Published online Aug 15, 2017. doi: 10.4291/wjgp.v8.i3.142
Peer-review started: November 22, 2016
First decision: February 30, 2017
Revised: May 24, 2017
Accepted: July 14, 2017
Article in press: July 17, 2017
Published online: August 15, 2017
Multiple endocrine neoplasia 2B (MEN2B) is a rare syndrome caused by an activating mutation of the RET gene, leading to enteric gangliomatosis. This child presented with constipation at 1-mo old, was diagnosed with MEN2B by rectal biopsy at 4 mo, had thyroidectomy at 9 mo and a colectomy at 4 years. We studied the extent of neuronal and nerve fibre proliferation and which classes of enteric nerves are affected by examining the colon with multiple neuronal antibodies. Resected transverse colon was fixed, frozen, sectioned and processed for fluorescence immunohistochemistry labelling with antibodies against TUJ1, Hu, ChAT, NOS, VIP, SP and CGRP and cKit. Control transverse colon was from the normal margin of Hirschsprung (HSCR) colon (4-year-old) and a child with familial adenomatous polyposis (FAP, 12 year). Myenteric ganglia were increased in size to as wide as the circular muscle. There was a large increase in nerve cells and nerve fibres. ChAT-, NOS-, VIP- and SP-immunoreactive nerve fibres all increased in the myenteric ganglia. NOS-IR nerves preferentially increased in the muscle, while VIP and SP increased in submucosal ganglia and mucosal nerve fibres. The density of ICC was normal. RET overactivation in MEN2B lead to a large increase in intrinsic nerve fibres in the myenteric and submucosal ganglia, with a relative increase in NOS-IR nerve fibres in the circular muscle and VIP and SP in the submucosal ganglia and mucosa. The changes were associated with severe constipation resulting in colectomy at 4 years.
Core tip: Multiple endocrine neoplasia (MEN) 2B is a rare anomaly caused by an activating mutation of the RET known to produce enteric gangliomatosis. Thyroidectomy is performed to avoid cancer and the colon removed to overcome the constipation. This child presented with constipation very early (1-mo-old) and had subtotal colectomy at 4 years. The classes of nerves affected in MEN2B colon have not been studied before. We used immunohistochemistry with multiple antibodies. There was a massive increase in intrinsic nerve fibres in myenteric and submucosal ganglia, with a differential increase in types of nerve fibres in the muscle and mucosa.
- Citation: Hutson JM, Farmer PJ, Peck CJ, Chow CW, Southwell BR. Multiple endocrine neoplasia 2B: Differential increase in enteric nerve subgroups in muscle and mucosa. World J Gastrointest Pathophysiol 2017; 8(3): 142-149
- URL: https://www.wjgnet.com/2150-5330/full/v8/i3/142.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v8.i3.142
Multiple endocrine neoplasia (MEN) 2B is a rare autosomal dominant anomaly characterised by marfanoid appearance, ganglioneuromatosis and gastrointestinal disorders[1-4]. There is a very high risk of medullary carcinoma of the thyroid (MTC) which increases with age, and a moderate risk of developing phaeochromocytoma[3]. MTC develops in infancy and originally determined the prognosis for MEN2B patients. In the last 15 years, it has been recognised that MTC is curable by surgical removal of the thyroid under 1-year-old[4].
The genetic anomaly in MEN2B is an activating mutation of RET[5-7], mostly M918T[5,8]. The RET gene codes for a membrane-bound tyrosine kinase receptor that is activated by glial-derived neurotrophic factor (GDNF), that regulates growth and development of the peripheral, autonomic and enteric nervous systems[9-11]. In MEN2B, activation of RET results in hyperganglionosis in the nervous system including the enteric nervous system[1,12,13].
Constipation is present in many MEN2B patients[1,2,7,12,14-16], and they may present in the neonatal period with symptoms suspiciously like Hirschsprung disease (HSCR)[2,16-20]. Indeed, MEN2B can be diagnosed in the neonatal period, by the presence of neurons among the enlarged nerves in submucosal ganglia in standard rectal biopsies[16,18,21]. Intestinal ganlioneuromatosis develops in an age-dependent fashion and not all develop severe constipation necessitating surgery. Removal of the thyroid does not alter the effect that RET activation has on the nervous system. We could not find any reports of the changes in the enteric nervous system post thyroidectomy. Also, despite numerous reports of hyperganglionosis, there are only a few histological images of the MEN2B intestine[12,15,16,19]. Most of the images are haematoxylin and eosin and while they clearly show enlarged ganglia, they do not reveal the nerve fibres or how neuronal subtypes are affected in MEN2B. Here we examined the enteric nervous system in bowel from a child with severe constipation from birth, who had a colectomy at 4 years.
Choline acetyl transferase (ChAT) and nitric oxide synthase (NOS) are present in 95% of myenteric neurons (ChAT+/NOS- 48%, ChAT-/NOS+ 43%, ChAT+/NOS+ 4%, ChAT-/NOS- 5%)[22,23]. Vasoactive intestinal peptide (VIP) is present in inhibitory motor and secretomotor neurons and substance P (SP) is in sensory, excitatory motor neurons and secretomotor neurons in the myenteric and submucosal ganglia[23,24]. In this study we examined enteric nerves in samples taken from a child 3.5 years after thyroidectomy. We labelled full thickness bowel with antibodies against all these different molecules to determine if all of the neuron types were increased. We compared the labelling in the MEN2B case to 2 controls: Bowel from a patient with familial adenomatous polyposis (FAP) and the normal margin of bowel in a patient operated for HSCR. To determine if changes were specific to nerves, we also labelled the interstitial cells of Cajal (ICC), cells that have a different developmental origin to neurons (they arise from the mesenchyme)[25].
This child (now 14 years) presented with chronic constipation at 1 mo (in 2003) and was investigated for HSCR with a rectal biopsy at 4 mo. A preliminary diagnosis of MEN2B was made from the mucosal biopsy stained for acetylcholinesterase and showing thickened nerve fibres with nerve cells present in the submucosal ganglia and we reported on this analysis in 2006[18]. Genetic analysis confirmed the RET M918T mutation and diagnosis of MEN2B and thyroidectomy was performed at 9 mo. At 4-year-old, the boy underwent subtotal colectomy for management of severe constipation. Terminal ileum and transverse colon were fixed and frozen. Blocks were sectioned (5 μm) and sections were processed for fluorescence immunohistochemistry[26,27] using a bank of antibodies to label nerve cells and fibres, different subtypes of neurons and ICC. Antibodies were chosen to label all nerve fibres (Tuj1), nerve cell bodies (Hu), the major excitatory (ChAT and SP) and inhibitory (NOS and VIP) neurons and nerve fibres and extrinsic sensory nerve fibres (CGRP) in the myenteric plexus and muscle layers, and the major secretomotor nerves (SP and VIP) in the muscosa.
Cryostat sections (10 μm thick) were air-dried at 4 °C overnight and then incubated overnight at room temperature with primary antibody (Table 1) followed by 2 h in secondary antibody (Table 1), rinsed and mounted in (Mowiol 4-88 (Hoechst/Aventis) in 25% glycerol/0.1 mol/L Tris HCl, with 2.5% DABCO (1,4-diazabicyclo-[2,2,2]-octane, Sigma). Negative controls excluded primary and secondary antibodies. Staining was covered by Royal Children’s Hospital Ethics Committee approval 23081B and 24105.
| Molecule | Company | Dilution |
| Primary antibody | ||
| Goat anti c ChAT | AB5966 Chemicon International, Temecula, CA, United States | 1:100 |
| Rabbit anti nNOS | AB 5380, Chemicon International, Temecula, CA, United States | 1:1000 |
| Rabbit anti VIP | NCL VIPp, Novocastra Laboratories, Newcastle-upon-Tyne, United Kingdom | 1:200 |
| Rabbit anti SP | 18-0091, Zymed Laboratories, South San Francisco, CA, United States | 1:50 |
| Goat anti CGRP | 1720-9-7, AbD Serotec, Biorad | 1:1000 |
| Rabbit anti-human cKit | Polyclonal CD117, Dako | 1:200 |
| Secondary antibody | ||
| Goat anti rabbit Alexa 488 | Molecular Probes, ThermoFisher Scientific A-11012 | 1:400 |
| Donkey anti rabbit Alexa 488 | Molecular Probes, ThermoFisher Scientific, A-21206 | 1:200 |
| Goat anti mouse Alexa 488 | Molecular Probes, ThermoFisher Scientific, A11001 | 1:400 |
| Donkey anti goat Alexa 568 | Molecular Probes, ThermoFisher Scientific, A11057 | 1:400 |
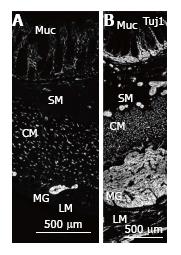
Sections were imaged on a Leica CSLM confocal microscope using 488 nm excitation with variable filters. For each antibody, the entire thickness of the colon wall was imaged to locate nerve fibres in the longitudinal and circular muscle, myenteric and submucosal ganglia, submucosa and mucosa (Figure 1). Quantitation was performed using NIH Image J. The thickness of the ganglia and muscle layers was measured in 24 sections from MEN2B patient and 15 sections from the controls. The density of nerve fibres labelled with each marker was measured in circular muscle cut in transverse section as previously described[26,27] in 3 sections for each antibody.
Normal paediatric bowel is rarely collected, so we used full-thickness biopsies of the normal ganglionic region of colon from 1 child with HSCR (4 years), the same age as the MEN2B child, and sigmoid colon from 1 child (12 years) with familial adenomatous polyposis (FAP).
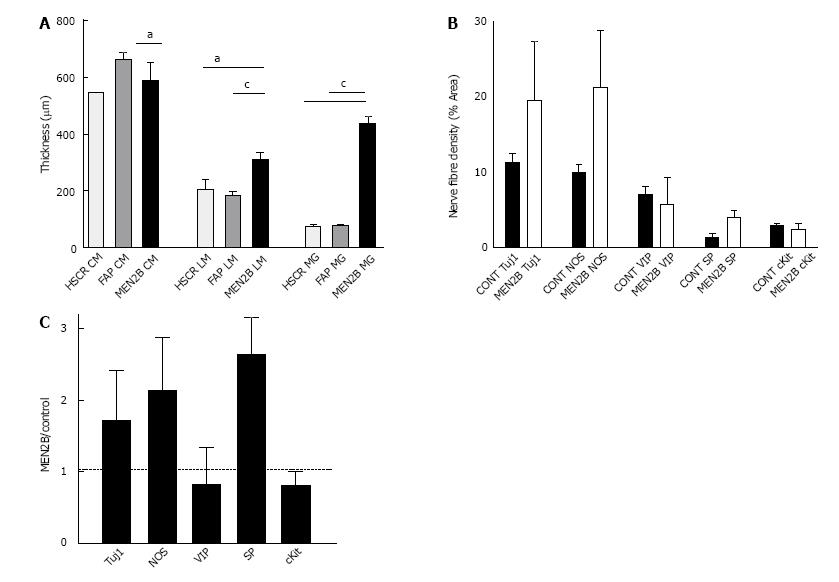
In the MEN2B colon, TUJ1-immunoreactivity (TUJ1-IR) showed overgrowth of enteric nerves in all layers (Figure 1). Myenteric ganglia were very large, with diameters equal to the thickness of the circular muscle layer (Figure 2A). The diameter of the myenteric ganglia was 5-fold more than control (P < 0.0001). The longitudinal muscle layer was significantly thicker than control (P < 0.01), while the circular muscle thickness was less than in controls (P < 0.05). Nerve fibres (Tuj1) in circular muscle were increased so they comprised 25% of the muscle area, compared with 10% in controls (Figure 2A). The submucosal ganglia were enlarged and there was a great increase in nerve fibres in the lamina propria (Figure 1B). The thickness of muscle layers and size of ganglia in the 2 “controls” were not statistically different, so we combined the results in the quantitation of different neuron types.
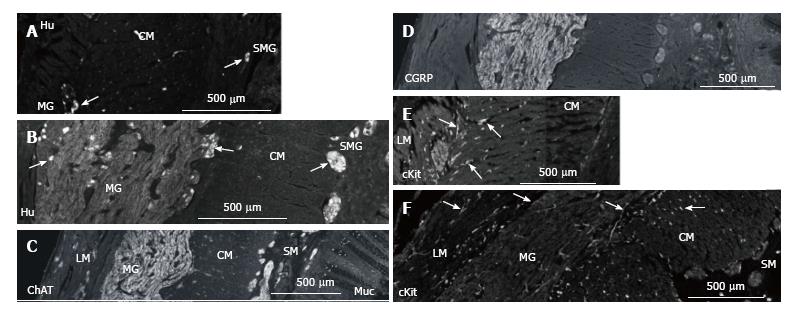
Hu-immunoreactivity (IR) shows neuronal cell bodies (Figure 3). In myenteric ganglia, there was an increase from 20 to 76 neurons per high power field. As the size of the ganglia increased, nerve fibres also increased greatly and the density of neurons (neurons/mm2 of ganglion area) decreased from 1.5/mm2 to 0.2/mm2. In the submucosa ganglia, neurons increased from 2-3 to 5-10 per ganglia (per high power field) with a great increase in nerve fibres (Figure 3).
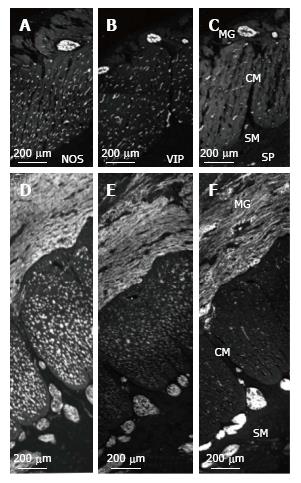
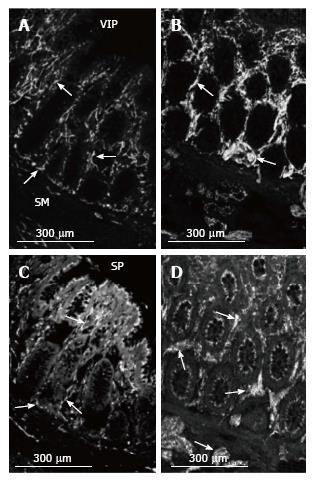
In control and MEN2B, NOS-IR was present in neurons in myenteric ganglia with nerve fibres abundant in the muscle (Figure 4), with no labelling in mucosa. NOS nerve fibre density increased 2 fold in the circular muscle (Figure 2). VIP-IR nerve fibres increased in the ganglia (Figure 4E), and mucosa (Figure 5) but proportionally did not change in density in the muscle (Figure 2). The increased density of NOS fibres in MEN2B accounted for the significant increase in density of all nerve fibres (Figure 2B).
In the MEN2B bowel, SP-IR showed increased nerve fibres in myenteric and submucosal ganglia (Figure 4) and in the mucosa (Figure 5). The relative amount of SP-IR fibres supplying the circular muscle was greater than in normal colon (Figure 2C).
ChAT-IR was high in nerve fibres in the enlarged myenteric and submucosal ganglia with relatively little labelling in the circular muscle or mucosa (Figure 3C). CGRP-IR was also high in the myenteric ganglia but low in the muscle, submucosa and mucosa (Figure 3D).
c-Kit-IR labelled a relatively normal number of ICC in the muscle layers and mast cells in the lamina propria (Figure 3E and F).
At follow-up at 14 years of age, this child is well. Early recognition and treatment (thyroidectomy and colectomy) have enabled normal growth. In addition to colectomy, he has had neuromas removed from his tongue and has nerves growing across his cornea that are monitored 6 monthly.
We established the normal ganglion size and area of nerve fibres in the circular muscle in 2 children (aged 4 and 12 years), then compared the area of ganglia and nerve fibres in a 4-year-old child who presented as an infant with constipation and genetic confirmation of MEN2B. We used a range of antibodies to identify nerve cells, fibres, nerve subgroups, and ICC, and found that in MEN2B ganglioneuromatosis, the overgrowth in myenteric ganglia was both nerve cells and fibres with most subpopulations of enteric nerves involved. There was an increase in nerve cell bodies in the submucosal ganglia, with large bundles of nerve fibres in the submucosa reminiscent of HSCR.
In the aganglionic region of Hirschsprung colon, extrinsic nerve fibres form thick cables in the submucosa and myenteric layer. This is thought to be because the lack of nerve cells provides no sites for the extrinsic nerves to synapse onto. In MEN2B submucosa, the nerve fibres also form large cables but with nerve cells present in many ectopic ganglia[18,20]. The presence of neurons among the enlarged nerve fibres has been reported previously as the diagnostic criterion to distinguish MEN2B from Hirschsprung's disease[18].
Myenteric interneurons and excitatory motor and sensory neurons that send processes to the muscle and secretomotor neurons in the submucosal ganglia that send processes to the mucosa are cholinergic[22,23]. There was a high level of ChAT labelling in the myenteric ganglia but not in the muscle. The antibody against ChAT only labels proximal processes (i.e., nerve fibres near the cell body)[28] and so does not display the nerves in the muscle or mucosa. Identifying the cholinergic nerve fibres in the muscle and mucosa requires labelling with vesicular acetyl choline (VAChT) or high affinity choline transporter (CHT)[28] and was not done in this study. This should be further investigated in future studies.
Nerve fibres containing the inhibitory neurotransmitters, nitric oxide and VIP, were unevenly distributed, with NOS prominent in the muscle layers and VIP prominent in the lamina propria. NOS is present in inhibitory motor neurons in the ganglia, forming connections within the ganglia and sending process into the muscle layers. More NOS-synthesizing fibres in the myenteric layer would increase the inhibitory pathways within the ganglia and more in the muscle layer might inhibit contraction or lead to relaxation. NOS immunoreactive neurons are normally sparse in the submucosal ganglia and their fibres innervate the inner muscle layer. NOS-immunoreactive nerve fibres are not normally present in the muscosa.
SP levels were especially high in the submucosal ganglia and mucosa. Thus, RET overactivation increased all nerve fibres in the myenteric ganglia, NOS in the circular muscle, and VIP and SP in the submucosal ganglia and mucosa. Previous studies have shown histology of ganglia and staining with neuron specific enolase (NSE)[12,15,19] but only one other study has reported labelling for specific neurotransmitters[20]. Previously, in seromuscular biopsies from 2 other MEN2B patients, we noted a decrease in SP in the circular muscle. This may reflect differences between patients in types of nerves that increase[20].
ICC arise from the mesoderm[25]. In this patient there was normal density of ICC suggesting that they are not influenced by RET. Interestingly, ICC numbers were not decreased in the MEN2B patient despite severe constipation. Reduced numbers of ICC are often associated with bowel dysmotility and chronic constipation[29,30].
Mouse models of the human RETMEN2B gene have produced thyroid and sympathetic ganglioneuroma phenotypes but to date have not reproduced intestinal gangliomatosis[31]. Thus it is difficult to investigate what the molecular mechanism of the RETMEN2B affects in the enteric nervous system. We can speculate that the activating mutation in RETMEN2B overrides an “OFF” switch that is normally induced by synapse formation and normally inhibits neuron proliferation and nerve fibre formation. This results in overproduction of nerve fibres in all layers of the bowel.
Different types of neurons differentiate at different times and environmental cues change over time. Neurturin, a GDNF family member, may play a role in development of cholinergic nerves[32]. There is particular interest in determining the factors that induce NOS expression in neuronal stem cells[32], as optimal conditions for differentiation will be needed for potential neuronal transplantation to treat HSCR. MEN2B shows that too much RET activation is not advantageous.
In this patient, overactivation of RET lead to a large increase in neurons and nerve fibres in the myenteric and submucosal ganglia, muscle and mucosa. This patient had a relative increase in NOS-IR nerve fibres in the circular muscle and of VIP-IR and SP-IR nerve fibres in the submucosal ganglia and mucosa. The RET gene test was performed after finding the enlarged ganglia in the rectal biopsies at 4 mo old and was positive resulting in a pre-emptive thyroidectomy at 9 mo. The samples studied were collected at 4 years during a partial colectomy. This study indicates that the neuronal growth continues due to the RET activation, independent of thyroid presence. So although removing the thyroid does prevent the MTC[4], the children require ongoing monitoring and maintenance and are not problem free. The child has 6-monthly screening for thyroid with annual ultrasound, annual review of corneal nerves and bowel function. He last had removal of tongue ganglia in 2015 and was seen 3 recently (May 2017) with no new nodules needing treatment. He is now 14 and doing well.
A 4-year-old boy with chronic intractable constipation since a few weeks after birth, diagnosed with multiple endocrine neoplasia 2B (MEN2B) via rectal biopsy to exclude Hirschsprung (HSCR) has partial colectomy.
MEN2B with proven M918T-RET mutation (activating).
Hirschsprung's disease was main differential, investigated in rectal biopsy at 4 wk of age, showing enlarged nerve fibres and enlarged ganglia rather than lack of enteric neurons.
Immunohistochemistry showed a large increase in intrinsic nerve fibres in both myenteric and submucosal ganglia, with a relative increase in NOS-IR nerve fibres in the circular muscle and VIP in the submucosal ganglia. GENE analysis identified M918T-RET mutation.
MEN2B caused by RET gene mutation.
Thryoidectomy at 9 mo to prevent medullary thyroid carcinoma, partial colectomy at 4 years to overcome constipation, removal of gangliomas from the tongue. Continued monitoring of thyroid and corneal nerves.
MEN2B is a rare syndrome caused by an activating mutation in the RET gene, which produces medullary thyroid cancer, adrenal phaeochromocytoma enlarged nerve fibres and ganglia, including overgrowth of the enteric nerves.
Nitric oxide synthase (NOS) is present in inhibitory motor nerves and interneurons in the myenteric and submucosal ganglia; VIP (vasoactive intestinal peptide) is also present in secretomotory nerves in the submucosal ganglia and mucosa.
Detailed immunohistochemistry has enabled us to understand the effects of an activating mutation of the RET gene in MEN2B, with the relative increase in inhibitory neurotransmitters (NOS and VIP) likely related to the clinical symptoms of intractable constipation.
Strengths: Endeavours to characterise morphological and immunohistochemical changes of MEN2B associated intestinal ganglioneuromatosis. Found that M918T-induced RET activation led to a large increase in intrinsic nerve fibres in myenteric and submucosal ganglia, specifically NOS and VIP. There is paucity of data on enteric neuronal content in MEN2B other than that ganglia are enlarged.
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cario E, Castinetti F, Machens A S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
| 1. | Erdogan MF, Gulec B, Gursoy A, Pekcan M, Azal O, Gunhan O, Bayer A. Multiple endocrine neoplasia 2B presenting with pseudo-Hirschsprung’s disease. J Natl Med Assoc. 2006;98:783-786. [PubMed] [Cited in This Article: ] |
| 2. | de Krijger RR, Brooks A, van der Harst E, Hofstra RM, Bruining HA, Molenaar JC, Meijers C. Constipation as the presenting symptom in de novo multiple endocrine neoplasia type 2B. Pediatrics. 1998;102:405-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Kaufman FR, Roe TF, Isaacs H Jr, Weitzman JJ. Metastatic medullary thyroid carcinoma in young children with mucosal neuroma syndrome. Pediatrics. 1982;70:263-267. [PubMed] [Cited in This Article: ] |
| 4. | Brauckhoff M, Machens A, Lorenz K, Bjøro T, Varhaug JE, Dralle H. Surgical curability of medullary thyroid cancer in multiple endocrine neoplasia 2B: a changing perspective. Ann Surg. 2014;259:800-806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 5. | Hofstra RM, Landsvater RM, Ceccherini I, Stulp RP, Stelwagen T, Luo Y, Pasini B, Höppener JW, van Amstel HK, Romeo G. A mutation in the RET proto-oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994;367:375-376. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 791] [Cited by in F6Publishing: 696] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 6. | Rajan I, Gestblom C, Kapur RP. RET(Men2B)-transgene produces sympathoadrenal tumors but does not prevent intestinal aganglionosis in gdnf-/- or gfr alpha-1(-/-) mice. Pediatr Dev Pathol. 2001;4:446-453. [PubMed] [Cited in This Article: ] |
| 7. | Romeo G, Ceccherini I, Celli J, Priolo M, Betsos N, Bonardi G, Seri M, Yin L, Lerone M, Jasonni V. Association of multiple endocrine neoplasia type 2 and Hirschsprung disease. J Intern Med. 1998;243:515-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 59] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Smith-Hicks CL, Sizer KC, Powers JF, Tischler AS, Costantini F. C-cell hyperplasia, pheochromocytoma and sympathoadrenal malformation in a mouse model of multiple endocrine neoplasia type 2B. EMBO J. 2000;19:612-622. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 9. | Uesaka T, Nagashimada M, Yonemura S, Enomoto H. Diminished Ret expression compromises neuronal survival in the colon and causes intestinal aganglionosis in mice. J Clin Invest. 2008;118:1890-1898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 10. | Jain S, Naughton CK, Yang M, Strickland A, Vij K, Encinas M, Golden J, Gupta A, Heuckeroth R, Johnson EM Jr, Milbrandt J. Mice expressing a dominant-negative Ret mutation phenocopy human Hirschsprung disease and delineate a direct role of Ret in spermatogenesis. Development. 2004;131:5503-5513. [PubMed] [Cited in This Article: ] |
| 11. | Puri P, Shinkai T. Pathogenesis of Hirschsprung’s disease and its variants: recent progress. Semin Pediatr Surg. 2004;13:18-24. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Smith VV, Eng C, Milla PJ. Intestinal ganglioneuromatosis and multiple endocrine neoplasia type 2B: implications for treatment. Gut. 1999;45:143-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 77] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 13. | Shimotake T, Kubota Y, Inoue K, Yanagihara J, Iwai N. Absence of rectoanal inhibitory reflex in a child with multiple endocrine neoplasia type 2B. J Pediatr Surg. 1998;33:1268-1271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Demos TC, Blonder J, Schey WL, Braithwaite SS, Goldstein PL. Multiple endocrine neoplasia (MEN) syndrome type IIB: gastrointestinal manifestations. AJR Am J Roentgenol. 1983;140:73-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Grobmyer SR, Guillem JG, O’Riordain DS, Woodruff JM, Shriver C, Brennan MF. Colonic manifestations of multiple endocrine neoplasia type 2B: report of four cases. Dis Colon Rectum. 1999;42:1216-1219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Gibbons D, Camilleri M, Nelson AD, Eckert D. Characteristics of chronic megacolon among patients diagnosed with multiple endocrine neoplasia type 2B. United European Gastroenterol J. 2016;4:449-454. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | O’Riordain DS, O’Brien T, Crotty TB, Gharib H, Grant CS, van Heerden JA. Multiple endocrine neoplasia type 2B: more than an endocrine disorder. Surgery. 1995;118:936-942. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 95] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Yin M, King SK, Hutson JM, Chow CW. Multiple endocrine neoplasia type 2B diagnosed on suction rectal biopsy in infancy: a report of 2 cases. Pediatr Dev Pathol. 2006;9:56-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Evans CA, Nesbitt IM, Walker J, Cohen MC. MEN 2B syndrome should be part of the working diagnosis of constipation of the newborn. Histopathology. 2008;52:646-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | King SK, Southwell BR, Hutson JM. An association of multiple endocrine neoplasia 2B, a RET mutation; constipation; and low substance P-nerve fiber density in colonic circular muscle. J Pediatr Surg. 2006;41:437-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Gfroerer S, Theilen TM, Fiegel H, Harter PN, Mittelbronn M, Rolle U. Identification of intestinal ganglioneuromatosis leads to early diagnosis of MEN2B: role of rectal biopsy. J Pediatr Surg. 2017;52:1161-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Porter AJ, Wattchow DA, Brookes SJ, Costa M. Cholinergic and nitrergic interneurones in the myenteric plexus of the human colon. Gut. 2002;51:70-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 93] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 23. | Murphy EM, Defontgalland D, Costa M, Brookes SJ, Wattchow DA. Quantification of subclasses of human colonic myenteric neurons by immunoreactivity to Hu, choline acetyltransferase and nitric oxide synthase. Neurogastroenterol Motil. 2007;19:126-134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Matsuda H, Hirato J, Kuroiwa M, Nakazato Y. Histopathological and immunohistochemical study of the enteric innervations among various types of aganglionoses including isolated and syndromic Hirschsprung disease. Neuropathology. 2006;26:8-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Young HM, Ciampoli D, Southwell BR, Newgreen DF. Origin of interstitial cells of Cajal in the mouse intestine. Dev Biol. 1996;180:97-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 147] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | King SK, Sutcliffe JR, Ong SY, Lee M, Koh TL, Wong SQ, Farmer PJ, Peck CJ, Stanton MP, Keck J. Substance P and vasoactive intestinal peptide are reduced in right transverse colon in pediatric slow-transit constipation. Neurogastroenterol Motil. 2010;22:883-892, e234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 27. | Southwell BR, Koh TL, Wong SQ, King SK, Ong SY, Lee M, Farmer PJ, Peck CJ, Sutcliffe JR, Stanton MP. Decrease in nerve fibre density in human sigmoid colon circular muscle occurs with growth but not aging. Neurogastroenterol Motil. 2010;22:439-445, e106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Harrington AM, Lee M, Ong SY, Yong E, Farmer P, Peck CJ, Chow CW, Hutson JM, Southwell BR. Immunoreactivity for high-affinity choline transporter colocalises with VAChT in human enteric nervous system. Cell Tissue Res. 2010;341:33-48. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Sutcliffe J, King SK, Clarke MC, Farmer P, Hutson JM, Southwell BR. Reduced distribution of pacemaking cells in dilated colon. Pediatr Surg Int. 2007;23:1179-1182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Huizinga JD, Chen JH. Interstitial cells of Cajal: update on basic and clinical science. Curr Gastroenterol Rep. 2014;16:363. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 31. | Sweetser DA, Froelick GJ, Matsumoto AM, Kafer KE, Marck B, Palmiter RD, Kapur RP. Ganglioneuromas and renal anomalies are induced by activated RET (MEN2B) in transgenic mice. Oncogene. 1999;18:877-886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Burns AJ, Pasricha PJ, Young HM. Enteric neural crest-derived cells and neural stem cells: biology and therapeutic potential. Neurogastroenterol Motil. 2004;16 Suppl 1:3-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |