JL, Van Laethem JL, Maurel J, Richardson G, Wolf M, Amado RG. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658-1664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1444] [Cited by in F6Publishing: 1424] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 73. | Saltz LB, Clarke S, Díaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzén F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013-2019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2147] [Cited by in F6Publishing: 2218] [Article Influence: 138.6] [Reference Citation Analysis (0)] |
| 74. | Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, Humblet Y, Bouché O, Mineur L, Barone C, Adenis A, Tabernero J, Yoshino T, Lenz HJ, Goldberg RM, Sargent DJ, Cihon F, Cupit L, Wagner A, Laurent D; CORRECT Study Group. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1808] [Cited by in F6Publishing: 1899] [Article Influence: 172.6] [Reference Citation Analysis (0)] |
| 75. | Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2901] [Cited by in F6Publishing: 3021] [Article Influence: 201.4] [Reference Citation Analysis (1)] |
| 76. | Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, Błasińska-Morawiec M, Šmakal M, Canon JL, Rother M, Oliner KS, Wolf M, Gansert J. Randomized, phase III trial of panitumumab with infusional fluorouracil, leucovorin, and oxaliplatin (FOLFOX4) versus FOLFOX4 alone as first-line treatment in patients with previously untreated metastatic colorectal cancer: the PRIME study. J Clin Oncol. 2010;28:4697-4705. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1296] [Cited by in F6Publishing: 1336] [Article Influence: 95.4] [Reference Citation Analysis (0)] |
| 77. | Bennouna J, Sastre J, Arnold D, Österlund P, Greil R, Van Cutsem E, von Moos R, Viéitez JM, Bouché O, Borg C, Steffens CC, Alonso-Orduña V, Schlichting C, Reyes-Rivera I, Bendahmane B, André T, Kubicka S; ML18147 Study Investigators. Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol. 2013;14:29-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 760] [Cited by in F6Publishing: 827] [Article Influence: 68.9] [Reference Citation Analysis (0)] |
| 78. | Thirion P, Michiels S, Pignon JP, Buyse M, Braud AC, Carlson RW, O'Connell M, Sargent P, Piedbois P; Meta-Analysis Group in Cancer. Modulation of fluorouracil by leucovorin in patients with advanced colorectal cancer: an updated meta-analysis. J Clin Oncol. 2004;22:3766-3775. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 241] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 79. | Fernández-Esparrach G, Bordas JM, Giráldez MD, Ginès A, Pellisé M, Sendino O, Martínez-Pallí G, Castells A, Llach J. Severe complications limit long-term clinical success of self-expanding metal stents in patients with obstructive colorectal cancer. Am J Gastroenterol. 2010;105:1087-1093. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 126] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 80. | Manes G, de Bellis M, Fuccio L, Repici A, Masci E, Ardizzone S, Mangiavillano B, Carlino A, Rossi GB, Occhipinti P, Cennamo V. Endoscopic palliation in patients with incurable malignant colorectal obstruction by means of self-expanding metal stent: analysis of results and predictors of outcomes in a large multicenter series. Arch Surg. 2011;146:1157-1162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 81. | Lee HJ, Hong SP, Cheon JH, Kim TI, Min BS, Kim NK, Kim WH. Long-term outcome of palliative therapy for malignant colorectal obstruction in patients with unresectable metastatic colorectal cancers: endoscopic stenting versus surgery. Gastrointest Endosc. 2011;73:535-542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 82. | Yamashita S, Tanemura M, Sawada G, Moon J, Shimizu Y, Yamaguchi T, Kuwai T, Urata Y, Kuraoka K, Hatanaka N, Yamashita Y, Taniyama K. Impact of endoscopic stent insertion on detection of viable circulating tumor cells from obstructive colorectal cancer. Oncol Lett. 2018;15:400-406. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 83. | Telford JJ. Covered or uncovered stents in the colon? Gastrointest Endosc. 2010;72:311-312. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 84. | Park S, Cheon JH, Park JJ, Moon CM, Hong SP, Lee SK, Kim TI, Kim WH. Comparison of efficacies between stents for malignant colorectal obstruction: a randomized, prospective study. Gastrointest Endosc. 2010;72:304-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 85. | Llano RC. Tecnicas en stents gastrointestinales endoscopicos: cómo, cuándo, manejo de complicaciones, selección del stent y costos. Rev Colomb Gastroenterol. 2012;27:33-44. [Cited in This Article: ] |
| 86. | Cwikiel W, Andrén-Sandberg A. Malignant stricture with colovesical fistula: stent insertion in the colon. Radiology. 1993;186:563-564. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 87. | Lim SG, Lee KJ, Lee HS. Mo1622 Preoperative Colonoscopy for Detection of Synchronous Neoplasm After Self-Expandable Metallic Stent Insertion in Occlusive Colorectal Cancer: Comparison of Covered and Uncovered Stents. Gastrointest Endosc. 2013;77:AB449. [DOI] [Cited in This Article: ] |
| 88. | Kim JS, Lee KM, Kim SW, Kim EJ, Lim CH, Oh ST, Choi MG, Choi KY. Preoperative colonoscopy through the colonic stent in patients with colorectal cancer obstruction. World J Gastroenterol. 2014;20:10570-10576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 89. | Yoo IK, Lee JS, Chun HJ, Jeen YT, Keum B, Kim ES, Choi HS, Lee JM, Kim SH, Nam SJ, Kang HS, Lee HS, Kim CD, Um SH, Seo YS, Ryu HS. A randomized, prospective trial on efficacy and tolerability of low-volume bowel preparation methods for colonoscopy. Dig Liver Dis. 2015;47:131-137. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 90. | de Moura DT, Guedes H, Tortoretto V, Arataque TP, de Moura EG, Román JP, Rodela GL, Artifon EL. [Comparison of colon-cleansing methods in preparation for colonoscopy-comparative of solutions of mannitol and sodium picosulfate]. Rev Gastroenterol Peru. 2016;36:293-297. [PubMed] [Cited in This Article: ] |
| 91. | Baron TH, Dean PA, Yates MR, Canon C, Koehler RE. Expandable metal stents for the treatment of colonic obstruction: techniques and outcomes. Gastrointest Endosc. 1998;47:277-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 206] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 92. | Trummel JM, Chandrasekhara V, Kochman ML. Anesthesia for Colonoscopy and Lower Endoscopic Procedures. Anesthesiol Clin. 2017;35:679-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 93. | Lin OS. Sedation for routine gastrointestinal endoscopic procedures: a review on efficacy, safety, efficiency, cost and satisfaction. Intest Res. 2017;15:456-466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 64] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 94. | Kochar R, Shah N. Enteral stents: from esophagus to colon. Gastrointest Endosc. 2013;78:913-918. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 95. | Coronel M, Korkischko N, Marques Bernardo W, Lordello Passos M, Cavalheiro Bonifacio P, Valente de Matos M, de Moura DTH, Ide E. Comparison between Carbon Dioxide and Air Insufflation in Colonoscopy: A Systematic Review and Meta-Analysis Based On Randomized Control Trials. J Gastroenterol Pancreatol Liver Disord. 2017;1-11. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 96. | Cadoni S, Hassan C, Frazzoni L, Ishaq S, Leung FW. Impact of water exchange colonoscopy on endoscopy room efficiency: a systematic review and meta-analysis. Gastrointest Endosc. 2019;89:159–167.e13. [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 97. | Samadder NJ, Bonin EA, Buttar NS, Baron TH, Gostout CJ, Topazian MD, Song LM. Placement of a covered stent for palliation of a cavitated colon cancer by using a novel over-the-scope technique (with video). Gastrointest Endosc. 2012;76:1275-1277. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 98. | Baron TH, Rey JF, Spinelli P. Expandable metal stent placement for malignant colorectal obstruction. Endoscopy. 2002;34:823-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 53] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 99. | Kim C, Park J-J, Seo Y-S, Jang Y-J, Lee J-Y, Kim J-Y, Kim J-S, Bak Y-T. Complications of Self-Expandable Colorectal Stenting for the Treatment of Acute Large Bowel Obstruction. Gastrointest Endosc. 2005;61:AB262 [doi:10.1016/S0016-5107(05)01376-3]. [Cited in This Article: ] |
| 100. | Stankiewicz R, Kozieł S, Pertkiewicz J, Zieniewicz K. Outcomes and complications of self-expanding metal stent placement for malignant colonic obstruction in a single-center study. Wideochir Inne Tech Maloinwazyjne. 2018;13:53-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 101. | Small AJ, Coelho-Prabhu N, Baron TH. Endoscopic placement of self-expandable metal stents for malignant colonic obstruction: long-term outcomes and complication factors. Gastrointest Endosc. 2010;71:560-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 192] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 102. | Yoon JY, Jung YS, Hong SP, Kim TI, Kim WH, Cheon JH. Outcomes of secondary stent-in-stent self-expandable metal stent insertion for malignant colorectal obstruction. Gastrointest Endosc. 2011;74:625-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 103. | Cao Y, Deng S, Wu K, Zheng H, Cheng P, Zhang J, Chen L, Tang S, Wang P, Liao X, Zhang Y, Zhu G, Tong Q, Wang J, Gao J, Shuai X, Tao K, Wang G, Li J, Cai K. Oncological consequence of emergent resection of perforated colon cancer with complete obstruction after stent insertion as a bridge to surgery. Int J Colorectal Dis. 2018;. [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |