Published online Sep 27, 2011. doi: 10.4254/wjh.v3.i9.228
Revised: August 15, 2011
Accepted: August 22, 2011
Published online: September 27, 2011
Despite significant advances in medicine, liver cancer, predominantly hepatocellular carcinoma remains a major cause of death in the United States as well as the rest of the world. As limited treatment options are currently available to patients with liver cancer, novel preventive control and effective therapeutic approaches are considered to be reasonable and decisive measures to combat this disease. Several naturally occurring dietary and non-dietary phytochemicals have shown enormous potential in the prevention and treatment of several cancers, especially those of the gastrointestinal tract. Terpenoids, the largest group of phytochemicals, traditionally used for medicinal purposes in India and China, are currently being explored as anticancer agents in clinical trials. Terpenoids (also called “isoprenoids”) are secondary metabolites occurring in most organisms, particularly plants. More than 40 000 individual terpenoids are known to exist in nature with new compounds being discovered every year. A large number of terpenoids exhibit cytotoxicity against a variety of tumor cells and cancer preventive as well as anticancer efficacy in preclinical animal models. This review critically examines the potential role of naturally occurring terpenoids, from diverse origins, in the chemoprevention and treatment of liver tumors. Both in vitro and in vivo effects of these agents and related cellular and molecular mechanisms are highlighted. Potential challenges and future directions involved in the advancement of these promising natural compounds in the chemoprevention and therapy of human liver cancer are also discussed.
- Citation: Thoppil RJ, Bishayee A. Terpenoids as potential chemopreventive and therapeutic agents in liver cancer. World J Hepatol 2011; 3(9): 228-249
- URL: https://www.wjgnet.com/1948-5182/full/v3/i9/228.htm
- DOI: https://dx.doi.org/10.4254/wjh.v3.i9.228
Hepatocellular carcinoma (HCC) is the most common form of primary hepatic carcinoma[1] and a pressing sociomedical problem in several countries, particularly in Asia and sub-Saharan Africa[2]. HCC is currently the fifth most common cancer and third leading cause of cancer-related deaths in the world[3]. HCC has a poor prognosis with the number of deaths almost equal to the number of cases being diagnosed annually (about 600 000) and the 5-year survival rate is below 9%[4]. In the United States, the incidence of HCC has been steadily rising with a 70% increase registered in the last 25 years[5]. The American Cancer Society[6], estimated that in 2010 alone, more than 24 000 new cases and nearly 19 000 deaths occurred in the United States due to liver cancer (including biliary cancers).
Major risk factors for HCC are well known and are dependent on the geographic area. In Europe, the United States, and Japan, the main risk factors are liver cirrhosis, Hepatitis B virus (HBV) and Hepatitis C virus (HCV), alcohol, and tobacco; in contrast, in Africa and Asia, the etiological factors include HBV and HCV, tobacco use, and aflatoxin exposure[7,8]. Treatment for HCC has been conventionally divided into curative and palliative. Curative treatments, such as resection, liver transplantation and percutaneous ablation, induce complete responses in a high proportion of patients and are expected to improve survival. Palliative treatments are not aimed to cure, but in some cases can obtain good response rates and even improve survival[9]. In the west, curative treatments are applied to 30%-40% of patients in referral centers[10], whereas in Japan 60%-90% of patients benefit because of widespread implementation of surveillance and a broad application of treatments[11,12]. There is no firm evidence to establish the optimum first-line treatment for patients who have a single small HCC and well-preserved liver function[9]. Resection and transplantation achieve the best outcomes in well-selected candidates (5-year survival 60%-70%)[13-16], and compete as the first option from an intention-to-treat perspective[17]. Percutaneous treatments provide good results (5-year survival 40%-50%)[11,18], but have not been able to achieve response rates and outcomes comparable to surgical treatments[11,12]. Liver transplantation has been suggested as the best treatment for patients with one tumor and decompensated cirrhosis or multicentric small tumors[19].
HCC prognosis remains dismal despite many treatment options. Overall, the cure rate among patients who undergo resection is not very high and for those patients who are not eligible for surgery or percutaneous procedures, only chemoembolization appears to improve survival. The need thus arises to test novel agents in large-scale randomized trials; these include intraarterial injection of radiolabeled microsphere and new systemic drugs, such as tyrosine kinase inhibitors, antivascular endothelial growth factor (VEGF) antibody, and antiepithelial growth factor receptor[7]. HCC is also widely considered to be a chemotherapy-resistant disease[20]. Sorafenib, the only drug approved by the United States Food and Drug Administration for the treatment of advanced HCC, increases the median survival time by less than 3 mo[21]. However, this drug does not defer the symptomatic progression of the disease, costs about $5400 per month for treatment[22], and exhibits severe adverse effects, including a significant risk of bleeding[23]. These drawbacks necessitate the search for novel preventive and therapeutic approaches for this disease. Chemoprevention has emerged as an ideal approach whereby the occurrence and progression of the disease can be prevented, slowed, or reversed by the administration of one or more naturally occurring and/or synthetic compounds[24-26].
Phytochemicals, including those obtained from fruits, vegetables, nuts and spices, have drawn a considerable amount of attention due to their ability to selectively kill tumor cells and suppress carcinogenesis in preclinical animal models[27-33]. A large number of these plant-derived substances have been shown to significantly prevent or delay cancer development in several high risk populations[34-36]. Mounting evidence, based on in vitro experiments and studies involving animal models as well as humans, support potential chemopreventive and therapeutic effects of diverse phytochemicals in liver cancer[37-41]. This review delves into the current use of terpenoids, the largest families of plant-derived natural products, for either chemoprevention or therapy of hepatic cancer, by examining an extensive number of studies conducted both in vitro and in vivo.
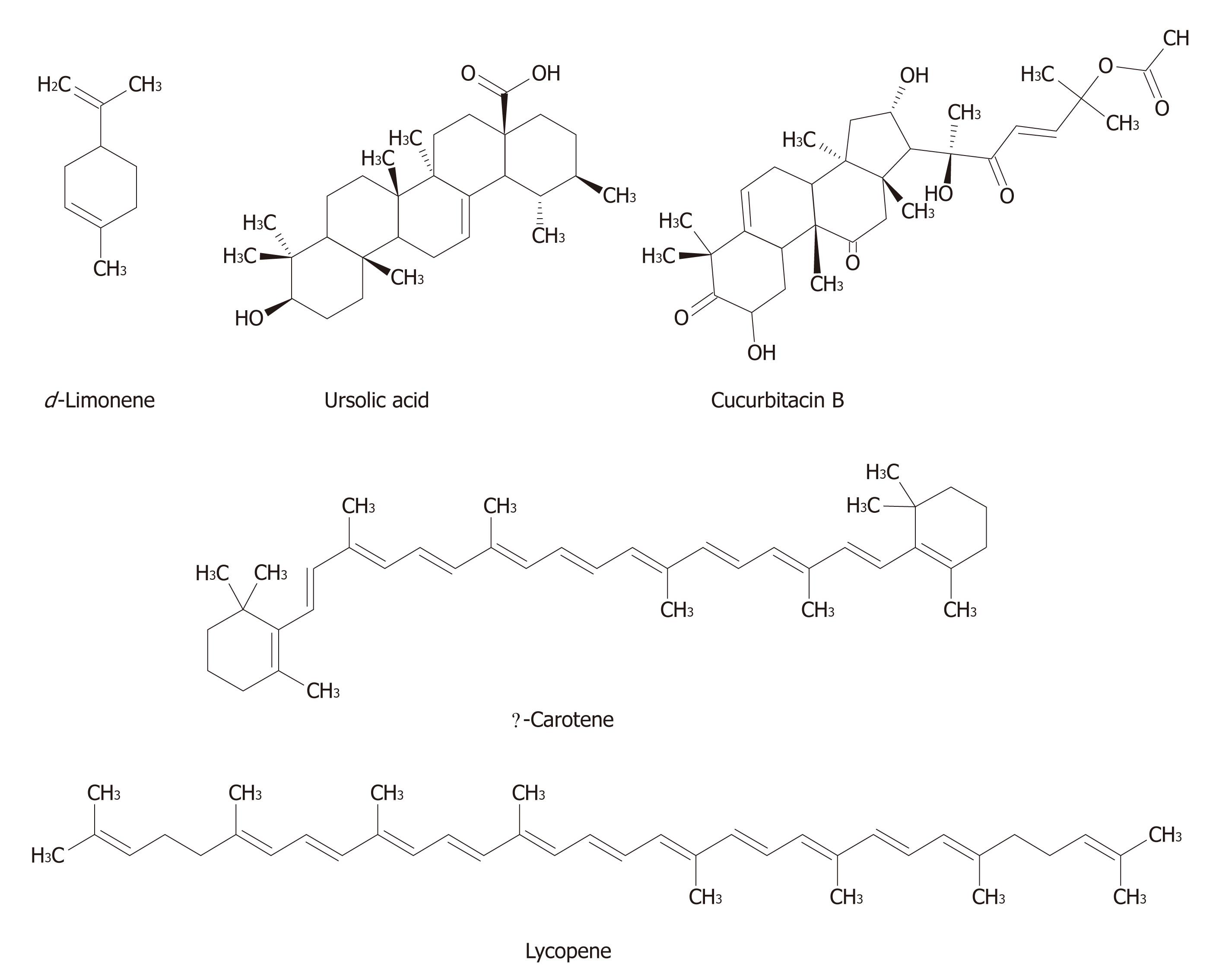
Terpenoids composed of “isoprenoid” units constitute one of the largest group of natural products accounting for more than 40 000 individual compounds, with several new compounds being discovered every year[42-44]. Most of the terpenoids are of plant origin; however, they are also synthesized by other organisms, such as bacteria and yeast as part of primary or secondary metabolism. Terpenoids are synthesized from two five-carbon building blocks, i.e., the isoprenoid units. Based on the number of building blocks, terpenoids are classified into several classes, such as monoterpenes (e.g., carvone, geraniol, d-limonene, and perillyl alcohol), diterpenes (e.g. retinol and trans-retinoic acid), triterpenes [e.g., betulinic acid (BA), lupeol, oleanic acid, and ursolic acid (UA)], and tetraterpenes (e.g., α-carotene, β-carotene, lutein, and lycopene)[45].
The diverse array of terpenoid structures and functions has provoked increased interest in their commercial use. Terpenoids have been found to be useful in the prevention and therapy of several diseases, including cancer, and also to have antimicrobial, antifungal, antiparasitic, antiviral, anti-allergenic, antispasmodic, antihyperglycemic, antiinflammatory, and immunomodulatory properties[45-48]. In addition, terpenoids can be used as protective substances in storing agriculture products as they are known to have insecticidal properties[49].
Epidemiological and experimental studies suggest that monoterpenes may be helpful in the prevention and therapy of several cancers, including mammary, skin, lung, forestomach, colon, pancreatic and prostate carcinomas[34,50-55]. Triterpenoids are the metabolites of isopentenyl phosphate oligomers and constitute the largest group of phytochemicals with more than 20 000 known compounds available in nature[56]. A large number of triterpenoids have been shown to suppress the growth of a variety of cancer cells without exerting any toxicity in normal cells[57-59]. Numerous preclinical efficacy studies have provided extensive evidence that both naturally occurring and synthetic derivatives of triterpenoids possess chemopreventive and therapeutic effects against colon, breast, prostate and skin cancer[56,60-64]. These triterpenoids and their derivatives act at various stages of tumor development, inhibit initiation and promotion of carcinogenesis, induce tumor cell differentiation and apoptosis, and suppress tumor angiogenesis, invasion and metastasis through regulation of various transcription and growth factors as well as intracellular signaling mechanisms[56,64-66]. Currently, several phase I/II clinical trials have been initiated to evaluate the chemopreventive as well as the anticancer efficacy of a number of triterpenoids[56,67]. Although several excellent articles provide an overview of the cancer preventive and antitumor potential of terpenoids against various cancers, the use of these phytoconstituents for either chemoprevention or therapy of liver cancer has not previously been discussed exclusively.
The following sections of this review showcase the in vitro and in vivo studies undertaken by a number of researchers around the world exploring the chemopreventive as well as chemotherapeutic potential of terpenoids in liver cancer.
There are a number of in vitro studies that demonstrate the cytotoxic effects of various terpenoids against proliferation, growth and invasion of a variety of liver cancer cell lines (Table 1).
| Terpenoids | Compounds | Cellular effects | Mechanisms | Conc. | Ref. |
| Monoterpenes | Geraniol | Inhibited the growth of HepG2 cells | ↓HMG-CoA reductase | 50-400 μmol/L | Polo et al[68] |
| Diterpenes | Andrographolide | Inhibited the growth of Hep3B cells | ↑apoptosis; ↑ MAPKs; ↑pJNK; ↑ERK1/2 | 3-100 μmol/L | Ji et al[69] |
| Excisanin A | Decreased viability of Hep3B cells | ↑apoptosis; ↓pAKT | 1-32 μmol/L | Deng et al[70] | |
| Gnidimacrin | Demonstrated tumor inhibition in PKC βII-transfected HLE cells | ⊥G2; ↓cdc2; ↑p21WAF1/CIP1 | 0.00001-0.1 μg/mL | Yoshida et al[71] | |
| Oridonin | Promoted cytotoxicity in HepG2 cells | ↑apoptosis; ↑ROS; ↑p53; ↑p38; ↓ΔΨm; ↑cyt. c; ↑caspase-3, -9 | 10-50 μmol/L | Huang et al[72] | |
| Triterpenes | Actein | Inhibited the growth of p53-positive HepG2 cells | 27 μg/mL [IC50] | Einbond et al[75] | |
| Ardisiacrispin (A+B) | Exhibited cytotoxicity against Bel-7402 cells | ↑apoptosis; ↓proliferation; microtubule disassembly | 1-10 μg/mL | Li et al[76] | |
| Astragaloside IV | Decreased colonogenic survival and inhibited anchorage-independent growth of HepG2 cells | ↓Vav3.1; ↑HSP70; ↑HSPA1A; ↑HSPA8; ↑BiP/GRP78 | 150-200 μg/mL | Qi et al[78] | |
| Asiatic acid | Decreased viability of HepG2 cells | ↑apoptosis; ↑Ca2+; ↑p53 | 10-100 μmol/L | Lee et al[79] | |
| Betulinic acid | Displayed cytotoxicity against HUH6, HepT1 and HepT3 cells | ↑apoptosis; ↑caspase 3; ↓PI3K/AKT; ↓GLI1; ↓PTCH1; ↓IGF2 | 1-50 μg/mL | Eichenmüller et al[80] | |
| Failed to induce apoptosis against HepG2 cells | ↑survivin; ↑Bcl-2 | 0.1-50 μg/mL | Eichenmüller et al[80] | ||
| Cucurbitacin B | Decreased the viability of HepG2 cells | ↑apoptosis; ↓Bcl-2; ↓pSTAT3 | 10 nmol/L-10 μmol/L | Zhang et al[83] | |
| Exhibited growth inhibitory effects on Bel-7402 cells | ↑apoptosis; ⊥S; ↓cyclin D1; ↓cdc2; ↓c-Raf; ↑ERK 1/2 | 0.01-1000 μmol/L | Chan et al[84] | ||
| Cucurbitacin D | Suppressed the growth of Hep3b cells | ↑apoptosis; ↑caspase-3; ↑pJNK | 0-10 μmol/L | Takahashi et al[85] | |
| Cucurbitacin D, I | Displayed cytotoxicity against Bel-7402 cells | < 1 μmol/L [IC50] | Meng et al[86] | ||
| Echinocystic acid | Exhibited antiproliferative effect against HepG2 cells | ↑apoptosis; ↓Bcl-2; ↑caspase-3, -9, -8; ↑PARP cleavage; ↓ΔΨm; ↓cyt. c; ↑p38; ↑JNK | 15-100 μmol/L | Tong et al[87] | |
| Escin | Demonstrated inhibitory effects on cell viability of HepG2 cells | ↑apoptosis; ⊥G1/S; ↑AIF; ↑cyt. c; ↑bax; ↓Bcl-2; ↓cyclin-E/cdk2; ↓pRb; ↓E2F | 10-60 μg/mL | Zhou et al[88] | |
| Ganoderic acid | Demonstrated an inhibition in the growth of BEL 7402 cells | ⊥G1/S | 50-500 mg/mL | Yang[89] | |
| Ganoderiol F | Displayed antiproliferative effects in HepG2 , Huh7 and Hep3B cells | ⊥G1; ↓DNA synthesis; ↓topo I and II; ↑p16; ↓p21; ↑pERK2 | 0.1-60 μmol/L | Chang et al[90] | |
| Ginsenoside-Rg1 | Accelerated the growth of SK-Hep-1 cells | ↑cyclin E; ↑cdk2 | 2.5-100 μmol/L | Lee et al[91] | |
| Ginsenoside-Rg5 | Suppressed the growth of SK-Hep-1 cells | ⊥G1/S; ↓cyclin E; ↓cdk2; ↑p21WAF1/CIP1; ↓cdc25A | 0.1-25 μmol/L | Lee et al[92] | |
| Ginsenoside Rh2 | Inhibited DNA synthesis in SK-Hep-1 cells | ⊥G1/S; ↓cyclin E; ↑p27kip1; ↓cdc25A | 0.25-100 μmol/L | Lee et al[93] | |
| Induced cell-death in SK-Hep-1 cells | ↑apoptosis; ↑caspase-3; ↑PARP | 2-12 mg/mL | Park et al[94] | ||
| Ginsenoside Rk1 | Inhibited the growth of HepG2 cells | ↑apoptosis; ↑caspase-3, -8; ↓FADD; ↓telomerase activity | 12.5-100 μmol/L | Kim et al[95] | |
| Exhibited antiproliferative effects on HepG2 cells | ↑autophagy; ⊥G1 | 0-100 μmol/L | Ko et al[96] | ||
| Ginsenoside Rs3 | Displayed suppressive effects against the growth of SK-Hep-1 cells | ↑apoptosis; ↓cyclin E, A; ↑p53; ↑p21WAF1/CIP1; ↓cdk2 | 0.1-25 μmol/L | Kim et al[97] | |
| Gypenosides | Exhibited cytotoxicity and decreased viability of Huh7, Hep3B and HA22T cells | ↑apoptosis; ↑Bax; ↑Bak; ↑Bcl-XL; ↓Bcl-2; ↓Bad; ↑cyt. c; ↑caspase-3, -9, -8 | 0.1-400 mg/mL | Wang et al[98] | |
| IH-901 | Conferred antiproliferative effects against HepG2 cells | ↑apoptosis; ↑caspase-3, -8, -9; ↑PARP; ↑cyt. c | 10-60 μmol/L | Oh et al[99] | |
| Inhibited the growth of SMMC7721 cells | ↑apoptosis; ⊥G0/G1; ↑Bax; ↑p53; ↑cyt. c; ↓pro-caspase-3, -9 | 5-100 μmol/L | Ming et al[100] | ||
| Kalopanaxsaponins A, I | Showed significant cytotoxicity against HepG2 and R-HepG2 cells | ↑apoptosis | 8.9-18.1 μmol/L | Tian et al[101] | |
| Keto- and acetyl-keto-boswellic acids | Exhibited antiproliferative effects in HepG2 cells | ↑apoptosis; ⊥G1; ↑caspase-3, -8, -9 | 25-200 μmol/L | Liu et al[102] | |
| Lucidenic acid A,B,C, N | Displayed antiproliferative and anti-invasive effects against HepG2 cells | ↓MMP-9; ↓ERK1/2; ↓NF-κB; ↓AP-1; ↓c-Jun; ↓c-Fos | 10-100 μmol/L | Weng et al[103,104] | |
| Lupeol | Exhibited growth inhibitory effects against SMMC7721 cells | ↑apoptosis; ↑caspase-3, -8; ↓DR3; ↑FADD | 6.25-200 μmol/L | Zhang et al[105] | |
| 25-Methoxyhispidol A | Showed antiproliferative effects against SK-Hep1 cells | ↑apoptosis; ⊥G0/G1; ↓cyclin D1; ↓CDK4; ↓c-myc; ↓pRb; ↑p21 | 0.8-100 μmol/L | Hong et al[106] | |
| Oleanolic acid | Decreased the viability of HepG2, Hep3B, Huh7 and HA22T cells | ↑apoptosis; ↓ΔΨm; ↑caspase-3, -8; ↓Na+-K+- ATPase; ↓ICAM-1; ↓VEGF | 2-8 μmol/L | Yan et al[107] | |
| Reduced the viability of Huh7 cells | ↑apoptosis; ↓ΔΨm; ↑Bax; ↓Bcl2; ↑cyt. c; ↑ caspase-3, -9; ↓NF-κB; ↓XIAP | 20-100 μmol/L | Shyu et al[108] | ||
| Ursolic acid | Inhibited the proliferation of HepG2 and R-HepG2 cells | ↑apoptosis; ⊥G1/G0; ↓COX-2; ↑HSP105 | 3.125-100 μmol/L | Tian et al[109] | |
| Suppressed the proliferation of HepG2 cells | ↑apoptosis; ↑p53; ↓Bcl-2; ↓survivin; ↑caspase-3; ↓PI3K/Akt | 5-80 μmol/L | Tang et al[110] | ||
| Decreased the viability of HepG2, Hep3B, Huh7 and HA22T cells | ↑apoptosis; ↓ΔΨm; ↑caspase-3, -8; ↓Na+-K+- ATPase; ↓ICAM-1; ↓VEGF | 2-8 μmol/L | Yan et al[107] | ||
| Reduced the viability of Huh7 cells | ↑apoptosis; ↓ΔΨm; ↑Bax; ↓Bcl2; ↑cyt. c; ↑ caspase-3, -9; ↓NF-κB; ↓XIAP | 20-100 μmol/L | Shyu et al[108] | ||
| Waltonitone | Conferred inhibitory effects on the growth of BEL-7402 cells | ↑apoptosis; ↑caspase-3, -8, -9; ↑Bax; ↑cyt. c; ↑Fas; ↑FasL; ↑apaf-1 | 0.4-100 μmol/L | Zhang et al[111] | |
| Miscellaneous | Induced reduction in the viability of HepG2 and Hep3B cells | 9.4-71.1 μmol/L [IC50] | Wang et al[112] | ||
| Exhibited cytotoxicity against HepG2 cells | ↑apoptosis; ↑p53; ⊥G1/S | 0.5-80 μmol/L | Huang et al[113] | ||
| Tetraterpenes | Astaxanthin, β-Carotene | Suppressed the invasive characteristic of AH109A cells | Antioxidant mechanisms | 2.5-20 μmol/L | Kozuki et al[114] |
| Inhibited the migration of SK-Hep-1 cells | 1-20 μmol/L | Huang et al[115] | |||
| Delayed the proliferation and improved the differentiation of oval cells obtained from neoplastic liver | ↑albumin; ↑fibrinogen; ↑hapatoglobin | 5 μmol/L | Wójcik et al[116] | ||
| Exerted genotoxic and cytotoxic effects in HepG2 cells | ↑apoptosis; ↑necrosis; ↑TBARS | 4-8 μmol/L | Yurtcu et al[117] | ||
| Fucoxanthin | Inhibited the growth of HepG2 cells | ⊥G0/G1; ↓cyclin D1, D3; ↓cdk4; ↓pRb | 10-50 μmol/L | Das et al[118] | |
| Exhibited antiproliferative effect against SK-Hep-1 cells | ↑apoptosis; ⊥G0-G1; ↑GJIC; ↑Cx43; ↑Cx32; ↓pJNK; ↓pERK; ↑[Ca]++ | 1-20 μmol/L | Liu et al[119] | ||
| Lycopene | Suppressed the invasive property of AH109A cells | 0.1-20 μmol/L | Kozuki et al[114] | ||
| Induced an inhibitory effect on the growth of Hep3b cells | ⊥G0/G1; DNA damage | 0.1-50 μmol/L | Park et al[120] | ||
| Displayed antimigration and anti-invasive activity in SK-Hep-1 cells | ↑nm23-H1 | 1-20 μmol/L | Huang et al[115] | ||
| Inhibited adhesion, invasion and migration of SK-Hep-1 cells | ↓MMP-9, -2 | 0.1-50 μmol/L | Hwang et al[121] | ||
| Inhibited SK-Hep-1 cell invasion | ↓MMP-9; ↓NF-κB; ↑IκBα; ↓Sp1; ↓IGF-1R; ↓ROS | 1-10 μmol/L | Huang et al[122] | ||
| Sesquiterpenes | α-Bisabolbol | Induced cytoxicity in HepG2 cells | ↑apoptosis; ↑caspase-8, -9; ↑cyt. c; ↑Bax; ↑Bak; ↓Bcl-2; ↑p53; ↑NF-κB; ↑Fas | 0.1-20 μmol/L | Chen et al[123] |
| Dehydrocostuslactone | Inhibited the proliferation of HepG2 and PLC/PRF/5 cells | ↑apoptosis; ↑Bax; ↑Bak; ↓Bcl-2; ↓Bcl-XL; ↑caspase-4, -9; ↑AIF; ↑Endo G; ↑ER stress; ↓CHOP/GADD153; ↑Bip; ↑MAPK; ↑pJNK; ↑ERK1/2; ↑p38 | 1-40 μmol/L | Hsu et al[124] | |
| Furanodiene | Inhibited cell growth of HepG2 cells | ↑apoptosis; ⊥G2/M; altered ΔΨm; ↑cyt. c; ↑caspase-3; ↑pp38; ↓pERK1/2 | 0.1-1000 μmol/L | Xiao et al[125] | |
| HOBS1, HOBS2 | Exhibited antiproliferative effects and induced redifferentiation in SMMC-7721 cells | ⊥G1; ↓AFP; ↓γ-GT; ↓TAT | 0.1-30 μg/mL | Miao et al[126] | |
| Zerumbone | Showed antiproliferative activity against HepG2 cells | ↑apoptosis; ↓Bcl-2; ↑Bax | 3.45 μg/mL [IC50] | Sakinah et al[127] |
Monoterpenes: Geraniol, an acyclic dietary monoterpene, represents the only monoterpene that has been studied in vitro against liver cancer cells. Geraniol was shown to inhibit the growth of HepG2 human hepatic carcinoma cells by decreasing 3-hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase, the major rate-limiting enzyme in cholesterol biosynthesis in mammals[68].
Diterpenes: Andrographolide, a potential antiinflammatory diterpenoid lactone isolated from the traditional medicinal plant Andrographis paniculata, demonstrated inhibitory effects against the growth of hepatoma-derived Hep3B cells. Apoptosis as well as activation of the c-Jun-N-terminal kinase (JNK) and mitogen-activated protein kinase (MAPK) pathways were shown to play an important role in the cytotoxic effect exerted by andrographolide[69].
Excisanin A, a diterpenoid compound purified from Isodon macrocalyxin D, was tested on human Hep3B liver cancer cells with positive results indicating growth inhibition mediated by apoptosis through inhibition of the AKT signaling pathway[70].
Gnidimacrin, a daphnane-type diterpenoid, was successfully shown to inhibit the growth of protein kinase C βII gene-transfected human hepatoma HLE cells through G2 phase arrest, not only by the suppression of cdc2 activity, but also by the subsequent transcriptional suppression of cdc2 itself. There was also an increase in p21WAF1/CIP1, a potent and universal inhibitor of the cyclin-dependent kinase-1 (cdk1)[71].
Oridonin, a diterpenoid isolated from Rabdosia rubescences, promoted cytotoxic activities against HepG2 cells through an increase in the apoptotic cell death process and reactive oxygen species (ROS) generation. Increase in the tumor suppressor gene p53, apoptotic proteins, such as caspase-3, and -9, cytochrome c (cyt. c), and p38 protein expression with decrease in mitochondrial membrane potential (ΔΨm) were involved in the cytotoxic effects of this compound[72].
Triterpenes: Actein is an active component from the herb black cohosh [Actaea racemosa L. syn. Cimicifuga racemosa (L) Nutt]. Recent studies indicate that black cohosh may have chemopreventive and chemotherapeutic potential[73,74]. Actein was found to inhibit the growth of p53-positive HepG2 cells with an IC50 value of 27 μg/mL[75].
Ardisiacrispin (A+B), a triterpenoid saponin mixture in the fixed proportion 2:1 of ardisiacrispin A and ardisiacrispin B, is derived from Ardisia crenata. This mixture exerted cytotoxic activity against Bel-7402 liver cancer cells through pro-apoptotic, anti-proliferative, and microtubule disruptive activities[76].
Astragaloside IV is the major active triterpenoid in Radix astragali, a dietary supplement widely used in traditional Chinese medicine to prevent and treat various cancers[77]. This compound decreased the colonogenic survival and inhibited anchorage-independent growth of HepG2 cells possibly by decreasing the expression of oncogene Vav3.1 and increasing the stress proteins, namely glucose-regulated protein BiP/GRP78, heat shock protein (HSP) 70, HSPA1A, and HSPA8[78].
Asiatic acid, a pentacyclic triterpene isolated from the Brahmi plant, Centella asiatica, was successfully tested for its apoptotic effects in HepG2 cells. This triterpene decreased the viablility of HepG2 cells mediated by an increase in intracellular calcium levels, which led to the increase in expression of the tumor suppressor gene p53[79].
BA, another pentacyclic triterpene, was significantly effective in inducing cytotoxicity in hepatoblastoma cell lines, namely HUH6, HepT1 and HepT3. Apoptosis through proteolytic cleavage of caspase-3 was found to be the primary mechanism involved in the antihepatoblastoma effects of this natural compound. BA also inhibited the phosphatidylinositol 3-kinase/AKT pathway and target genes of hedgehog signaling, e.g. protein patched homolog 1, insulin growth factor 2, and glioma-associated oncogene homolog 1[80]. Deregulation of the hedgehog signaling pathway plays a fundamental role in an increasing number of malignancies[81,82]. However, BA failed to induce apoptosis in HepG2 cells (known to lack activation of the hedgehog signaling pathway) but increased the protein levels of survivin and anti-apoptotic Bcl-2[80].
Cucurbitacins, a group of oxygenated triterpenes, are characterized by the presence of the tetracyclic cucurbitane skeleton. Cucurbitacin B, one of the most abundant forms of cucurbitacins, was found to effectively decrease the viability of HepG2 cells through apoptosis with a simultaneous decrease in the levels of the pro-apoptotic protein Bcl-2. Suppression of transcription factor signal transducer and activator of transcription-3 (STAT3) phosphorylation was associated with the cell killing effect of this natural compound[83]. Chan et al[84] were successful in achieving similar growth inhibitory effects of cucurbitacin B in Bel-7402 cells. Treatment of Bel-7402 cells with cucurbitacin B also induced S phase arrest and apoptosis associated with down regulation of cyclin D1 and cdc-2. Additional studies indicated cucurbitacin B mediated the inhibition of c-Raf activation without any alteration of the STAT3 phosphorylation. Cucurbitacin D, isolated from Trichosanthes kirilowii, was shown to suppress the growth of Hep3b cells by apoptosis through caspase-3 and phosphorylation of JNK protein[85]. Meng et al[86] isolated cucurbitacins D and I from Elaeocarpus hainanensis and reported strong cytoxic effects of these compounds against Bel-7402 cells.
Echinocystic acid, a triterpene present in various herbs, is used for medicinal purposes in many Asian countries. This compound was shown to exhibit antiproliferative effects against HepG2 cells through typical apoptosis mechanisms, characterized by DNA fragmentation, activation of caspase-3, -8 and -9, polypeptide poly(ADP-ribose) polymerase (PARP) cleavage, truncation of Bid, reduction of Bcl-2, loss of ΔΨm, cyt. c release and activation of JNK and p38 kinase[87].
Escin, a mixture of triterpene saponins extracted from Aesculus wilsonii Rehd., displayed its potency against HepG2 cell viability through disruption of the G1/S phase of cell cycle progression and caspase-independent cell death via apoptosis-inducing factor/cyt. c translocation from the mitochondria to the nucleus[88].
Ganoderic acid, produced by the submerged culture of Ganoderma lucidum, was effective in inhibiting the growth of Bel-7402 cells by blocking the transition of cells from G1 to S phase[89]. Ganoderiol F, a tetracyclic triterpene isolated from Ganoderiol amboinense, induced antiproliferative effects through senescence in HepG2, Huh7 and Hep3b. Ganoderiol F treatment in HepG2 and Huh7 cells resulted in inhibition of DNA synthesis and arrest of cell cycle progression in G1 phase. The inhibition of DNA synthesis in HepG2 cells was attributed to the suppression of topoisomerases I and II[90].
The triterpenoid ginsenoside-Rg1 failed to confer cytotoxicity against SK-Hep-1 cells and instead stimulated its growth through an increase in cyclin E and cdk2 protein expression[91]. Ginsenoside-Rg5, a new diol-containing ginsenoside, is isolated from red ginseng. This triterpenoid saponin was found to suppress the growth of SK-Hep-1 cells through cell cycle arrest in the G1/S phase associated with downregulation of cdk2 activity. This was caused by selective induction of p21WAF1/CIP1 with a concurrent decrease in cyclin E, cdk2 and cdc25A[92].
Ginsenoside-Rh2 inhibited DNA synthesis in SK-Hep-1 cells through cell cycle arrest in the G1/S phase by selectively inducing p27kip1 expression consequently downregulating cyclin E-dependent kinase activity[93]. A similar study conducted by Park et al[94] described an inhibitory effect of ginsenoside-Rh2 on the growth of SK-Hep-1 cells by apoptosis through Bcl-2-insensitive activation of caspase-3 followed by proteolytic cleavage of PARP.
Ginsenoside Rk1, obtained from heat-processed Panax ginseng C.A. MEYER, suppressed the growth of HepG2 cells associated with a significant inhibition of telomerase activity 48 h following the treatment. Moreover, this compound induced apoptosis through activation of caspase-8 and -3 with a decrease in Fas-associated death domain expression[95]. Interestingly, when HepG2 cells were incubated with ginsenoside Rk1 for 24 h, cell growth inhibition, G1 cell cycle arrest and autophagy were observed[96].
Kim et al[97] reported the growth suppressive effects of ginsenoside Rs3 in SK-Hep-1 cells by apoptosis, cell cycle arrest in the G1/S phase and selective elevation of the protein levels of p53 and p21WAF1/CIP1 with a concurrent decrease in the activities of cyclin E- and A-associated kinases.
Gypenosides, triterpenoid saponins present in the extract from Gynostemma pentaphyllum Makino, were found to exhibit cytoxicity against HUH7, Hep3B and HA22T cancer cell lines through the intrinsic cell death pathway mediated by alteration of apoptosis-associated proteins, such as Bax, Bak, Bcl-XL, Bcl-2, Bad, cyt. c, caspase-3, -9 and -8[98].
IH-901, a novel metabolite of the ginseng saponin, exhibited cytotoxicity against HepG2 cells with simultaneous induction of apoptosis via mitochondrial-mediated pathway, which resulted in the activation of caspase-9 and subsequent mitochondrial pathway activation. Caspase-8 was shown to cleave Bid which subsequently relocated to the mitochondria and amplified the mitochondrial pathway activation[99]. IH-901 was also tested against SMCC7721 cells with antiproliferative and apoptotic effects via the mitochondrial-mediated pathway, which resulted in activation of caspase-9 and subsequently caspase-3 through release of cyt. c[100].
Kalopanaxsaponins A and I are two oleanane triterpene saponins isolated from the seeds of Nigella glanulifera. Through apoptotic mechanisms, these triterpenes displayed significant cyotoxicity against HepG2 as well as drug-resistant HepG2 (R-HepG2) cells[101].
Boswellic acids, natural compounds isolated from the gum resin of Bosewellia serrata, have recently drawn attention because of their potential as chemopreventive and therapeutic agents. Keto- and acetyl-keto-boswellic acids exhibited antiproliferative effects against HepG2 cells, with apoptosis accompanied by activation of caspase-3, -8 and -9 being the mechanisms by which the boswellic acids exerted their liver cancer cell killing effects[102].
Lucidenic acids A, B, C and N were isolated from Ganoderma lucidum, a well-known mushroom with pharmacological effects. These triterpenoid components exhibited antiproliferative and anti-invasive effects against HepG2 cells as evidenced by lucidenic acid-mediated inhibition of phorbol-12-myristate-13-acetate-induced matrix metalloproteinase-9 (MMP-9) activity[103]. Subsequent studies from the same group demonstrated that the anti-invasive effects of lucidenic acid B might be effected through inhibiting the phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 and downregulating MMP-9 expression by suppressing activator protein-1- and nuclear factor κB (NF-κB)-DNA binding activities[104].
Lupeol, a novel dietary triterpene found in several fruits and vegetables as well as in a number of medicinal plants, greatly inhibited the growth of SMMC7721 cells with apoptotic death through activation of caspase-3 mediated by the downregulation of death receptor 3[105].
25-Methoxyhispidol A, a novel triterpenoid isolated from the fruit of Poncirus trifolata, was successfully shown to display antiproliferative effects against SK-Hep-1 cells. Apoptosis and G0/G1 phase arrest were suggested as the mechanisms of action. This correlated well with the downregulation of cyclin D1, CDK4, c-myc, and retinoblastoma protein expressions, along with the upregulation of p21[106].
The pentacyclic triterpene oleanolic acid (OA) proved its beneficial chemotherapeutic properties against HCC by inducing significant decrease in viability of various liver cancer cell lines, namely HepG2, Hep3B, Huh7 and HA22T. The observed elevation in caspase activities and DNA fragmentation suggested apoptosis to be a key mechanism in OA action. Concentration-dependent inhibition of intercellular adhesion molecule 1 (ICAM-1) levels along with that of VEGF, a pro-angiogenic protein, provided insight into specific targets of the anti-cancer effects of OA against liver cancer[107]. A similar study also reported the antiproliferative effects of OA in Huh7 cells through apoptosis associated with alteration in Bcl-2 family proteins and downregulation of NF-κB and the X-linked inhibitor of apoptotic protein (XIAP)[108].
UA, isolated from Aralia decaisneana, displayed anti-hepatoma activity against HepG2 and R-HepG2 cells through apoptosis and G0/G1 cell cycle arrest. UA-mediated downregulation of cyclooxygenase-2 and upregulation of HSP105 provided additional mechanisms[109]. UA was also found to exert antiproliferative effects against HepG2 cells through activation of apoptosis accompanied by a significant decrease in Bcl-2 and survivin expression[110]. Two recent studies confirmed the anti-tumor effects of UA using HepG2 as well as additional cell lines, such as Hep3B, Huh7 and HA22T. Ancillary studies revealed mitochondrial-mediated apoptosis and inhibition of ICAM, VEGF, NF-κB and XIAP as underlying molecular mechanisms of UA action[107,108].
Waltonitone, a new pentacyclic triterpene isolated from Gentian waltonii Burkill, was found to inhibit the growth of BEL-7402 human hepatic carcinoma cells through apoptosis via the intrinsic and extrinsic cell death pathways. Waltonitone was found to upregulate the mRNA expressions of caspase-3, -8, -9, Bax, apoptotic protease activating factor 1 (apaf-1), Fas and FasL in BEL-7402 cells[111].
Two new triterpenoid saponins along with five other known triterpene compounds were extracted from Androsace umbellate and tested for their anti-tumor efficacies in HepG2 and Hep3B cells. The new triterpenoid saponins, namely 3-O-{β-D-glucopyranosyl-(1→2)-β-D-glucopyranosul-(1→4)-[β-D-glucopyranosyl-(1→2)-α-L-arabinopyranosl}-3-β-hydroxy13β,28-epoxy-16-oxo-oleanan-30-al and 3-O-β-D-xylopyranosyl-(1→2)-β-D-glucopyranosyl-(1→4)-α-L- arabinopyranosl-3β-hydroxy-13β,28-epoxy-16-oxo-oleanan-30-al, as well as the known triterpene compounds [3-O-β-D-glucopyranosyl-(1→2)-α-L-arabinopyranoslcyclamiretin A, primulanin, saxifragifolin B, C and D were found to exert cytotoxity in the aforementioned liver cancer cell lines although the mechanisms of such effects were not elucidated[112]. A new triterpene compound, 20(R), 22(ζ), 24(S)-dammar-25(26)-ene-3β, 6α, 12β, 20, 22, 24-hexanol, and three known triterpenoids, β-D-glucopyranoside,(3β,12β)-12,20-dihydroxydammar-24-en-3-yl,6-acetate, 20(R)ginsenoside Rg3, and 20(R)-ginsenoside Rh2, isolated from the leaves of Panax ginseng, exhibited various degrees of cytoxicity against HepG2 cells through p53-mediated cell cycle arrest and apoptosis via activation of the caspase signaling pathway[113].
Tetraterpenes: The effect of carotenoids, including astaxanthin and β-carotene, on the invasion of AH109A rat ascites hepatoma cells was investigated by co-culturing the hepatoma cells with rat mesentery-derived mesothelial cells. Both carotenoids inhibited AH109A invasion through antioxidant mechanisms[114]. Huang et al[115] showed that β-carotene effectively inhibited cell migration in SK-Hep-1 cell lines. The effects of astaxanthin and β-carotene on the proliferation and differentiation of isolated rat oval cells were investigated. Oval cells were isolated from the livers of rats subjected to partial hepatectomy or chronic diethylnitrosamine (DENA) treatment. Both compounds decreased the proliferation and intensified the differentiation of oval cells with increased expression of albumin, hapatoglobin and fibrinogen[116]. Recently, Yurtcu et al[117] reported the genotoxic and cytotoxic effects of β-carotene in HepG2 cells as evidenced from increased apoptosis and necrosis. A concurrent increased in thiobarbituric acid-reactive substances indicated elevated oxidative damage of β-carotene-exposed cells.
Fucoxanthin, an oxygenated carotenoid present in several types of edible seaweed, such as Laminaria japonica, Undaria pinnatifida and Hijikia fusiformis, was found to suppress the growth of HepG2 cells with inhibition of cell cycle arrest in the G0/G1 phase being the typical mechanism of action. Other mechanisms observed were decrease in the cyclin proteins D1, D3 and cdk4 with the inhibition of the phosphorylation of the retinoblastoma[118]. A separate study by Liu et al[119] described similar antiproliferative effects of fucoxanthin against SK-Hep-1 cells. Apoptosis was induced in these cells as well as cell cycle phase arrest at G0/G1. An upregulation of the connexin genes 43 (Cx43) and 32 (Cx32) provided evidence for the enhancement of gap junctional intercellular communication resulting in an increase in intracellular calcium concentrations. A decrease in the phosphorylation of ERK and JNK was also associated with fucoxanthin action.
Lycopene, the major carotenoid present in tomatoes and tomato-derived products, was found to decrease the invasive properties of AH109A rat ascites cells[114]. Park et al[120] demonstrated the inhibitory effect exerted by lycopene in Hep3B cells through mechanisms involving cell cycle arrest in the G0/G1 phase and DNA damage. This tetraterpenoid also effectively displayed antimigration and anti-invasive properties against highly invasive SK-Hep-1 cells which were associated with the increase of the metastasis suppressor gene, nm23-H1[115]. The study by Hwang and Lee[121] concluded with similar results displaying lycopene’s inhibitory effects on adhesion, invasion and migration of SK-Hep-1 cells. Additionally, these investigators showed a decrease in the activities of MMP-2 and MMP-9. Additional studies by Huang et al[122] demonstrated that lycopene strongly inhibited the invasion of SK-Hep-1 cells and this effect was mediated by inhibition of NF-κB and stimulatory protein-1 binding activity leading to a decrease in the secretion of MMP-9. Lycopene also decreased the level of ROS and insulin-like growth factor-1 (IGF-1R), albeit to a lesser extent[122].
Sesquiterpenes:α-Bisabolbol, a sesquiterpene, was found to induce cytotoxicity in HepG2 cells. Apoptosis, through both the intrinsic and extrinsic pathway, was identified as the mechanism of action exhibited by this natural compound. Associated proteins of the apoptotic pathways, namely caspase-3, -8, Bax, Bak, cyt c., p53 and Fas were found to be increased with a concomitant decrease in the Bcl-2[123].
Dehydrocostuslactone (DHE), sesquiterpene lactone, exhibits its antitumor activity against different human hepatoma cell lines, namely HepG2 and PLC/PRF/5, through a wide variety of mechanisms. The anti-proliferative effects exerted by DHE in these cell lines involved apoptotic death of liver cancer cells through the mitochondrial pathway mediated through increases in the pro-apoptotic proteins, decreases in the anti-apoptotic proteins, increases of caspase-3, -4, apoptosis-inducing factor and endonuclease G. DHE also triggered endoplasmic reticulum stress, as evidenced by changes in cytosol calcium levels, double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase phosphorylation, inositol-requiring protein 1 and CHOP/GADD153 upregulation, X-box transcription factor-1 mRNA splicing and caspase-4 activation[124].
Furanodiene, a sesquiterpene compound isolated from the essential oil of the Chinese medicinal plant Curcuma wenyujin, was found to inhibit the growth of HepG2 cells by causing cell cycle arrest at G2/M phase and inducing apoptosis related to mitochondrial transmembrane depolarization, release of cyt. c, activation of caspase-3 and cleavage of PARP. These effects were associated with the activation of p38 and inactivation of ERK1/2 MAPK signaling cascades[125].
Antiproliferative effects along with redifferentiation were induced in SMMC-7721 human hepatoma cells by two highly oxygenated bisabolane-type sesquiterpenes, namely HOBS1 and HOBS2, isolated from Cremanthodium discoideum. These sequiterpenes arrested cell growth in the G1 phase of the cell cycle, increased tyrosine-α-ketoglutarate transaminase activity, decreased α-fetoprotein (AFP) level and γ-glutamyl transferase activity[126].
Zerumbone (ZER), a cytotoxic component isolated from the wild ginger, Zingiber zerumbet Smith, was shown to induce significant antiproliferative activity against HepG2 cells through mechanisms involving an elevation of the apoptotic process with increase in the level of pro-apoptotic protein Bax and decrease of anti-apoptotic protein Bcl-2 without involving p53[127].
In vivo studies
Although the various aforementioned in vitro studies demonstrated the antiproliferative and cytotoxic effects of various terpenoids against a broad spectrum of liver cancer cells, surprisingly very few compounds from these studies have been further investigated for similar effects in vivo. Nevertheless, a large number of other natural terpenoids have been tested in vivo to show positive chemopreventive and antitumor potential against several animal models of liver cancer, with a few studies describing the lack of any beneficial effect produced by these compounds (Table 2). Most studies have been conducted in animals with chemically-induced liver tumors while other studies were conducted using xenografted animal models of liver cancer to test the potential beneficial effects of these natural terpenoid compounds.
| Terpenoids | Compounds | Effects | Mechanisms | Dose/duration | Route | Ref. |
| Monoterpenoids | Auraptene | Suppressed DENA-induced TGF-α foci and carcinoma in male F344 rats | ↑apoptosis; ↓cell proliferation | 100-500 ppm; 7 wk | Diet | Sakata et al[128] |
| Geraniol | Decreased GSTp foci and nodules during DENA-initiated and 2-AAF-promoted hepatocarcinogenesis in male Wistar rats | ↓cell proliferation; ↑apoptosis | 25 mg/100 g; 8 wk | po | Ong et al[129] | |
| Inhibited liver carcinogenesis with decrease in mean number of GSTp foci and nodule induced by DENA and promoted by PB in male Wistar rats | ↑apoptosis; ↓RhoA activation | 25 mg/100 g; 5 wk | po | Cardozo et al[130] | ||
| d-Limonene | Decreased the number of GSTp foci in DENA-initiated and Glu-P-1-promoted hepatocarcinogenesis in male F344 rats | 0.5%; 6 wk | Diet | Hirose et al[131] | ||
| Exhibited chemopreventive effects against DENA/PB hepatocarcinogenesis in male AKR mice | ↓c-jun; ↓c-myc | 5%; 80 d | Diet | Giri et al[132]; Parija et al[133] | ||
| Reduced the incidence, number and size of GSTp foci and neoplastic nodules induced by NNM in male Sprague-Dawley rats | ↑apoptosis; ↓cell proliferation | 1%, 2%; 7 wk | Diet | Kaji et al[134] | ||
| Suppressed hepatic preneoplasia and tumor growth induced by DENA in male WR rats | ↑GJIC | 3 mL/kg per day; 5 times/wk; 5.5 mo | po | Bodake et al[135] | ||
| Perillyl alcohol | Inhibited liver tumor growth induced by DENA in male F344 rats | ↑ apoptosis; ↑TGFβ; ↑M6P/IGFII R; ↑TGFβ type I, II, III R | 1% w/w for 1 wk and 2% w/w for 18 wk | Diet | Mills et al[136] | |
| Diterpenoids | Andrographolide | Suppressed the formation of BHC- induced nodules in Swiss male albino mice | ↑GSH; ↑GR; ↑GPX; ↑SOD; ↑CAT; ↓GGT; ↓GST | 5-10 mg/kg per day; 1-8 mo | po | Trivedi et al[137] |
| Reversed histomorphological and ultrastructural changes during BHC-induced hepato-carcinogenesis male Swiss mice | ↑G-6-Pase; ↑ATPase; ↑SDH; ↓SGPT; ↓SGOT; ↓ALP; ↓OCT; ↓ACP | 5,7 and 10 mg/kg; 1-8 mo | Diet | Trivedi et al[138] | ||
| Epoxy clerodane diterpene | Reduced incidence, multiplicity and size of nodules induced by DENA in male Wistar rats | ↓SGOT; ↓SGPT; ↓LDH; ↑SOD; ↑CAT; ↓GST; ↓GGT; ↑GSH; ↑GPX | 10 mg/kg per day; 8 wk | po | Dhanasekaran et al[139] | |
| Excisanin A | Suppressed tumor growth in BALB/c nude mice implanted with Hep3B cells | ↓apoptosis; ↓pAKT | 10-20 mg/kg; 12 d | ip | Deng et al[70] | |
| Geranylgeraniol | Reduced the incidence, number and size of visible nodules and GSTp foci in DENA/2-AAF hepatocarcinogenesis in male Wistar rats | ↓cell proliferation; ↓DNA strand break; ↓plasma cholesterol; ↓NF-κB p65 | 8-16 mg/kg per day; 7 wk | po | de Moura Espíndola et al[140] | |
| Triterpenoids | Bacoside A | Delayed the development and growth of neoplastic nodules induced by DENA in male Wistar albino rats | ↓lipid peroxidation; ↑Vit A; ↑Vit E; ↑GSH; ↓ROS; ↓AFP; ↓CEA; ↓5’-nucleotidase; ↓ALT; ↓AST; ↓LDH; ↓ALP; ↓GGT; ↑SOD; ↑CAT; ↑GPX; ↑GR; ↓MMP-2; ↓MMP-9 | 15 mg/kg; 16 wk | po | Janani et al[141,142] |
| Cucurbitacin B | Decreased HepG2 tumor volume and inhibited tumor growth in mice xenograft model | 26-110 µg/kg per day; 12 d | po | Zhang et al[83] | ||
| Inhibited tumor growth in BEL-7402 xenografted mice model | 0.1-3 mg/kg; twice per day for 26 d | po | Chan et al[84] | |||
| Escin | Demonstrated inhibitory effects on tumor growth in H22 tumor- bearing female Kunming mice | 1.4-2.8 mg/kg; 7 d | ip | Zhou et al[88] | ||
| Ginseng extract | Inhibited AFB1-initiated and FB-promoted hepatocarcinogenesis in female SD rats | ↓ALT; ↓AST; ↑albumin; ↓TG; ↓cholesterol; ↓LDL; ↑HDL; ↓AFP, ↓CEA, ↓MDA, ↑TAC; ↓fibrosis | 150 mg/kg; 1-12 wk | po | Abdel-Wahhab et al[145] | |
| Glycyrrhizin | Decreased the incidence of nodules and HCC induced by DENA in BALB/c mice | ↑albumin; ↑AST | 2 mg/animal; 3 d/wk for 12-32 wk | im | Shiota et al[146] | |
| Reduced the size, volume and number of preneoplastic liver lesions initiated by DENA and promoted with 2-AAF in male SD rats | 0.1% 25 mg/kg; 1 wk | po | Wan et al[147] | |||
| EC-2, EC-4 | Failed to show modifying effects against DENA-induced hepato-carcinogenesis in male F-344 rats | 1 mg/kg; 5 times a week for 2-8 wk | po | Karim et al[148] | ||
| Squalene | Failed to exhibit chemoprevention against DENA-initiated and 2-AAF- promoted hepatocarcinogenesis in male Wistar rats | ↑ plasma cholesterol | 1-1.5 g/kg; 8 wk | po | Scolastici et al[149] | |
| Ursolic acid | Inhibited the growth of H22 hepatoma implanted in male CD-1 mice | 15-30 mg/kg; 10 d | ip | Tian et al[109] | ||
| Suppressed DENA-initiated and PB- promoted hepatocarcinogenesis in male Wistar rats | ↓MDA; ↓PC; ↓membrane damage; ↑Na+K+ ATPase; ↑Mg2+ ATPase; ↑Ca2+ ATPase | 20 mg/kg per day; 6 wk | po | Gayathri et al[150] | ||
| Waltonitone | Delayed the growth of tumors in athymic nude BALB/c nu/nu mice implanted with BEL-7402 cells | ↑cleaved caspase-3, -9; ↓pro-caspase-9 | 20-50 mg/kg; Once in 2 d for 15 d | iv | Zhang et al[111] | |
| Tetraterpenoids | α-Carotene | Decreased the number of hepatomas during spontaneous hepatocarcinogenesis in male C3H/He mice | 0.005%-0.05%; 40 wk | dw | Murakoshi et al[151] | |
| Reduced the number of liver tumors in multi-organ carcinogenesis in male and female B6C3F1 mice | 0.4 mg/mouse; three times a week for 24 wk | po | Tsuda et al[152] | |||
| β-Carotene | Failed to have an effect on the number and size of preneoplastic liver foci induced by DENA/2-AAF/PB in male Wistar rats | 300 mg/kg diet or 10 mg/kg bw (3 times/wk); 3 wk | Diet; ip | Astorg et al[153] | ||
| Failed to exhibit any effect on 2-NP- or DENA-initiated hepato- carcinogenesis in male Wistar rats | 300 mg/kg diet; 3 wk | Diet | Astorg et al[154] | |||
| Did not modify DENA-initiated and PCB-promoted hepatocarcinogene-sis in female Sprague-Dawley rats | 0.5%; 12 wk | Diet | Tharappel et al[155] | |||
| Inhibited the development of preneoplastic foci and nodules initiated with DENA and promoted by 2-AAF in male Wistar rats | 70 mg/kg; alternate d for 2-8 wk | po | Moreno et al[156] | |||
| Reduced the generation of nodules and GGT foci induced by DENA/2-AAF in male Wistar rats | 70 mg/kg; 8 wk | po | Moreno et al[157] | |||
| Decreased the incidence and total number of GGT foci and nodules in DENA-initiated and 2-AAF-promoted hepatocarcinogenesis in male Wistar rats | Remodeling of lesions | 70 mg/kg; 5 wk | po | Rizzi et al[158] | ||
| Attenuated the development of GSTp foci in the resistant hepatocyte model in male Wistar rats | ↓oval cell reaction | 70 mg/kg; alternate d for 8 wk | po | Dagli et al[160] | ||
| Suppressed progression of hepatocarcinogenesis induced by DENA in male Wistar rats | ↓cell proliferation | 70 mg/kg; 8 wk | po | Moreno et al[161] | ||
| Exerted chemoprevention of initiation phage of DENA-induced hepatocarcinogenesis in male Wistar rats | ↓cell proliferation; ↓DNA damage; remodeling of lesions | 70 mg/kg; 8 wk | po | Ambrogi et al[162] | ||
| Conferred a weak chemopreventive effect against DMH-initiated hepatocarcinogenesis in male Wistar rats | 70 mg/kg; 9 wk | po | Sampaio et al[163] | |||
| Decreased the incidence, number and size of nodules induced by 2-AAF in male Sprague-Dawley rats | ↑cyt. b5; ↑CYP; ↑NADPH cyt. c reductase; ↑AHH; ↓UDPGT; ↓GST | 100 mg/kg diet; 4-20 wk | Diet | Sarkar et al[164] | ||
| Suppressed 3’-Met-DAB-induced nodulogenesis in male Sprague-Dawley rats | ↓GSH; ↓GGT; ↓GST; ↓GPX; ↓GR; ↑Vit A | 120 mg/kg diet; 4-20 wk | Diet | Sarkar et al[165] | ||
| Decreased DENA-initiated and PB-promoted nodule incidence and area in male Sprague-Dawley rats | ↓RBC protein damage; ↓lipid peroxidation; ↑G-6-Pase; ↓SOD; ↓CAT | 120 mg/kg diet; 4-20 wk | Diet | Sarkar et al[166] | ||
| Suppressed DENA-induced hepatic chromosomal aberrations in male Sprague-Dawley rats | ↓frequency of chromosomal aberrations; ↓DNA chain breaks | 120 mg/kg per day; 15-45 d | Diet | Sarkar et al[167] | ||
| Reduced the incidence, total number, multiplicity and size of nodules in DENA/PB hepato-carcinogenesis in male Sprague-Dawley rats | ↓GSH; ↓GGT; ↓GPX; ↑CYP; ↑GST | 500 mg/kg; 4-20 wk | po | Bishayee et al[168] | ||
| Decreased the number and size of GSTp foci in IQ-initiated and PB- promoted hepatocarcinogenesis in male Fischer rats | 0.02%; 8 d | Diet | Tsuda et al[169] | |||
| Inhibited the development of GSTp foci and HCC induced by EE in female Wistar rats | 250 mg/kg diet; 1-12 mo | Diet | Ogawa et al[170] | |||
| Reduced the development of GSTp foci in Glu-P-1-induced hepato-carcinogenesis in male F344 rats | 0.1%; 6 wk | Diet | Hirose et al[131] | |||
| Inhibited carcinogen-DNA adduct formation induced by IQ in male F344 rats | ↓CYP1A1; ↓CYP1A2 | 0.02%; 8 d | Diet | Uehara et al[171] | ||
| Reduced the number of liver tumors in multi-organ carcinogenesis in male B6C3F1 mice | 0.4 mg/mouse; three times a week for 24 wk | po | Tsuda et al[152] | |||
| Reduced AFB1-induced hepato-carcinogenesis in male Wistar rats | 300 ppm; 3 wk | Diet | Gradelet et al[172] | |||
| Decreased the number and volume of GSTp foci initiated by AFB1 in male Wistar rats | 300 mg/kg diet; wk | Diet | Gradelet et al[173] | |||
| Inhibited liver tumor formation induced by DMBA in both male and female toads | 0.05 mg/toad; twice a week for 12 wk | sc | Sadek et al[174] | |||
| Suppressed the formation of GSTp foci and nodules initiated with DENA and promoted by PB in male Sprague-Dawley rats | ↓GSH; ↓GST; ↑O2 consumption | 120 mg/kg; 16 wk | Diet | Chattopadhyay et al[175] | ||
| Reversed histomorphological changes during DENA-induced hepatocarcinogenesis in male Sprague-Dawley rats | ↓DNA SSB; ↓K; ↑Ca; ↓Mn; ↓Fe; ↑Cu; ↑Zn; ↓Se | 120 mg/kg; 16 wk | Diet | Chattopadhyay et al[176] | ||
| Carotenoids (astaxanthin, β-apo-8’-carotenal, canthaxanthin) | Did not modify DENA/2-AAF/PB hepatocarcinogenesis in male Wistar rats | 300 mg/kg diet; 3 wk | Diet | Astorg et al[153] | ||
| Failed to exhibit any effect on 2-NP- or DENA-initiated liver carcinogenesis in male Wistar rats | 300 mg/kg diet; 3 wk | Diet | Astorg et al[154] | |||
| Attenuated the number and size of GSTp foci induced by AFB1/2-AAF in male Wistar rats | ↓DNA single strand breaks | 300 ppm; 3 wk | Diet | Gradelet et al[172] | ||
| Reduced initiation of liver carcinogenesis by AFB1 in male Wistar rats | ↓AFB1-DNA binding; ↓DNA single strand breaks; ↑AFB1 metabolism | 300 mg/kg diet; 3 wk | Diet | Gradelet et al[173] | ||
| β-Ionone | Reduced the incidence, number and size of GSTp foci and nodules in the initial phase of DENA/2-AAF hepatocarcinogenesis in male Wistar rats | ↓cell proliferation; ↓plasma cholesterol; ↓DNA damage | 80 160 mg/kg; 7 wk | po | de Moura Espíndola et al[140] | |
| Inhibited GSTp foci and nodules during promotional phase of DENA/2-AAF hepatocarcino-genesis in male Wistar rats | ↓cell proliferation; ↓HMGCoA reductase | 160 mg/kg; 5 wk | po | Cardozo et al[130] | ||
| Lutein | Reduced the number and size of GSTp foci initiated by DENA and promoted with 2-AAF in male Wistar rats | ↓DNA strand breaks | 70 mg/kg per day; 8 wk | po | Toledo et al[181] | |
| Reduced the size of nodules during the promotional phase of DENA/2-AAF hepatocarcinogenesis in male Wistar rats | ↓DNA damage | 70 mg/kg; 2-6 wk | po | Moreno et al[182] | ||
| Lycopene | Failed to modify AFB1-induced hepatocarcinogenesis in male Wistar rats | 300 ppm; 3 wk | Diet | Gradelet et al[172] | ||
| Exhibited no effect on the initiation of hepatocarcinogenesis induced by AFB1 in male Wistar rats | 300 mg/kg diet; 3 wk | Diet | Gradelet et al[173] | |||
| Failed to influence the risk of developing spontaneous HCC in male Long-Evans Cinnamon rats | 0.005% w/w; 70 wk | Diet | Watanabe et al[183] | |||
| Decreased the volume of GGT and GSTp foci in DENA-initiated and 2-AAF-promoted liver carcinogenesis in male Wistar rats | ↓CYP2E1 | 300 mg/kg diet; 3 wk | Diet | Astorg et al[154] | ||
| Attenuated the number and size of GSTp foci initiated by DENA and promoted with 2-AAF in male Wistar rats | ↓DNA strand breaks | 70 mg/kg per day; 8 wk | po | Toledo et al[181] | ||
| Decreased the number of GSTp foci in DENA-initiated and PCB-promoted hepatocarcinogenesis in female Sprague-Dawley rats | 1%; 12 wk | Diet | Tharappel et al[155] | |||
| Inhibited DENA-initiated and NASH-promoted GSTp foci development in male Sprague-Dawley rats | ↓PCNA; ↓cyclinD1; ↓lipid peroxidation; ↑Nrf2; ↑HO-1; ↓pERK; ↓NF-κB p65 | 15 mg/kg per day; 6 wk | Diet | Wang et al[184] | ||
| Tomato extract | Reduced the number of GSTp foci initiated by DENA and promoted by NASH in male Sprague-Dawley rats | ↓PCNA; ↓cyclinD1; ↓TNF-α; ↓IL-1β; ↓IL-12; ↓lipid peroxidation; ↓CYP2E1; ↓pERK; ↓NF-κB | 250 mg/kg per day; 6 wk | Diet | Wang et al[184] | |
| Sesquiterpenoids | Dehydrocostus-lactone | Inhibited tumor growth in nude mouse implanted with PLC/PRF/5 cells | ↑apoptosis; ↑pPERK; ↑IRE-1 | 10 mg/kg per day; 45 | ip | Hsu et al[124] |
| Farnesol | Suppressed the development of GSTp foci and nodules initiated with DENA and promoted by 2-AAF in male Wistar rats | ↓cell proliferation; ↓DNA damage; ↓plasma cholesterol; ↓HMG-CoA reductase | 250 mg/kg; 8 wk | po | Ong et al[129] | |
| Zerumbone | Reduced DENA/2-AAF- induced hepatocarcinogenesis in Sprague- Dawley rats | ↓ALP; ↓ALT; ↓AST; ↓AFP; ↓lipid peroxidation; ↓GSH; ↓PCNA; ↑Bax; ↓Bcl-2 | 15, 30 or 60 mg/kg; twice a week for 11 wk | ip | Taha et al[186] |
Monoterpenes: Auraptene, a citrus antioxidant, was found to be effective in suppressing the DENA-induced hepatocarcinogenesis in male F344 rats upon dietary supplementation. A dose range of 100-500 ppm over a period of 7 wk inhibited the development of DENA-induced transforming growth factor (TGF)-α-positive foci and placental glutathione S-transferase (GSTp)-positive foci through mechanisms that include increase in apoptosis and decrease in cell proliferation[128].
Atleast two studies on the isoprenoid geraniol have contributed to its chemopreventive effects against hepatocarcinogenesis. Ong et al[129] found that oral geraniol treatment (250 mg/kg) for 8 wk in Wistar rats, initiated with DENA and promoted by 2-acetylaminofluorene (2-AAF), decreased both hepatic remodeling and persistent GSTp-positive preneoplastic lesions. A recent and similar study by Cardozo et al[130] observed the same chemopreventive benefits of geraniol in male Wistar rats initiated with DENA promoted by phenobarbital (PB). Geraniol was found to suppress liver carcinogenesis by inhibiting the promotion phase through induction of apoptosis and reduction of hepatic membrane protein RhoA.
A number of in vivo studies have been performed with d-limonene showing its efficacy against HCC. From earlier studies, it has been shown that 0.5% d-limonene in diet has been responsible for a 32% inhibition of the GSTp foci in DENA-initiated and 2-amino-6-methyldipyrido [1,2-α:3’,2’-d] imidazole (Glu-P-1)-promoted hepatocarcinogenesis in male F-344 rats[131]. Later studies demonstrated that d-limonene’s chemopreventive efficacy arises from its capability to block alterations in the level of oncogene expression both at the RNA and protein levels brought about by DENA alone or along with PB in the liver of AKR mice. Inhibition of the overexpression of c-myc and c-jun proteins, the characteristics of DENA hepatocarcinogenesis, is one mechanism by which d-limonene exhibits its anticarcinogenic effect[132]. According to a subsequent study, a potential role of d-limonene might involve modulation of the ying yang 1 protein which was found to be correlated with c-myc in DENA-induced hepatocarcinogenesis[133]. Kaji et al[134] confirmed d-limonene’s antitumor effect in Sprague Dawley rat hepatocarcinogenesis induced by N-nitrosomorpholine. d-Limonene was found to reduce the incidence, number as well as the size of GSTp foci without the involvement of p21RAS plasma membrane association. In 2002, the chemopreventive effect of orange oil (containing 90%-95% d-limonene) was tested and proved by Bodake et al[135]. Oral administration of orange oil for more than 5 mo following DENA treatment in male Wistar rats significantly suppressed the growth of liver tumors with the restoration of the normal phenotype and upregulation of gap junctional complexes.
Significant inhibition of liver tumors through a marked increase in the frequency of apoptosis was achieved by perillyl alcohol upon treatment of F344 rats challenged with DENA. In addition, the elevated expressions of mannose 6-phosphate/IGF receptor II and TGF-β type I, II and III receptors were also demonstrated as underlying mechanisms of perillyl alcohol’s chemopreventive activity[136].
Diterpenes: The antihepatocarcinogenic effect exerted by the antioxidant andrographolide extracted from Andrographis paniculata (Kalmegh) was demonstrated in male Swiss-albino mice challenged with benzene hexachloride (BHC). The oral administration of andrographolide resulted in suppression of BHC-induced hepatic nodules. Spectrometric analysis showed significant increases in glutathione (GSH) and antioxidant enzyme activities of glutathione reductase (GR), glutathione peroxidase (GPX), superoxide dismutase (SOD) and catalase (CAT) with concurrent decreases in γ-glutamyl transpeptidase (GGT) and GST[137]. A follow-up study confirmed the potent anti-tumor activity possessed by andrographolide. This study indicated a reversal of the histomorphological and ultrastructural changes induced by BHC, along with improved glycogenesis in the liver due to increased activities of glucose-6-phosphate and phosphorylase. Regenerative effects elicited by andrographolide were the result of decreased activities of serum glutamate pyruvate transaminase, serum glutamate oxalate transaminase, alkaline phosphatase (AP), ornithine carbomoyl transferase and acid phosphatase, all of which are markers of liver damage[138].
A recent investigation by Dhanasekaran et al[139] on the epoxy clerodane diterpene extracted from Tinospora cordifolia proposed increases in the hepatic antioxidant status of Wistar rats administered with DENA to induce HCC. Treatment for 8 wk at a dose of 8 mg/kg per day resulted in reduced incidence, multiplicity and size of hepatic nodules. The treatment was instrumental in increasing the level of detoxifying enzymes and bringing down the elevated levels of serum transaminase and hepatic marker enzymes to near normal.
Excisanin A, a diterpenoid compound extracted from Isodon MacrocalyxinD, was found to be an effective and potent tumor growth inhibitor in BALB/c nude mice implanted with Hep3B cells. A daily intraperitoneal (ip) injection of 10-20 mg/kg of excisanin A in this xenografted mouse model and the subsequent analysis of the tumor samples after sacrifice established the inhibition of the AKT signaling pathway in tumor cells. Upon performing the terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay on frozen liver sections, TUNEL-positive cells were identified, indicating apoptotic activity in the tumor cells[70].
de Moura Espíndola et al[140] studied the anticarcinogenic effects of geranylgeraniol and showed the potential usefulness of this diterpenoid as a chemopreventive agent in hepatocarcinogenesis. Geranylgeraniol treatment administration to male Wistar rats subjected to DENA/2-AAF regimen resulted in significant reduction of nodule incidence and size. The antitumor activity of geranylgeraniol was closely interlinked to decreases in cell proliferation and DNA damage. Decreases in the transcription factor NF-κB and plasma cholesterol levels were also interesting hallmarks of the chemopreventive activity exhibited by geranylgeraniol in the initiation phase of hepatocarcinogenesis.
Triterpenes: Bacoside A, a triterpenoid saponin isolated from Bacopa monniera Linn., was studied for its chemopreventive potential against rat liver carcinogenesis induced by DENA. Treatment with bacoside A demonstrated the delayed development and growth of neoplastic nodules. The levels of AFP, carcinoembryonic antigen (CEA), aspartate transaminase (AST), alanine transaminase (ALT), ROS, lipid peroxides, GSH antioxidant enzymes, SOD and CAT, vitamins A and E and MMP-2 and -9 were decreased in the bacoside A-treated rats[141,142].
Two groups of researchers have investigated the chemopreventive effects of cucurbitacin B using xenografted mouse models of liver cancer. Zhang et al[83] showed the decrease in tumor volume along with tumor growth inhibition exerted by cucurbitacin B in mice xenografted with HepG2 cells, whereas Chan et al[84] demonstrated the inhibitory activity of cucurbitacin B on the growth of xenografted BEL-7402 tumor. Nevertheless, the in vivo mechanisms of such antitumor effects of cucurbitacin B were not studied.
Escin, a natural mixture of triterpene saponins, was evaluated by Zhou et al[88] to determine its antitumor efficacy against mice xenografted with H22 malignant HCC cells. Therapy with escin at a concentration of 1.4 mg/kg per day or 2.8 mg/kg per day for 7 d was conducted via ip injections in these animals. The highest dose of escin therapy was effective in significantly lowering the tumor weights compared to the controls.
Korean ginseng is one of the most widely used medicinal plants, particularly in traditional oriental medicine, and has a wide range of pharmacological and physiological actions[143,144]. These along with other beneficial effects prompted Abdel-Wahhab et al[145] to evaluate the chemopreventive effects of ginseng extract (GE) against precancerous lesions in female Sprague-Dawley rats treated with aflatoxin B1 (AFB1) and fumonisin. A dose of 150 mg/kg of GE for 12 wk inhibited hepatocarcinogenesis in these rats and decreased the levels of ALT, AST, CEA, malondialdehyde, and AFP with concurrent increases in serum albumin and high density lipoprotein levels compared to the positive controls. This study concluded that GE administration before or after treatment with mycotoxins could be effective in preventing hepatocellular carcinogenesis.
Shiota et al[146] earn the credit as the first group of researchers to have used glycyrrhizin as a chemopreventive agent against HCC in an experimental animal study. Their work demonstrated that glycyrrhizin decreased nodule incidence and HCC induced by DENA. Serum albumin and AST, markers of liver function, were found to be normalized following glycyrrhizin treatment. A later study conducted by Wan et al[147] evaluated the hepatoprotective and antihepatocarcinogenic effects of glycyrrhizin. This study reported the reduction in size, volume and number of hepatic lesions initiated by DENA and promoted with 2-AAF in male Sprague Dawley rats.
In contrary to all the above studies on triterpenes, the study conducted by Karim et al[148] on two fernane-type triterpenes, namely EC-2 and EC-4, did not observe any modifying effects on DENA-induced liver cancer in rats. No significant differences were observed in the number and area/cm2 of GSTp-positive foci induced by DENA between the treatment and control groups. Neither was there any treatment-related variation in cell proliferation as demonstrated by 5-bromo-2’-deoxyuridine (BrdU) labeling.
Squalene, a triterpenoid present in olive oil, also failed to exhibit chemopreventive activities against DENA/2-AAF rat hepatocarcinogenesis model. Squalene induced an increase in levels of plasma cholesterol in the treated rats leading to hypercholesterolemia[149].
The in vivo chemotherapeutic efficacy of UA was first demonstrated by Tian et al[109] who used CD-1 mice implanted with H22 hepatoma cell lines. Results showed an inhibition in the growth of hepatoma in UA-treated mice. The drawback on using UA as a potential anti-hepatoma agent, as presented in this study, was the poor water solubility of UA which confines its potential use. A later study evaluated UA’s efficacy in DENA-initiated and PB-promoted hepatocarcinogenesis rats. Oral administration of UA at 20 mg/kg per day for 6 wk suppressed hepatocellular carcinogenesis and decreased lipid peroxidation and protein carbonyls by about 52%, along with reversing the membrane damage and elevated glycoprotein levels[150].
Athymic nude BALB/c nu/nu mice implanted with BEL-7402 cells was used to generate the HCC model by Zhang et al[111] in order to study the chemopreventive characteristics exhibited by waltonitone, a new ursane-type pentacyclic triterpene isolated from Gentian waltonii Burkill. Results of this study showed a delay in tumor growth and inhibition of tumor weight in waltonitone-treated mice with HCC compared to the control. Western blotting analysis of tumor tissue indicated an increase in expression of proapoptotic proteins, such as cleaved caspase-3 and caspase-9, suggesting the involvement of the mitochondrial apoptotic pathway in the chemopreventive effect of waltitonine.
Tetraterpenes: Tetraterpenes represent one of the most widely studied classes of terpenes for the chemopreventive and therapeutic treatment potential for HCC. Murakoshi
et al[151] described a study evaluating α-carotene’s chemopreventive effects against spontaneous liver carcinogenesis. Their study showed that α-carotene, obtained from palm oil, significantly decreased the number of hepatomas. α-carotene’s chemopreventive capability was confirmed by Tsuda et al[152] when they tested its effects (0.4 mg/animal) in both male and female B6C3F mice injected intraperitoneally with DENA and N-methyl-N-nitrosourea to produce a multiorgan carcinogenesis model. The results demonstrated that α-carotene reduced the number of liver tumors in both sexes.
Astorg et al[153,154] demonstrated in two studies that β-carotene failed to exhibit any chemopreventive activity against a variety of chemically-induced hepatocarcinogenesis models in rats. This includes absence of effects on the number and size of preneoplastic foci in male Wistar rats that were induced with DENA/2-AAF, DENA or 2-nitropropane (2-NP) to develop preneoplastic liver foci. A similar study, conducted by Tharappel et al[155] using the DENA hepatocarcinogenesis model in female Wistar rats, showed that β-carotene treatment for 12 wk failed to produce any modifying effect, reiterating the results obtained by the previous investigators. Contrary to these studies, Moreno et al[156], have provided substantial evidence, over two decades, on the chemopreventive as well as chemotherapeutic efficacies of β-carotene. In one of the early studies, a dose of 70 mg/kg of β-carotene for 2-8 wk
in the resistant hepatocyte model significantly reduced the incidence, multiplicity as well as the total number and size of hepatocyte nodules. β-carotene also attenuated the number of foci, average focal area and percentage of liver parenchyma occupied by these foci. These inhibitory effects were primarily exerted in the initiation phase of the hepatocarcinogenic process[156]. In another study, chronic administration of β-carotene throughout the study resulted in a drastic reduction in hepatocyte nodule incidence, total number of nodules and nodule multiplicity as well as the number and size of GGT-positive foci in DENA-initiated and 2-AAF-promoted hepatocarcinogenesis in rats[157]. A similar study described decreases in the incidence and total number of hepatocyte nodules and GGT-positive foci upon treatment with β-carotene during early promotional phase of DENA-initiated 2-AAF-promoted hepatocarcinogenesis in rats. Additionally, this study showed remodeling of persistent hepatic lesions as a mechanism by which β-carotene exhibited its chemopreventive activity[158]. Along with the initiated hepatocytes, another group of cells known as “oval cells”, proliferate in the liver during rat and mouse hepatocarcinogenesis[159]. Dagli et al[160] showed that β-carotene was responsible for attenuating the development of GSTp foci along with decreasing oval cell reaction in Wistar rats submitted to the resistant hepatocyte model of carcinogenesis. Moreno et al[161] reported that β-carotene treatment during progression of chemically-induced liver cancer exhibited a lower of incidence of neoplastic lesions with reduction in hepatic BrdU labeling indexes indicating an inhibitory action of β-carotene on cell proliferation. β-Carotene’s chemopreventive activity was further explored during the initial phases of DENA-induced hepatocarcinogenesis in rats, with results showing a decrease in cell proliferation and DNA damage in conjunction with remodeling of GSTp-positive lesions as likely mechanisms of action[162]. In a study with the 1, 2-dimethylhydrazine-induced hepatocarcinogenesis rat model, β-carotene in conjunction with anticancer drug, 5-azacytidine[163]. Conferred only weak chemoprotective effects. Studies by Sarkar and associates[164-167] using three different chemically-induced hepatocarcinogenesis models added extensively to the existing knowledge on mechanism-based chemopreventive properties of β-carotene. A treatment dose of β-carotene at 100-120 mg/kg per day from 4 to 20 wk in all these studies notably decreased nodulogenesis and hepatic chromosomal aberrations in male Sprague Dawley rats. This group of researchers provided extensive evidence of the antioxidant effects of β-carotene as the underlying mechanism of action. Decreases in hepatic DNA strand breaks, frequency of chromosomal aberrations, red blood cell protein damage and lipid peroxidation were observed. There were also decreases in hepatic GSH, GGT, GST, GPX, GR, SOD and CAT. Increased cytochrome b5, vitamin A and glucose-6-phosphatase (G6Pase) levels also appeared as potential mechanisms by which β-carotene exhibited its chemopreventive activity. Bishayee et al[168] reported β-carotene to have a superior chemopreventive action to that of retinoic acid. As described in this study, β-carotene greatly reduced the incidence and multiplicity of nodules in DENA/PB hepatocarcinogenesis in rats with decreased levels of GSH, GGT, GPX and increased levels of cytochrome P-450 (CYP) and GST. Several other studies also demonstrated the chemopreventive efficacy exerted by β-carotene based on reduced number and size of GSTp foci. Interestingly, all these studies showed positive results in several rat hepatocarcinogenesis model induced by IQ, ethynlyestradiol or GLU-P-1[140,169,170]. Inhibition in the formation of IQ-DNA adducts with decreases in CYPA1A2 protein expressions was a characteristic effect of β-carotene treatment in male F344 rats challenged with IQ[171]. Utilizing a multiorgan carcinogenesis model in B6C3F1 mice, Tsuda et al[152] were successful in showing a reduction in the number of liver tumors with β-carotene treatment. However, the results of this study were only significant in male B6C31 mice and not in the female mice model. Gradelet et al[172,173] investigated the chemopreventive effect of dietary β-carotene during initiation of AFB1-induced hepatocarcinogenesis in male Wistar rats. They demonstrated a reduction in hepatocarcinogenesis as evidenced from a decrease in number and volume of GSTp foci possibly due to deactivation of AFB1 metabolism towards detoxification pathways. Results of an interesting study on toad liver carcinogenesis suggested β-carotene inhibition in liver tumor formation induced by 7, 12, dimethylbenz(a)anthracene in female toads[174]. Chattopadhyay et al[175,176] conducted two similar studies that involved β-carotene treatment in male Sprague-Dawley rats initiated with DENA. The authors effectively proved β-carotene’s chemopreventive capability as indicated by suppression in the formation of GSTp foci along with reversal of hepatic histomorphological changes in DENA-initiated animals[175]. Mechanistic studies revealed that dietary supplementation of β-carotene decreased the levels of hepatic GSH, GST and DNA single strand breaks[175,176]. The latter study also investigated various mineral levels following β-carotene treatment and found that levels of calcium, copper and zinc were increased while levels of manganese, iron, potassium and selenium appeared to have decreased in the blood of experimental animals[176]. Another interesting observation from these studies was that anticarcinogenic micronutrient vanadium[177] was found to augment the effects of β-carotene[175,176].
Studies have been also been performed with various carotenoid triterpenes, such as β-apo-8’carotenal, astaxanthin and canthaxanthin, to determine their possible chemopreventive efficacies against liver cancer. However, only two studies were successful in achieving any beneficial effects exerted by these carotenoids. Astorg et al[153,154] conducted two studies with canthaxanthin and astaxanthin, and did not observe any preventive effects exerted by them against chemically-induced hepatocarcinogenesis in male Wistar rats. A dietary treatment regimen of either carotenoid at 300 mg/kg for 3 wk did not modify the number and size of GGT or GSTp foci in rats subjected to DENA or 2-NP hepatocarcinogenic regimen. On the contrary, two other studies[172,173] demonstrated chemopreventive effects of dietary β-apo-8’carotenal, astaxanthin and canthaxanthin against AFB1-initiated hepatocarcinogenesis in rats as evidenced by a reduction in the number and size of GSTp-positive preneoplastic foci. An improved detoxification of AFB1 metabolism has been proposed as the basis of the observed protective effects of these carotenoids against rat liver carcinogenesis.
Dietary isoprenic derivatives, including β-ionone, a cyclic isoprenoid present in grapes and wine, represent a promising class of chemopreventive agents[178-180]. β-Ionone was found to inhibit hepatic preneoplastic lesions with a decrease in cell proliferation, inhibition of plasma cholesterol and amelioration of DNA damage during the initial phases of hepatocarcinogenesis initiated with DENA and promoted by 2-AAF in rats[140]. A later study conducted using a similar hepatocarcinogenesis model documented the antihepatocarcinogenic effects of β-ionone during the promotional phase with a concurrent inhibition of cell proliferation and modulation of HMGCoA reductase[130]. These two studies warrant further investigations into the chemopreventive efficacies of β-ionone against liver cancer.
The carotenoid lutein was successfully shown to be an effective chemopreventive agent against DENA-initiated and 2-AAF promoted hepatocarcinogenesis by two studies in Wistar rats. Lutein when administered at 70 mg/kg per day for 8 wk played an important role in reducing the number and size of GSTp foci with a concurrent decrease in DNA damage[181]. Moreno et al[182] however showed that lutein presented its inhibitory actions during promotional stage but not during the initiation phase of hepatocarcinogenesis. During the initiation phase lutein did not inhibit nor induce hepatic preneoplastic lesions or DNA damage. However, treatment during the promotional phase inhibited the size of nodules with reduced DNA damage. Accordingly, lutein has been classified as a suppressing agent.
Another carotenoid studied with great expectations for its chemopreventive potential is lycopene. While several laboratories reported potential beneficial effects, others demonstrated the lack of effects exerted by lycopene. Studies conducted by Gradelet et al[172,173] showed that dietary lycopene treatment failed to show any modifying effects, either on the initiation or on the promotional phases of hepatocarcinogenesis induced by AFB1 in male Wistar rats. Similar results were reported by Watanabe et al[183]
indicating that lycopene failed to produce any chemopreventive effect against spontaneous HCC in Long-Evans Cinnamon rats. Few other studies conducted with different treatment regimens of lycopene arrived at conclusions that were contrary to the aforementioned studies. Astorg et al[154] showed significant decreases in GSTp foci including decrease in liver volume occupied by the foci. They also concluded that lycopene’s chemopreventive effects were due to its modulating effect on the liver enzyme activating DENA, namely CYP2E1. Three other research groups[155,181,184] conducted their studies using several chemical hepatocarcinogenesis model and observed similar end points as Astorg et al[154]. All three research groups observed the chemopreventive effects of lycopene on hepatocellular carcinogenesis induced by DENA in rats as indicated by inhibition of GSTp foci. Wang et al[184] confirmed the efficacy of lycopene against nonalcoholic steatohepatitis (NASH)-promoted hepatocarcinogenesis and provided several molecular mechanisms, such as lowering of protein expression of proliferating cell nuclear antigen, cyclin D1, and NF-κB as well as induction in nuclear NF-E2 related factor-2 and heme oxygenase-1 protein expressions. The efficacy of tomato extract (TE) as a chemopreventive agent against DENA-initiated NASH-promoted hepatocarcinogenesis in male rats was also investigated. TE supplemented in the diet at a concentration of 250 mg/kg per day for 6 wk produced similar inhibitory effects to lycopene on the development of GSTp foci. Ancillary studies showed decreases in CYP2E1, inflammatory foci and mRNA expression of proinflammatory cytokines, namely TNF-α, IL-1β and IL-12.
Sesquiterpenes: The chemotherapeutic effects of DHE, a medicinal plant-derived sesquiterpene lactone, have been investigated in male nude mice subcutaneously (sc) injected with PLC/PRF/5 human liver cancer cells. DHE treatment (10 mg/kg per day carried out for 45 d) elicited a significant decrease in tumor volume. Tumor samples analyzed from DHE-treated animals displayed TUNEL-positive cells indicative of apoptosis within the tumor. ER-stress related proteins, namely inositol-requiring protein-1 and phospho-PKR-like ER kinase, were found to be increased in DHE-treated animals[124].
Ong et al[129] studied the chemopreventive effects of the sesquiterpene farnesol using Wistar rats submitted to the RH model of hepatocarcinogenesis. Farnesol inhibited the incidence and mean number of visible hepatocyte nodules, as well as the size of total, persistent and remodeling GSTp-positive preneoplastic lesions. Farnesol was also found to inhibit tumor cell proliferation and HMG-CoA reductase activity, thus reducing synthesis of cholesterol and intermediates of the mevalonate pathway, such as farnesyl pyrophosphates[185]. The study also highlighted that the chemopreventive effects exerted by farnesol did not involve the farnesoid X activated receptor.
ZER, a monosesquiterpene, was assessed for its chemopreventive properties against DENA-initiated and 2-AAF-promoted liver carcinogenesis in rats. ZER was administered (ip) for at 15-60 mg/kg body weight for
11 wk. Histopathological observation showed the antihepatocarcinogenic effects of ZER. Serum levels of ALT, AST, AP and AFP were found to be lower in ZER-treated rats. ZER treatment also reduced oxidative stress and proliferation accompanied with apoptosis. An increase in Bax and decrease in Bcl-2 protein expression have been postulated as the mechanistic basis of proapoptotic properties of ZER during experimental hepatocarcinogenesis[186].
Liver cancer remains the third leading cause of cancer mortality throughout the world with few therapeutic options to combat its prevalance. Natural compounds obtained from different sources such as fruits, vegetables, herbs and even fungi, open up a novel and exciting prospective on liver cancer prevention as well as treatment. Among the various secondary metabolites produced by plants and other organisms, terpenoids have emerged as a promising group of phytochemicals. Terpenoids selectively kill liver cancer cells with a pleiotropic mode of action while sparing normal cells. The various studies, conducted in vitro and in vivo by numerous research groups, describe the use of these phytoconstituents as potential chemopreventive and therapeutic agents in the fight against liver cancer. Based on available data, the terpenoids which may have the greatest therapeutic potential for liver cancer prevention and treatment include d-limonene, cucurbitacin B, ursolic acid, β-carotene and lycopene (Figure 1).
Terpenoids have not only been shown to exert biological activities that are a far cry from any single chemotherapeutic drug but have also exhibited little or no toxicity. These phytochemicals have been found to induce a wide spectrum of activities such as reduction in oxidative stress, suppression of inflammation, induction of apoptosis, regulation of cell cycle, inhibition of cell proliferation and also modulation of multiple signal transduction pathways. In essence, these pleiotropic mechanisms could explain their antineoplastic properties against liver cancer.
From the studies discussed here, it is apparent that terpenoids of various classes are unique as they have characteristic mechanisms of action. However, in most of the studies, individual compounds have been tested against in vitro or in vivo liver cancer models. There remains the indefinite yet unending possibility that there exists better preventive or therapeutic potential in the synergistic action of these terpenoid molecules. Emerging studies have shown that a combination of phytochemicals may be more effective against cancer than individual components[187-191]. Further studies are needed to explore the full potential of these multifunctional terpenoids in the combinational setting.
Possible combinations of two or more compounds could prove to be much more effective, in many cases, where use of a single agent is not effective enough. However, further studies are needed in order to provide more insight into this assumption. It is well known that the combination of several chemotherapeutic drugs offers the possibility of lowering doses of individual compounds, consequently reducing unwanted adverse effects. Considering these advantages, terpenoids may be used in combination with other chemotherapeutic drugs and radiation therapy to enhance their therapeutic efficacy while limiting chemo- and radio-therapy-associated unwanted side effects. However, more studies are required to validate these premises.
Scanning of pertinent and extensive literature reveals a large number of in vitro studies demonstrating the cytotoxicity of terpenoid molecules against various liver cancer cells, yet very few compounds have been evaluated in preclinical animal models of liver cancer. The same follows suit for those natural terpenoid compounds effectively tested in animal models moving up to phase I clinical trials. One of the reasons for the limited number of preclinical liver cancer studies on triterpenoids, including lack of in vivo studies on agents which have already showed efficacy in cell culture systems, could be that most terpenoids are insoluble in aqueous media limiting their bioavailability in the body, an important aspect for in vivo efficacy. One approach to enhance the water solubility of triterpenoids could be the structural modification of naturally occurring compounds to generate more water-soluble analogs. Several other possibilities of improving the hydrophilicity of triterpenoids include the design and generation of formulations containing cyclodextrin complexes, liposomes, colloids, micelles as well as nanoparticles[192-194].
As highlighted in this review, a large number of investigations have unraveled many unique biological properties of terpenoids with the goal of evaluating their clinical potential in the prevention and therapy of liver cancer. Nevertheless, a considerable amount of work remains to be done, such as identification of novel target proteins and intercellular pathways in which they function, and development of selective end-points and surrogate biomarkers for evaluating efficacy. Long-standing epidemiological studies and well-designed clinical trials are also necessary. In conclusion, substantial experimental results from in vitro and in vivo studies presented in this review suggest that terpenoids are novel candidates in the chemopreventive and chemotherapeutic strategies to combat liver cancer.
The authors sincerely thank Altaf S Darvesh, PhD, for carefully review of the manuscript and providing valuable comments, and Werner J Geldenhuys, PhD, for assistance with the chemical structures.
Peer reviewer: Tanios Bekaii Saab, MD, Medical Director, Gastrointestinal Oncology, Assistant Professor of Medicine and Pharmacology, Arthur James Cancer Hospital, The Ohio State University, B407 Starling Loving Hall, 320 West 10th Avenue, Columbus, OH 43210, United States
S- Editor Zhang SJ L- Editor Hughes D E- Editor Zheng XM
| 1. | Nakashima T, Okuda K, Kojiro M. Pathology of hepatocellular carcinoma in Japan: two hundred thirty-two consecutive caws autopsied in 10 years. Cancer. 1983;51:863-877. [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 102] [Reference Citation Analysis (0)] |
| 2. | Bosch FX, Ribes J, Díaz M, Cléries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5-S16. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1799] [Cited by in F6Publishing: 1760] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 3. | Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970-2002. JAMA. 2005;294:1255-1259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 666] [Cited by in F6Publishing: 643] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 4. | Sherman M. Hepatocellular carcinoma: epidemiology, risk factors, and screening. Semin Liver Dis. 2005;25:143-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 301] [Cited by in F6Publishing: 324] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 5. | El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27-S34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 738] [Cited by in F6Publishing: 702] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 6. | Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10002] [Cited by in F6Publishing: 10353] [Article Influence: 739.5] [Reference Citation Analysis (0)] |
| 7. | Raoul JL. Natural history of hepatocellular carcinoma and current treatment options. Semin Nucl Med. 2008;38:S13-S18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Schütte K, Bornschein J, Malfertheiner P. Hepatocellular carcinoma--epidemiological trends and risk factors. Dig Dis. 2009;27:80-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 305] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 9. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3241] [Cited by in F6Publishing: 3212] [Article Influence: 153.0] [Reference Citation Analysis (0)] |
| 10. | Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology. 2002;35:519-524. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 837] [Cited by in F6Publishing: 829] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 11. | Arii S, Yamaoka Y, Futagawa S, Inoue K, Kobayashi K, Kojiro M, Makuuchi M, Nakamura Y, Okita K, Yamada R. Results of surgical and nonsurgical treatment for small-sized hepatocellular carcinomas: a retrospective and nationwide survey in Japan. The Liver Cancer Study Group of Japan. Hepatology. 2000;32:1224-1229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 568] [Cited by in F6Publishing: 532] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 12. | Sato S, Shiratori Y, Imamura M, Teratani T, Obi S, Koike Y, Imai Y, Yoshida H, Shiina S, Omata M. Power Doppler signals after percutaneous ethanol injection therapy for hepatocellular carcinoma predict local recurrence of tumors: a prospective study using 199 consecutive patients. J Hepatol. 2001;35:225-234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomised trial. Lancet. 2000;356:802-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 630] [Cited by in F6Publishing: 626] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 14. | Wayne JD, Lauwers GY, Ikai I, Doherty DA, Belghiti J, Yamaoka Y, Regimbeau JM, Nagorney DM, Do KA, Ellis LM. Preoperative predictors of survival after resection of small hepatocellular carcinomas. Ann Surg. 2002;235:722-30; discussion 730-1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 158] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 15. | Jonas S, Bechstein WO, Steinmüller T, Herrmann M, Radke C, Berg T, Settmacher U, Neuhaus P. Vascular invasion and histopathologic grading determine outcome after liver transplantation for hepatocellular carcinoma in cirrhosis. Hepatology. 2001;33:1080-1086. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 723] [Cited by in F6Publishing: 684] [Article Influence: 29.7] [Reference Citation Analysis (0)] |
| 16. | Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001;33:1394-1403. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1594] [Cited by in F6Publishing: 1586] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 17. | Llovet JM. Updated treatment approach to hepatocellular carcinoma. J Gastroenterol. 2005;40:225-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 388] [Cited by in F6Publishing: 383] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 18. | Lencioni R, Pinto F, Armillotta N, Bassi AM, Moretti M, Di Giulio M, Marchi S, Uliana M, Della Capanna S, Lencioni M. Long-term results of percutaneous ethanol injection therapy for hepatocellular carcinoma in cirrhosis: a European experience. Eur Radiol. 1997;7:514-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 132] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Tanaka Y, Hanada K, Mizokami M, Yeo AE, Shih JW, Gojobori T, Alter HJ. A comparison of the molecular clock of hepatitis C virus in the United States and Japan predicts that hepatocellular carcinoma incidence in the United States will increase over the next two decades. Proc Natl Acad Sci U S A. 2002;99:15584-15589. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 249] [Cited by in F6Publishing: 260] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 20. | Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3252] [Cited by in F6Publishing: 3170] [Article Influence: 137.8] [Reference Citation Analysis (0)] |
| 21. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9016] [Cited by in F6Publishing: 9507] [Article Influence: 594.2] [Reference Citation Analysis (1)] |
| 22. | Lu SC. Where are we in the chemoprevention of hepatocellular carcinoma? Hepatology. 2010;51:734-736. [PubMed] [Cited in This Article: ] |
| 23. | Je Y, Schutz FA, Choueiri TK. Risk of bleeding with vascular endothelial growth factor receptor tyrosine-kinase inhibitors sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. Lancet Oncol. 2009;10:967-974. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 215] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 24. | Sporn MB, Suh N. Chemoprevention of cancer. Carcinogenesis. 2000;21:525-530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 331] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 25. | Jordan VC. Chemoprevention of breast cancer with selective oestrogen-receptor modulators. Nat Rev Cancer. 2007;7:46-53. [PubMed] [Cited in This Article: ] |
| 26. | Castrellon AB, Glück S. Chemoprevention of breast cancer. Expert Rev Anticancer Ther. 2008;8:443-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 27. | Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71:1397-1421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1174] [Cited by in F6Publishing: 1068] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 28. | Russo GL. Ins and outs of dietary phytochemicals in cancer chemoprevention. Biochem Pharmacol. 2007;74:533-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 213] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 29. | Naithani R, Huma LC, Moriarty RM, McCormick DL, Mehta RG. Comprehensive review of cancer chemopreventive agents evaluated in experimental carcinogenesis models and clinical trials. Curr Med Chem. 2008;15:1044-1071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Kaefer CM, Milner JA. The role of herbs and spices in cancer prevention. J Nutr Biochem. 2008;19:347-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 270] [Cited by in F6Publishing: 234] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 31. | Moiseeva EP, Manson MM. Dietary chemopreventive phytochemicals: too little or too much? Cancer Prev Res (Phila). 2009;2:611-616. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 32. | Huang WY, Cai YZ, Zhang Y. Natural phenolic compounds from medicinal herbs and dietary plants: potential use for cancer prevention. Nutr Cancer. 2010;62:1-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 528] [Cited by in F6Publishing: 466] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 33. | Bishayee A, Darvesh AS. Oxidative stress in cancer and neurodegenrative diseases: prevention and treatment by dietary antioxidants. Free radicals: formation, types and effects. Hauppauge: Nova Science Publishers, Inc 2010; 1-55. [Cited in This Article: ] |
| 34. | Kris-Etherton PM, Hecker KD, Bonanome A, Coval SM, Binkoski AE, Hilpert KF, Griel AE, Etherton TD. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. Am J Med. 2002;113 Suppl 9B:71S-88S. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1508] [Cited by in F6Publishing: 1164] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 35. | Riboli E, Norat T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am J Clin Nutr. 2003;78:559S-569S. [PubMed] [Cited in This Article: ] |
| 36. | World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: a Global Perspective. Washington DC: AICR; 2007; . [Cited in This Article: ] |
| 37. | Nishino H. Phytochemicals in hepatocellular cancer prevention. Nutr Cancer. 2009;61:789-791. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 38. | Mann CD, Neal CP, Garcea G, Manson MM, Dennison AR, Berry DP. Phytochemicals as potential chemopreventive and chemotherapeutic agents in hepatocarcinogenesis. Eur J Cancer Prev. 2009;18:13-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 39. | Glauert HP, Calfee-Mason K, Stemm DN, Tharappel JC, Spear BT. Dietary antioxidants in the prevention of hepatocarcinogenesis: a review. Mol Nutr Food Res. 2010;54:875-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 40. | Bishayee A, Politis T, Darvesh AS. Resveratrol in the chemoprevention and treatment of hepatocellular carcinoma. Cancer Treat Rev. 2010;36:43-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 41. | Darvesh AS, Aggarwal BB, Bishayee A. Curcumin and Liver Cancer: A Review. Curr Pharm Biotechnol. 2011;Epub ahead of print. [PubMed] [Cited in This Article: ] |
| 42. | Sacchettini JC, Poulter CD. Creating isoprenoid diversity. Science. 1997;277:1788-1789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 410] [Cited by in F6Publishing: 368] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 43. | Peñuelas J, Munné-Bosch S. Isoprenoids: an evolutionary pool for photoprotection. Trends Plant Sci. 2005;10:166-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 44. | Withers ST, Keasling JD. Biosynthesis and engineering of isoprenoid small molecules. Appl Microbiol Biotechnol. 2007;73:980-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 166] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 45. | Rabi T, Bishayee A. Terpenoids and breast cancer chemoprevention. Breast Cancer Res Treat. 2009;115:223-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 46. | Wagner KH, Elmadfa I. Biological relevance of terpenoids. Overview focusing on mono-, di- and tetraterpenes. Ann Nutr Metab. 2003;47:95-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 47. | Sultana N, Ata A. Oleanolic acid and related derivatives as medicinally important compounds. J Enzyme Inhib Med Chem. 2008;23:739-756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 48. | Shah BA, Qazi GN, Taneja SC. Boswellic acids: a group of medicinally important compounds. Nat Prod Rep. 2009;26:72-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 49. | Theis N, Lerdau M. The evolution of function in plant secondary metabolites. Int J Plant Sci. 2003;164:S93-S103. [DOI] [Cited in This Article: ] |
| 50. | Gould MN. Cancer chemoprevention and therapy by monoterpenes. Environ Health Perspect. 1997;105 Suppl 4:977-979. [PubMed] [Cited in This Article: ] |
| 51. | Reddy BS, Wang CX, Samaha H, Lubet R, Steele VE, Kelloff GJ, Rao CV. Chemoprevention of colon carcinogenesis by dietary perillyl alcohol. Cancer Res. 1997;57:420-425. [PubMed] [Cited in This Article: ] |
| 52. | Vigushin DM, Poon GK, Boddy A, English J, Halbert GW, Pagonis C, Jarman M, Coombes RC. Phase I and pharmacokinetic study of D-limonene in patients with advanced cancer. Cancer Research Campaign Phase I/II Clinical Trials Committee. Cancer Chemother Pharmacol. 1998;42:111-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 53. | Crowell PL. Prevention and therapy of cancer by dietary monoterpenes. J Nutr. 1999;129:775S-778S. [PubMed] [Cited in This Article: ] |
| 54. | Burke YD, Ayoubi AS, Werner SR, McFarland BC, Heilman DK, Ruggeri BA, Crowell PL. Effects of the isoprenoids perillyl alcohol and farnesol on apoptosis biomarkers in pancreatic cancer chemoprevention. Anticancer Res. 2002;22:3127-3134. [PubMed] [Cited in This Article: ] |
| 55. | Carvalho CC, Fonseca MM. Carvone: Why and how should one bother to produce this terpene. Food Chem. 2006;95:413-422. [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 257] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 56. | Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357-369. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 474] [Cited by in F6Publishing: 473] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 57. | Setzer WN, Setzer MC. Plant-derived triterpenoids as potential antineoplastic agents. Mini Rev Med Chem. 2003;3:540-556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 131] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 58. | Laszczyk MN. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009;75:1549-1560. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 321] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 59. | Petronelli A, Pannitteri G, Testa U. Triterpenoids as new promising anticancer drugs. Anticancer Drugs. 2009;20:880-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 253] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 60. | Chaturvedi PK, Bhui K, Shukla Y. Lupeol: connotations for chemoprevention. Cancer Lett. 2008;263:1-13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 61. | Rabi T, Gupta S. Dietary terpenoids and prostate cancer chemoprevention. Front Biosci. 2008;13:3457-3469. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 62. | Mullauer FB, Kessler JH, Medema JP. Betulinic acid, a natural compound with potent anticancer effects. Anticancer Drugs. 2010;21:215-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 63. | Bishayee A, Ahmed S, Brankov N, Perloff M. Triterpenoids as potential agents for the chemoprevention and therapy of breast cancer. Front Biosci. 2011;16:980-996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 64. | Patlolla JM, Rao CV. Triterpenoids for Cancer Prevention and Treatment: Current Status and Future Prospects. Curr Pharm Biotechnol. 2011;in press. [PubMed] [Cited in This Article: ] |
| 65. | Liu J. Oleanolic acid and ursolic acid: research perspectives. J Ethnopharmacol. 2005;100:92-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 525] [Cited by in F6Publishing: 464] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 66. | Ovesná Z, Vachálková A, Horváthová K, Tóthová D. Pentacyclic triterpenoic acids: new chemoprotective compounds. Minireview. Neoplasma. 2004;51:327-333. [PubMed] [Cited in This Article: ] |
| 67. | Fulda S. Betulinic acid: a natural product with anticancer activity. Mol Nutr Food Res. 2009;53:140-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 68. | Polo MP, de Bravo MG. Effect of geraniol on fatty-acid and mevalonate metabolism in the human hepatoma cell line Hep G2. Biochem Cell Biol. 2006;84:102-111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Ji L, Liu T, Liu J, Chen Y, Wang Z. Andrographolide inhibits human hepatoma-derived Hep3B cell growth through the activation of c-Jun N-terminal kinase. Planta Med. 2007;73:1397-1401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 70. | Deng R, Tang J, Xia LP, Li DD, Zhou WJ, Wang LL, Feng GK, Zeng YX, Gao YH, Zhu XF. ExcisaninA, a diterpenoid compound purified from Isodon MacrocalyxinD, induces tumor cells apoptosis and suppresses tumor growth through inhibition of PKB/AKT kinase activity and blockade of its signal pathway. Mol Cancer Ther. 2009;8:873-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 71. | Yoshida M, Matsui Y, Iizuka A, Ikarashi Y. G2-phase arrest through p21(WAF1 / Cip1) induction and cdc2 repression by gnidimacrin in human hepatoma HLE cells. Anticancer Res. 2009;29:1349-1354. [PubMed] [Cited in This Article: ] |
| 72. | Huang J, Wu L, Tashiro S, Onodera S, Ikejima T. Reactive oxygen species mediate oridonin-induced HepG2 apoptosis through p53, MAPK, and mitochondrial signaling pathways. J Pharmacol Sci. 2008;107:370-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 73. | Rebbeck TR, Troxel AB, Norman S, Bunin GR, DeMichele A, Baumgarten M, Berlin M, Schinnar R, Strom BL. A retrospective case-control study of the use of hormone-related supplements and association with breast cancer. Int J Cancer. 2007;120:1523-1528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 74. | Zepelin HH, Meden H, Kostev K, Schröder-Bernhardi D, Stammwitz U, Becher H. Isopropanolic black cohosh extract and recurrence-free survival after breast cancer. Int J Clin Pharmacol Ther. 2007;45:143-154. [PubMed] [Cited in This Article: ] |
| 75. | Einbond LS, Soffritti M, Esposti DD, Park T, Cruz E, Su T, Wu HA, Wang X, Zhang YJ, Ham J. Actein activates stress- and statin-associated responses and is bioavailable in Sprague-Dawley rats. Fundam Clin Pharmacol. 2009;23:311-321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 76. | Li M, Wei SY, Xu B, Guo W, Liu DL, Cui JR, Yao XS. Pro-apoptotic and microtubule-disassembly effects of ardisiacrispin (A+B), triterpenoid saponins from Ardisia crenata on human hepatoma Bel-7402 cells. J Asian Nat Prod Res. 2008;10:739-746. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 77. | Wang GL, Chen CB, Gao JM, Ni H, Wang TS, Chen L. [Investigation on the molecular mechanisms of anti-hepatocarcinoma herbs of traditional Chinese medicine by cell cycle microarray]. Zhongguo Zhong Yao Za Zhi. 2005;30:50-54. [PubMed] [Cited in This Article: ] |
| 78. | Qi H, Wei L, Han Y, Zhang Q, Lau AS, Rong J. Proteomic characterization of the cellular response to chemopreventive triterpenoid astragaloside IV in human hepatocellular carcinoma cell line HepG2. Int J Oncol. 2010;36:725-735. [PubMed] [Cited in This Article: ] |
| 79. | Lee YS, Jin DQ, Kwon EJ, Park SH, Lee ES, Jeong TC, Nam DH, Huh K, Kim JA. Asiatic acid, a triterpene, induces apoptosis through intracellular Ca2+ release and enhanced expression of p53 in HepG2 human hepatoma cells. Cancer Lett. 2002;186:83-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 102] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 80. | Eichenmüller M, von Schweinitz D, Kappler R. Betulinic acid treatment promotes apoptosis in hepatoblastoma cells. Int J Oncol. 2009;35:873-879. [PubMed] [Cited in This Article: ] |
| 81. | Ruiz i Altaba A, Sánchez P, Dahmane N. Gli and hedgehog in cancer: tumours, embryos and stem cells. Nat Rev Cancer. 2002;2:361-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 557] [Cited by in F6Publishing: 594] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 82. | Evangelista M, Tian H, de Sauvage FJ. The hedgehog signaling pathway in cancer. Clin Cancer Res. 2006;12:5924-5928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 83. | Zhang M, Zhang H, Sun C, Shan X, Yang X, Li-Ling J, Deng Y. Targeted constitutive activation of signal transducer and activator of transcription 3 in human hepatocellular carcinoma cells by cucurbitacin B. Cancer Chemother Pharmacol. 2009;63:635-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 84. | Chan KT, Meng FY, Li Q, Ho CY, Lam TS, To Y, Lee WH, Li M, Chu KH, Toh M. Cucurbitacin B induces apoptosis and S phase cell cycle arrest in BEL-7402 human hepatocellular carcinoma cells and is effective via oral administration. Cancer Lett. 2010;294:118-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 85. | Takahashi N, Yoshida Y, Sugiura T, Matsuno K, Fujino A, Yamashita U. Cucurbitacin D isolated from Trichosanthes kirilowii induces apoptosis in human hepatocellular carcinoma cells in vitro. Int Immunopharmacol. 2009;9:508-513. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 86. | Meng D, Qiang S, Lou L, Zhao W. Cytotoxic cucurbitane-type triterpenoids from Elaeocarpus hainanensis. Planta Med. 2008;74:1741-1744. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 87. | Tong X, Lin S, Fujii M, Hou DX. Molecular mechanisms of echinocystic acid-induced apoptosis in HepG2 cells. Biochem Biophys Res Commun. 2004;321:539-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 88. | Zhou XY, Fu FH, Li Z, Dong QJ, He J, Wang CH. Escin, a natural mixture of triterpene saponins, exhibits antitumor activity against hepatocellular carcinoma. Planta Med. 2009;75:1580-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 89. | Yang HL. Ganoderic acid produced from submerged culture of Ganoderma lucidum induces cell cycle arrest and cytotoxicity in human hepatoma cell line BEL7402. Biotechnol Lett. 2005;27:835-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 90. | Chang UM, Li CH, Lin LI, Huang CP, Kan LS, Lin SB. Ganoderiol F, a ganoderma triterpene, induces senescence in hepatoma HepG2 cells. Life Sci. 2006;79:1129-1139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 91. | Lee KY, Lee SK. Ginsenoside-Rg1 positively regulates cyclin E-dependent kinase activity in human hepatoma SK-HEP-1 cells. Biochem Mol Biol Int. 1996;39:539-546. [PubMed] [Cited in This Article: ] |
| 92. | Lee KY, Lee YH, Kim SI, Park JH, Lee SK. Ginsenoside-Rg5 suppresses cyclin E-dependent protein kinase activity via up-regulating p21Cip/WAF1 and down-regulating cyclin E in SK-HEP-1 cells. Anticancer Res. 1997;17:1067-1072. [PubMed] [Cited in This Article: ] |
| 93. | Lee KY, Park JA, Chung E, Lee YH, Kim SI, Lee SK. Ginsenoside-Rh2 blocks the cell cycle of SK-HEP-1 cells at the G1/S boundary by selectively inducing the protein expression of p27kip1. Cancer Lett. 1996;110:193-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 61] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 94. | Park JA, Lee KY, Oh YJ, Kim KW, Lee SK. Activation of caspase-3 protease via a Bcl-2-insensitive pathway during the process of ginsenoside Rh2-induced apoptosis. Cancer Lett. 1997;121:73-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 111] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 95. | Kim YJ, Kwon HC, Ko H, Park JH, Kim HY, Yoo JH, Yang HO. Anti-tumor activity of the ginsenoside Rk1 in human hepatocellular carcinoma cells through inhibition of telomerase activity and induction of apoptosis. Biol Pharm Bull. 2008;31:826-830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 96. | Ko H, Kim YJ, Park JS, Park JH, Yang HO. Autophagy inhibition enhances apoptosis induced by ginsenoside Rk1 in hepatocellular carcinoma cells. Biosci Biotechnol Biochem. 2009;73:2183-2189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 97. | Kim SE, Lee YH, Park JH, Lee SK. Ginsenoside-Rs3, a new diol-type ginseng saponin, selectively elevates protein levels of p53 and p21WAF1 leading to induction of apoptosis in SK-HEP-1 cells. Anticancer Res. 1999;19:487-491. [PubMed] [Cited in This Article: ] |
| 98. | Wang QF, Chen JC, Hsieh SJ, Cheng CC, Hsu SL. Regulation of Bcl-2 family molecules and activation of caspase cascade involved in gypenosides-induced apoptosis in human hepatoma cells. Cancer Lett. 2002;183:169-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 67] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Oh SH, Lee BH. A ginseng saponin metabolite-induced apoptosis in HepG2 cells involves a mitochondria-mediated pathway and its downstream caspase-8 activation and Bid cleavage. Toxicol Appl Pharmacol. 2004;194:221-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 100. | Ming YL, Song G, Chen LH, Zheng ZZ, Chen ZY, Ouyang GL, Tong QX. Anti-proliferation and apoptosis induced by a novel intestinal metabolite of ginseng saponin in human hepatocellular carcinoma cells. Cell Biol Int. 2007;31:1265-1273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 101. | Tian Z, Liu YM, Chen SB, Yang JS, Xiao PG, Wang L, Wu E. Cytotoxicity of two triterpenoids from Nigella glandulifera. Molecules. 2006;11:693-699. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 102. | Liu JJ, Nilsson A, Oredsson S, Badmaev V, Duan RD. Keto- and acetyl-keto-boswellic acids inhibit proliferation and induce apoptosis in Hep G2 cells via a caspase-8 dependent pathway. Int J Mol Med. 2002;10:501-505. [PubMed] [Cited in This Article: ] |
| 103. | Weng CJ, Chau CF, Chen KD, Chen DH, Yen GC. The anti-invasive effect of lucidenic acids isolated from a new Ganoderma lucidum strain. Mol Nutr Food Res. 2007;51:1472-1477. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 104. | Weng CJ, Chau CF, Hsieh YS, Yang SF, Yen GC. Lucidenic acid inhibits PMA-induced invasion of human hepatoma cells through inactivating MAPK/ERK signal transduction pathway and reducing binding activities of NF-kappaB and AP-1. Carcinogenesis. 2008;29:147-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 122] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 105. | Zhang L, Zhang Y, Zhang L, Yang X, Lv Z. Lupeol, a dietary triterpene, inhibited growth, and induced apoptosis through down-regulation of DR3 in SMMC7721 cells. Cancer Invest. 2009;27:163-170. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 106. | Hong J, Min HY, Xu GH, Lee JG, Lee SH, Kim YS, Kang SS, Lee SK. Growth inhibition and G1 cell cycle arrest mediated by 25-methoxyhispidol A, a novel triterpenoid, isolated from the fruit of Poncirus trifoliata in human hepatocellular carcinoma cells. Planta Med. 2008;74:151-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 107. | Yan SL, Huang CY, Wu ST, Yin MC. Oleanolic acid and ursolic acid induce apoptosis in four human liver cancer cell lines. Toxicol In Vitro. 2010;24:842-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 161] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 108. | Shyu MH, Kao TC, Yen GC. Oleanolic acid and ursolic acid induce apoptosis in HuH7 human hepatocellular carcinoma cells through a mitochondrial-dependent pathway and downregulation of XIAP. J Agric Food Chem. 2010;58:6110-6118. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 132] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 109. | Tian Z, Lin G, Zheng RX, Huang F, Yang MS, Xiao PG. Anti-hepatoma activity and mechanism of ursolic acid and its derivatives isolated from Aralia decaisneana. World J Gastroenterol. 2006;12:874-879. [PubMed] [Cited in This Article: ] |
| 110. | Tang C, Lu YH, Xie JH, Wang F, Zou JN, Yang JS, Xing YY, Xi T. Downregulation of survivin and activation of caspase-3 through the PI3K/Akt pathway in ursolic acid-induced HepG2 cell apoptosis. Anticancer Drugs. 2009;20:249-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 77] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 111. | Zhang Z, Wang S, Qiu H, Duan C, Ding K, Wang Z. Waltonitone induces human hepatocellular carcinoma cells apoptosis in vitro and in vivo. Cancer Lett. 2009;286:223-231. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 112. | Wang Y, Zhang D, Ye W, Yin Z, Fung KP, Zhao S, Yao X. Triterpenoid saponins from Androsace umbellata and their anti-proliferative activities in human hepatoma cells. Planta Med. 2008;74:1280-1284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 113. | Huang J, Tang XH, Ikejima T, Sun XJ, Wang XB, Xi RG, Wu LJ. A new triterpenoid from Panax ginseng exhibits cytotoxicity through p53 and the caspase signaling pathway in the HepG2 cell line. Arch Pharm Res. 2008;31:323-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 114. | Kozuki Y, Miura Y, Yagasaki K. Inhibitory effects of carotenoids on the invasion of rat ascites hepatoma cells in culture. Cancer Lett. 2000;151:111-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 84] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 115. | Huang CS, Shih MK, Chuang CH, Hu ML. Lycopene inhibits cell migration and invasion and upregulates Nm23-H1 in a highly invasive hepatocarcinoma, SK-Hep-1 cells. J Nutr. 2005;135:2119-2123. [PubMed] [Cited in This Article: ] |
| 116. | Wójcik M, Bobowiec R, Martelli F. Effect of carotenoids on in vitro proliferation and differentiation of oval cells during neoplastic and non-neoplastic liver injuries in rats. J Physiol Pharmacol. 2008;59 Suppl 2:203-213. [PubMed] [Cited in This Article: ] |
| 117. | Yurtcu E, Iseri OD, Sahin FI. Effects of ascorbic acid and β-carotene on HepG2 human hepatocellular carcinoma cell line. Mol Biol Rep. 2011;38:4265-4272. [PubMed] [Cited in This Article: ] |
| 118. | Das SK, Hashimoto T, Kanazawa K. Growth inhibition of human hepatic carcinoma HepG2 cells by fucoxanthin is associated with down-regulation of cyclin D. Biochim Biophys Acta. 2008;1780:743-749. [PubMed] [Cited in This Article: ] |
| 119. | Liu CL, Huang YS, Hosokawa M, Miyashita K, Hu ML. Inhibition of proliferation of a hepatoma cell line by fucoxanthin in relation to cell cycle arrest and enhanced gap junctional intercellular communication. Chem Biol Interact. 2009;182:165-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 120. | Park YO, Hwang ES, Moon TW. The effect of lycopene on cell growth and oxidative DNA damage of Hep3B human hepatoma cells. Biofactors. 2005;23:129-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 121. | Hwang ES, Lee HJ. Inhibitory effects of lycopene on the adhesion, invasion, and migration of SK-Hep1 human hepatoma cells. Exp Biol Med (Maywood). 2006;231:322-327. [PubMed] [Cited in This Article: ] |
| 122. | Huang CS, Fan YE, Lin CY, Hu ML. Lycopene inhibits matrix metalloproteinase-9 expression and down-regulates the binding activity of nuclear factor-kappa B and stimulatory protein-1. J Nutr Biochem. 2007;18:449-456. [PubMed] [Cited in This Article: ] |
| 123. | Chen W, Hou J, Yin Y, Jang J, Zheng Z, Fan H, Zou G. alpha-Bisabolol induces dose- and time-dependent apoptosis in HepG2 cells via a Fas- and mitochondrial-related pathway, involves p53 and NFkappaB. Biochem Pharmacol. 2010;80:247-254. [PubMed] [Cited in This Article: ] |
| 124. | Hsu YL, Wu LY, Kuo PL. Dehydrocostuslactone, a medicinal plant-derived sesquiterpene lactone, induces apoptosis coupled to endoplasmic reticulum stress in liver cancer cells. J Pharmacol Exp Ther. 2009;329:808-819. [PubMed] [Cited in This Article: ] |
| 125. | Xiao Y, Yang FQ, Li SP, Gao JL, Hu G, Lao SC, Conceição EL, Fung KP, Wangl YT, Lee SM. Furanodiene induces G2/M cell cycle arrest and apoptosis through MAPK signaling and mitochondria-caspase pathway in human hepatocellular carcinoma cells. Cancer Biol Ther. 2007;6:1044-1050. [PubMed] [Cited in This Article: ] |
| 126. | Miao R, Wei J, Zhang Q, Sajja V, Yang J, Wang Q. Redifferentiation of human hepatoma cells (SMMC-7721) induced by two new highly oxygenated bisabolane-type sesquiterpenes. J Biosci. 2008;33:723-730. [PubMed] [Cited in This Article: ] |
| 127. | Sakinah SA, Handayani ST, Hawariah LP. Zerumbone induced apoptosis in liver cancer cells via modulation of Bax/Bcl-2 ratio. Cancer Cell Int. 2007;7:4. [PubMed] [Cited in This Article: ] |
| 128. | Sakata K, Hara A, Hirose Y, Yamada Y, Kuno T, Katayama M, Yoshida K, Zheng Q, Murakami A, Ohigashi H. Dietary supplementation of the citrus antioxidant auraptene inhibits N,N-diethylnitrosamine-induced rat hepatocarcinogenesis. Oncology. 2004;66:244-252. [PubMed] [Cited in This Article: ] |
| 129. | Ong TP, Heidor R, de Conti A, Dagli ML, Moreno FS. Farnesol and geraniol chemopreventive activities during the initial phases of hepatocarcinogenesis involve similar actions on cell proliferation and DNA damage, but distinct actions on apoptosis, plasma cholesterol and HMGCoA reductase. Carcinogenesis. 2006;27:1194-1203. [PubMed] [Cited in This Article: ] |
| 130. | Cardozo MT, de Conti A, Ong TP, Scolastici C, Purgatto E, Horst MA, Bassoli BK, Moreno FS. Chemopreventive effects of β-ionone and geraniol during rat hepatocarcinogenesis promotion: distinct actions on cell proliferation, apoptosis, HMGCoA reductase, and RhoA. J Nutr Biochem. 2011;22:130-135. [PubMed] [Cited in This Article: ] |
| 131. | Hirose M, Hasegawa R, Kimura J, Akagi K, Yoshida Y, Tanaka H, Miki T, Satoh T, Wakabayashi K, Ito N. Inhibitory effects of 1-O-hexyl-2,3,5-trimethylhydroquinone (HTHQ), green tea catechins and other antioxidants on 2-amino-6-methyldipyrido[1,2-a: 3',2'-d]imidazole (Glu-P-1)-induced rat hepatocarcinogenesis and dose-dependent inhibition by HTHQ of lesion induction by Glu-P-1 or 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx). Carcinogenesis. 1995;16:3049-3055. [PubMed] [Cited in This Article: ] |
| 132. | Giri RK, Parija T, Das BR. d-limonene chemoprevention of hepatocarcinogenesis in AKR mice: inhibition of c-jun and c-myc. Oncol Rep. 1999;6:1123-1127. [PubMed] [Cited in This Article: ] |
| 133. | Parija T, Das BR. Involvement of YY1 and its correlation with c-myc in NDEA induced hepatocarcinogenesis, its prevention by d-limonene. Mol Biol Rep. 2003;30:41-46. [PubMed] [Cited in This Article: ] |
| 134. | Kaji I, Tatsuta M, Iishi H, Baba M, Inoue A, Kasugai H. Inhibition by d-limonene of experimental hepatocarcinogenesis in Sprague-Dawley rats does not involve p21(ras) plasma membrane association. Int J Cancer. 2001;93:441-444. [PubMed] [Cited in This Article: ] |
| 135. | Bodake HB, Panicker KN, Kailaje VV, Rao KV. Chemopreventive effect of orange oil on the development of hepatic preneoplastic lesions induced by N-nitrosodiethylamine in rats: an ultrastructural study. Indian J Exp Biol. 2002;40:245-251. [PubMed] [Cited in This Article: ] |
| 136. | Mills JJ, Chari RS, Boyer IJ, Gould MN, Jirtle RL. Induction of apoptosis in liver tumors by the monoterpene perillyl alcohol. Cancer Res. 1995;55:979-983. [PubMed] [Cited in This Article: ] |
| 137. | Trivedi NP, Rawal UM, Patel BP. Hepatoprotective effect of andrographolide against hexachlorocyclohexane-induced oxidative injury. Integr Cancer Ther. 2007;6:271-280. [PubMed] [Cited in This Article: ] |
| 138. | Trivedi NP, Rawal UM, Patel BP. Potency of andrographolide as an antitumor compound in BHC-induced liver damage. Integr Cancer Ther. 2009;8:177-189. [PubMed] [Cited in This Article: ] |
| 139. | Dhanasekaran M, Baskar AA, Ignacimuthu S, Agastian P, Duraipandiyan V. Chemopreventive potential of Epoxy clerodane diterpene from Tinospora cordifolia against diethylnitrosamine-induced hepatocellular carcinoma. Invest New Drugs. 2009;27:347-355. [PubMed] [Cited in This Article: ] |
| 140. | de Moura Espíndola R, Mazzantini RP, Ong TP, de Conti A, Heidor R, Moreno FS. Geranylgeraniol and beta-ionone inhibit hepatic preneoplastic lesions, cell proliferation, total plasma cholesterol and DNA damage during the initial phases of hepatocarcinogenesis, but only the former inhibits NF-kappaB activation. Carcinogenesis. 2005;26:1091-1099. [PubMed] [Cited in This Article: ] |
| 141. | Janani P, Sivakumari K, Geetha A, Ravisankar B, Parthasarathy C. Chemopreventive effect of bacoside A on N-nitrosodiethylamine-induced hepatocarcinogenesis in rats. J Cancer Res Clin Oncol. 2010;136:759-770. [PubMed] [Cited in This Article: ] |
| 142. | Janani P, Sivakumari K, Geetha A, Yuvaraj S, Parthasarathy C. Bacoside A downregulates matrix metalloproteinases 2 and 9 in DEN-induced hepatocellular carcinoma. Cell Biochem Funct. 2010;28:164-169. [PubMed] [Cited in This Article: ] |
| 143. | Hu DN, Ritch R. Tissue culture of adult human retinal ganglion cells. J Glaucoma. 1997;6:37-43. [PubMed] [Cited in This Article: ] |
| 144. | Slifman NR, Obermeyer WR, Aloi BK, Musser SM, Correll WA, Cichowicz SM, Betz JM, Love LA. Contamination of botanical dietary supplements by Digitalis lanata. N Engl J Med. 1998;339:806-811. [PubMed] [Cited in This Article: ] |
| 145. | Abdel-Wahhab MA, Hassan NS, El-Kady AA, Khadrawy YA, El-Nekeety AA, Mohamed SR, Sharaf HA, Mannaa FA. Red ginseng extract protects against aflatoxin B1 and fumonisins-induced hepatic pre-cancerous lesions in rats. Food Chem Toxicol. 2010;48:733-742. [PubMed] [Cited in This Article: ] |
| 146. | Shiota G, Harada K, Ishida M, Tomie Y, Okubo M, Katayama S, Ito H, Kawasaki H. Inhibition of hepatocellular carcinoma by glycyrrhizin in diethylnitrosamine-treated mice. Carcinogenesis. 1999;20:59-63. [PubMed] [Cited in This Article: ] |
| 147. | Wan XY, Luo M, Li XD, He P. Hepatoprotective and anti-hepatocarcinogenic effects of glycyrrhizin and matrine. Chem Biol Interact. 2009;181:15-19. [PubMed] [Cited in This Article: ] |
| 148. | Karim R, Iwai S, Morimura K, Wanibuchi H, Tanaka R, Matsunaga S, Yoshitake A, Fukushima S. Lack of modification of rat hepatocarcinogenesis by fernane-type triterpenoids, isolated from a Euphorbia genus. Teratog Carcinog Mutagen. 2002;22:293-299. [PubMed] [Cited in This Article: ] |
| 149. | Scolastici C, Ong TP, Moreno FS. Squalene does not exhibit a chemopreventive activity and increases plasma cholesterol in a Wistar rat hepatocarcinogenesis model. Nutr Cancer. 2004;50:101-109. [PubMed] [Cited in This Article: ] |
| 150. | Gayathri R, Priya DK, Gunassekaran GR, Sakthisekaran D. Ursolic acid attenuates oxidative stress-mediated hepatocellular carcinoma induction by diethylnitrosamine in male Wistar rats. Asian Pac J Cancer Prev. 2009;10:933-938. [PubMed] [Cited in This Article: ] |
| 151. | Murakoshi M, Nishino H, Satomi Y, Takayasu J, Hasegawa T, Tokuda H, Iwashima A, Okuzumi J, Okabe H, Kitano H. Potent preventive action of alpha-carotene against carcinogenesis: spontaneous liver carcinogenesis and promoting stage of lung and skin carcinogenesis in mice are suppressed more effectively by alpha-carotene than by beta-carotene. Cancer Res. 1992;52:6583-6587. [PubMed] [Cited in This Article: ] |
| 152. | Tsuda H, Iwahori Y, Asamoto M, Baba-Toriyama H, Hori T, Kim DJ, Uehara N, Iigo M, Takasuka N, Murakoshi M. Demonstration of organotropic effects of chemopreventive agents in multiorgan carcinogenesis models. IARC Sci Publ. 1996;143-150. [PubMed] [Cited in This Article: ] |
| 153. | Astorg P, Gradelet S, Bergès R, Suschetet M. No evidence for an inhibitory effect of beta-carotene or of canthaxanthin on the initiation of liver preneoplastic foci by diethylnitrosamine in the rat. Nutr Cancer. 1996;25:27-34. [PubMed] [Cited in This Article: ] |
| 154. | Astorg P, Gradelet S, Bergès R, Suschetet M. Dietary lycopene decreases the initiation of liver preneoplastic foci by diethylnitrosamine in the rat. Nutr Cancer. 1997;29:60-68. [PubMed] [Cited in This Article: ] |
| 155. | Tharappel JC, Lehmler HJ, Srinivasan C, Robertson LW, Spear BT, Glauert HP. Effect of antioxidant phytochemicals on the hepatic tumor promoting activity of 3,3',4,4'-tetrachlorobiphenyl (PCB-77). Food Chem Toxicol. 2008;46:3467-3474. [PubMed] [Cited in This Article: ] |
| 156. | Moreno FS, Rizzi MB, Dagli ML, Penteado MV. Inhibitory effects of beta-carotene on preneoplastic lesions induced in Wistar rats by the resistant hepatocyte model. Carcinogenesis. 1991;12:1817-1822. [PubMed] [Cited in This Article: ] |
| 157. | Moreno FS, Wu TS, Penteado MV, Rizzi MB, Jordão Júnior AA, Almeida-Muradian LB, Dagli ML. A comparison of beta-carotene and vitamin A effects on a hepatocarcinogenesis model. Int J Vitam Nutr Res. 1995;65:87-94. [PubMed] [Cited in This Article: ] |
| 158. | Rizzi MB, Dagli ML, Jordão AA, Penteado MV, Moreno FS. beta-carotene inhibits persistent and stimulates remodeling gamma GT-positive preneoplastic lesions during early promotion of hepatocarcinogenesis. Int J Vitam Nutr Res. 1997;67:415-422. [PubMed] [Cited in This Article: ] |
| 159. | FARBER E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956;16:142-148. [PubMed] [Cited in This Article: ] |
| 160. | Dagli ML, Guerra JL, Sinhorini IL, Wu TS, Rizzi MB, Penteado MV, Moreno FS. Beta-carotene reduces the ductular (oval) cell reaction in the liver of Wistar rats submitted to the resistant hepatocyte model of carcinogenesis. Pathology. 1998;30:259-266. [PubMed] [Cited in This Article: ] |
| 161. | Moreno FS, S-Wu T, Naves MM, Silveira ER, Oloris SC, da Costa MA, Dagli ML, Ong TP. Inhibitory effects of beta-carotene and vitamin a during the progression phase of hepatocarcinogenesis involve inhibition of cell proliferation but not alterations in DNA methylation. Nutr Cancer. 2002;44:80-88. [PubMed] [Cited in This Article: ] |
| 162. | Ambrogi F, Biganzoli E, Boracchi P. Multiple correspondence analysis in S-PLUS. Comput Methods Programs Biomed. 2005;79:161-167. [PubMed] [Cited in This Article: ] |
| 163. | Sampaio AR, Chagas CE, Ong TP, Moreno FS. Vitamin A and beta-carotene inhibitory effect during 1,2-dimethylhydrazine induced hepatocarcinogenesis potentiated by 5-azacytidine. Food Chem Toxicol. 2007;45:563-567. [PubMed] [Cited in This Article: ] |
| 164. | Sarkar A, Mukherjee B, Chatterjee M. Inhibitory effect of beta-carotene on chronic 2-acetylaminofluorene induced hepatocarcinogenesis in rat: reflection in hepatic drug metabolism. Carcinogenesis. 1994;15:1055-1060. [PubMed] [Cited in This Article: ] |
| 165. | Sarkar A, Mukherjee B, Chatterjee M. Inhibition of 3'-methyl-4-dimethylaminoazobenzene-induced hepatocarcinogenesis in rat by dietary beta-carotene: changes in hepatic anti-oxidant defense enzyme levels. Int J Cancer. 1995;61:799-805. [PubMed] [Cited in This Article: ] |
| 166. | Sarkar A, Bishayee A, Chatterjee M. Beta-carotene prevents lipid peroxidation and red blood cell membrane protein damage in experimental hepatocarcinogenesis. Cancer Biochem Biophys. 1995;15:111-125. [PubMed] [Cited in This Article: ] |
| 167. | Sarkar A, Basak R, Bishayee A, Basak J, Chatterjee M. Beta-carotene inhibits rat liver chromosomal aberrations and DNA chain break after a single injection of diethylnitrosamine. Br J Cancer. 1997;76:855-861. [PubMed] [Cited in This Article: ] |
| 168. | Bishayee A, Sarkar A, Chatterjee M. Further evidence for chemopreventive potential of beta-carotene against experimental carcinogenesis: diethylnitrosamine-initiated and phenobarbital-promoted hepatocarcinogenesis is prevented more effectively by beta-carotene than by retinoic acid. Nutr Cancer. 2000;37:89-98. [PubMed] [Cited in This Article: ] |
| 169. | Tsuda H, Uehara N, Iwahori Y, Asamoto M, Iigo M, Nagao M, Matsumoto K, Ito M, Hirono I. Chemopreventive effects of beta-carotene, alpha-tocopherol and five naturally occurring antioxidants on initiation of hepatocarcinogenesis by 2-amino-3-methylimidazo[4,5-f]quinoline in the rat. Jpn J Cancer Res. 1994;85:1214-1219. [PubMed] [Cited in This Article: ] |
| 170. | Ogawa T, Higashi S, Kawarada Y, Mizumoto R. Role of reactive oxygen in synthetic estrogen induction of hepatocellular carcinomas in rats and preventive effect of vitamins. Carcinogenesis. 1995;16:831-836. [PubMed] [Cited in This Article: ] |
| 171. | Uehara N, Iwahori Y, Asamoto M, Baba-Toriyama H, Iigo M, Ochiai M, Nagao M, Nakayama M, Degawa M, Matsumoto K. Decreased levels of 2-amino-3-methylimidazo[4,5-f]quinoline-DNA adducts in rats treated with beta-carotene, alpha-tocopherol and freeze-dried aloe. Jpn J Cancer Res. 1996;87:342-348. [PubMed] [Cited in This Article: ] |
| 172. | Gradelet S, Astorg P, Le Bon AM, Bergès R, Suschetet M. Modulation of aflatoxin B1 carcinogenicity, genotoxicity and metabolism in rat liver by dietary carotenoids: evidence for a protective effect of CYP1A inducers. Cancer Lett. 1997;114:221-223. [PubMed] [Cited in This Article: ] |
| 173. | Gradelet S, Le Bon AM, Bergès R, Suschetet M, Astorg P. Dietary carotenoids inhibit aflatoxin B1-induced liver preneoplastic foci and DNA damage in the rat: role of the modulation of aflatoxin B1 metabolism. Carcinogenesis. 1998;19:403-411. [PubMed] [Cited in This Article: ] |
| 174. | Sadek IA, Hayat LG. Initiation and post-initiation chemopreventive effects of beta-carotene in toad liver carcinogenesis. Histol Histopathol. 1996;11:357-360. [PubMed] [Cited in This Article: ] |
| 175. | Chattopadhyay MB, C B MK, Kanna PS, Ray RS, Roy S, Chatterjee M. Combined supplementation of vanadium and beta-carotene suppresses placental glutathione S-transferase-positive foci and enhances antioxidant functions during the inhibition of diethylnitrosamine-induced rat liver carcinogenesis. J Gastroenterol Hepatol. 2004;19:683-693. [PubMed] [Cited in This Article: ] |
| 176. | Chattopadhyay MB, Mukherjee S, Kulkarni I, Vijayan V, Doloi M, Kanjilal N, Chatterjee M. Proton-Induced X-ray Emission (PIXE) Analysis and DNA-chain Break study in rat hepatocarcinogenesis: A possible chemopreventive role by combined supplementation of vanadium and beta-carotene. Cancer Cell Int. 2005;5:16. [PubMed] [Cited in This Article: ] |
| 177. | Bishayee A, Waghray A, Patel MA, Chatterjee M. Vanadium in the detection, prevention and treatment of cancer: the in vivo evidence. Cancer Lett. 2010;294:1-12. [PubMed] [Cited in This Article: ] |
| 178. | Singletary K. Diet, natural products and cancer chemoprevention. J Nutr. 2000;130:465S-466S. [PubMed] [Cited in This Article: ] |
| 179. | Mo H, Elson CE. Studies of the isoprenoid-mediated inhibition of mevalonate synthesis applied to cancer chemotherapy and chemoprevention. Exp Biol Med (Maywood). 2004;229:567-585. [PubMed] [Cited in This Article: ] |
| 180. | Ohizumi H, Masuda Y, Nakajo S, Sakai I, Ohsawa S, Nakaya K. Geranylgeraniol is a potent inducer of apoptosis in tumor cells. J Biochem. 1995;117:11-13. [PubMed] [Cited in This Article: ] |
| 181. | Toledo LP, Ong TP, Pinho AL, Jordão A, Vanucchi H, Moreno FS. Inhibitory effects of lutein and lycopene on placental glutathione S-transferase-positive preneoplastic lesions and DNA strand breakage induced in Wistar rats by the resistant hepatocyte model of hepatocarcinogenesis. Nutr Cancer. 2003;47:62-69. [PubMed] [Cited in This Article: ] |
| 182. | Moreno FS, Toledo LP, de Conti A, Heidor R, Jordão A, Vannucchi H, Cardozo MT, Ong TP. Lutein presents suppressing but not blocking chemopreventive activity during diethylnitrosamine-induced hepatocarcinogenesis and this involves inhibition of DNA damage. Chem Biol Interact. 2007;168:221-228. [PubMed] [Cited in This Article: ] |
| 183. | Watanabe S, Kitade Y, Masaki T, Nishioka M, Satoh K, Nishino H. Effects of lycopene and Sho-saiko-to on hepatocarcinogenesis in a rat model of spontaneous liver cancer. Nutr Cancer. 2001;39:96-101. [PubMed] [Cited in This Article: ] |
| 184. | Wang Y, Ausman LM, Greenberg AS, Russell RM, Wang XD. Dietary lycopene and tomato extract supplementations inhibit nonalcoholic steatohepatitis-promoted hepatocarcinogenesis in rats. Int J Cancer. 2010;126:1788-1796. [PubMed] [Cited in This Article: ] |
| 185. | McAnally JA, Jung M, Mo H. Farnesyl-O-acetylhydroquinone and geranyl-O-acetylhydroquinone suppress the proliferation of murine B16 melanoma cells, human prostate and colon adenocarcinoma cells, human lung carcinoma cells, and human leukemia cells. Cancer Lett. 2003;202:181-192. [PubMed] [Cited in This Article: ] |
| 186. | Taha MM, Abdul AB, Abdullah R, Ibrahim TA, Abdelwahab SI, Mohan S. Potential chemoprevention of diethylnitrosamine-initiated and 2-acetylaminofluorene-promoted hepatocarcinogenesis by zerumbone from the rhizomes of the subtropical ginger (Zingiber zerumbet). Chem Biol Interact. 2010;186:295-305. [PubMed] [Cited in This Article: ] |
| 187. | Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004;134:3479S-3485S. [PubMed] [Cited in This Article: ] |
| 188. | Lansky EP. Beware of pomegranates bearing 40% ellagic Acid. J Med Food. 2006;9:119-122. [PubMed] [Cited in This Article: ] |
| 189. | de Kok TM, van Breda SG, Manson MM. Mechanisms of combined action of different chemopreventive dietary compounds: a review. Eur J Nutr. 2008;47 Suppl 2:51-59. [PubMed] [Cited in This Article: ] |
| 190. | Bode AM, Dong Z. Epigallocatechin 3-gallate and green tea catechins: United they work, divided they fail. Cancer Prev Res (Phila). 2009;2:514-517. [PubMed] [Cited in This Article: ] |
| 191. | Korkina LG, De Luca C, Kostyuk VA, Pastore S. Plant polyphenols and tumors: from mechanisms to therapies, prevention, and protection against toxicity of anti-cancer treatments. Curr Med Chem. 2009;16:3943-3965. [PubMed] [Cited in This Article: ] |
| 192. | Guo M, Zhang S, Song F, Wang D, Liu Z, Liu S. Studies on the non-covalent complexes between oleanolic acid and cyclodextrins using electrospray ionization tandem mass spectrometry. J Mass Spectrom. 2003;38:723-731. [PubMed] [Cited in This Article: ] |
| 193. | Chen Y, Liu J, Yang X, Zhao X, Xu H. Oleanolic acid nanosuspensions: preparation, in-vitro characterization and enhanced hepatoprotective effect. J Pharm Pharmacol. 2005;57:259-264. [PubMed] [Cited in This Article: ] |
| 194. | Kang HS, Park JE, Lee YJ, Chang IS, Chung YI, Tae G. Preparation of liposomes containing oleanolic acid via micelle-to-vesicle transition. J Nanosci Nanotechnol. 2007;7:3944-3948. [PubMed] [Cited in This Article: ] |