Published online May 26, 2020. doi: 10.4330/wjc.v12.i5.192
Peer-review started: January 3, 2020
First decision: February 19, 2020
Revised: March 27, 2020
Accepted: May 12, 2020
Article in press: May 12, 2020
Published online: May 26, 2020
Tobacco-related products, containing the highly addictive nicotine together with numerous other harmful toxicants and carcinogens, have been clearly associated with coronary artery disease, heart failure, stroke, and other heart diseases. Among the mechanisms by which nicotine contributes to heart disease is elevation of the renin-angiotensin-aldosterone system (RAAS) activity. Nicotine, and its major metabolite in humans cotinine, have been reported to induce RAAS activation, resulting in aldosterone elevation in smokers. Aldosterone has various direct and indirect adverse cardiac effects. It is produced by the adrenal cortex in response to angiotensin II (AngII) activating AngII type 1 receptors. RAAS activity increases in chronic smokers, causing raised aldosterone levels (nicotine exposure causes the same in rats). AngII receptors exert their cellular effects via either G proteins or the two βarrestins (βarrestin1 and-2).
Since adrenal ßarrestin1 is essential for adrenal aldosterone production and nicotine/cotinine elevate circulating aldosterone levels in humans, we hypothesized that nicotine activates adrenal ßarrestin1, which contributes to RAAS activation and heart disease development.
We studied human adrenocortical zona glomerulosa H295R cells and found that nicotine and cotinine upregulate βarrestin1 mRNA and protein levels, thereby enhancing AngII-dependent aldosterone synthesis and secretion.
In contrast, siRNA-mediated βarrestin1 knockdown reversed the effects of nicotine on AngII-induced aldosterone production in H295R cells. Importantly, nicotine promotes hyperaldosteronism via adrenal βarrestin1, thereby precipitating cardiac dysfunction, also in vivo, since nicotine-exposed experimental rats with adrenal-specific βarrestin1 knockdown display lower circulating aldosterone levels and better cardiac function than nicotine-exposed control animals with normal adrenal βarrestin1 expression.
Adrenal βarrestin1 upregulation is one of the mechanisms by which tobacco compounds, like nicotine, promote cardio-toxic hyperaldosteronism in vitro and in vivo. Thus, adrenal βarrestin1 represents a novel therapeutic target for tobacco-related heart disease prevention or mitigation.
Core tip: Adrenal βarrestin1 is a novel molecular target for mitigation of the aldosterone-dependent cardiotoxic effects of tobacco. Angiotensin II induces aldosterone production in adrenocortical zona glomerulosa (AZG) cells by binding to its adrenal angiotensin II type 1 receptor, which then activates βarrestin1. Nicotine and cotinine are known to activate the renin-angiotensin-aldosterone-system (RAAS), promoting hyperaldosteronism. We report herein that these main tobacco compounds chronically upregulate adrenal βarrestin1, promoting excessive aldosterone synthesis and secretion from human AZG cells in vitro and from adrenal glands in vivo. Thus, adrenal βarrestin1 critically mediates tobacco-induced RAAS activation, which contributes to heart disease development/progression.
- Citation: Cora N, Ghandour J, Pollard CM, Desimine VL, Ferraino KE, Pereyra JM, Valiente R, Lymperopoulos A. Nicotine-induced adrenal beta-arrestin1 upregulation mediates tobacco-related hyperaldosteronism leading to cardiac dysfunction. World J Cardiol 2020; 12(5): 192-202
- URL: https://www.wjgnet.com/1949-8462/full/v12/i5/192.htm
- DOI: https://dx.doi.org/10.4330/wjc.v12.i5.192
Aldosterone exerts various deleterious effects on the failing heart, while elevated in chronic heart failure (HF)[1-4]. Accordingly, aldosterone levels serve as biomarker of HF severity[5] and mineralocorticoid receptor antagonists have several beneficial effects in HF[6,7]. Aldosterone is produced upon renin-angiotensin-aldosterone-system (RAAS) activation[8]. Together with Angiotensin II (AngII), it exerts a variety of cardiovascular effects in order to maintain renal perfusion and correct electrolyte/blood volume imbalances[1]. In the presence of heart disease however, aldosterone is markedly elevated, hindering cardiac function[1-4].
The main compound in tobacco, nicotine, and its major metabolite in humans, cotinine[9], have been reported to initially inhibit adrenal aldosterone production, causing compensatory RAAS activation upon chronic use in humans (i.e., in chronic smokers)[10-14]. This chronic RAAS activation leads to chronic elevation of aldosterone levels in smokers[10-14]. Given the harmful effects of both AngII and aldosterone in the heart and blood vessels, RAAS activation contributes to HF development in chronic tobacco smokers.
Aldosterone is produced by adrenocortical zona glomerulosa (AZG) cells in response to AngII acting through its type 1 receptors (AT1Rs)[8,15,16]. AT1Rs are G protein-coupled receptors[8] that can also signal through G protein-independent pathways[17-19]. The two universal receptor adaptor proteins β arrestin-1 and -2 (also known as arrestin-2 and -3, respectively) play a central role in mediating this G protein-independent signaling[17,18]. AngII stimulates aldosterone production via Gq/11-mediated activation of the extracellular signal-regulated kinase (ERK)1/2[20]. ERKs upregulate Steroidogenic Acute Regulatory (StAR) protein, which increases mitochondrial uptake of cholesterol to initiate steroid biosynthesis[15,21,22]. βarrestin1 is a crucial mediator of AT1R signaling to aldosterone production and secretion from human AZG cells[22-29]. The molecular signaling mechanism underlying this crucial role of βarrestin1 in adrenal aldosterone production also involves activation of ERK1/2, which upregulate StAR and, ultimately, aldosterone synthesis and release[22,28].
Since nicotine and cotinine activate RAAS and promote AngII actions at its various tissue targets, including aldosterone production in the adrenal cortex, we hypothesized that these tobacco compounds may chronically increase AngII-dependent aldosterone production in AZG cells, possibly via adrenal βarrestin1 upregulation. Indeed, we found that this is the case both in AZG cells in vitro and in vivo.
All chemicals (nicotine, cotinine, AngII, DMSO) were from Sigma-Aldrich (St. Louis, MO, United States; ≥ 98% purity, as assessed by High Performance Liquid Chromatography).
H295R cells were purchased from American Type Culture Collection (Manassas, VA, United States; RRID: CVCL_0458) and cultured, as previously described[22,26]. For siRNA-mediated knockdown, cells were transfected via the Lipofectamine method (Invitrogen, Carlsbad, CA, United States) with a custom-ordered rat βarrestin1 (Arrb1)-specific siRNA or control scrambled siRNA constructs (custom-made by Sirion Biotech, Cambridge, MA, United States). 48 h after transfection, cells were placed in serum-free medium and treated with the indicated agents for the indicated times.
In vitro aldosterone secretion in the culture medium of H295R cells and aldosterone levels in rat blood serum were measured by EIA (Aldosterone EIA kit, Cat. #: 11-AD2HU-E01; ALPCO Diagnostics, Salem, NH, United States), as described[22-26].
Total RNA isolation with TRIzol reagent (Life Technologies, Grand Island, NY, United States), reverse transcription and real-time polymerase chain reaction (RT-PCR) were carried out as previously described[30-32]. The following primer pairs were used: 5′‐GGCCCCGAGACTTCGTAA‐3′ and 5′‐TGGCAGCCACCCCTTGA‐3′ for rat StAR; 5′‐CCACATCGGGAAGTTCCAGA-3′ and 5′-CAGGCCGCTGACGAGCAA-3′ for rat βarrestin1; 5′-TCAAGAACGAAAGTCGGAGG-3′ and 5′-GGA CAT CTA AGGGCATCAC-3′ for 18S rRNA. Real time PCR was performed using SYBR® Green Supermix (Bio-Rad Laboratories, Hercules, CA, United States). Normalization was done with the housekeeping gene 18S rRNA levels. No bands were seen in the absence of reverse transcriptase.
H295R cell and rat adrenal protein extracts were prepared as described previously[22,23], in a 20 mmol/L Tris pH 7.4 buffer containing 1% Nonidet P-40, 20% glycerol, 10 mmol/L PMSF, 1 mmol/L Na3VO4, 10 mmol/L NaF, 2.5 µg/mL aprotinin, and 2.5 µg/mL leupeptin. Protein concentration was determined via the BCA method and equal amounts of protein per sample were loaded. The following antibodies were used for immunoblotting: sc-28869 (Santa Cruz Biotechnology, Santa Cruz, CA, United States) for βarrestin1; sc-25806 (Santa Cruz Biotechnology) for StAR; and sc-47724 (Santa Cruz Biotechnology) for GAPDH. Immunoblots were revealed by enhanced chemiluminescence (ECL, Life Technologies, Grand Island, NY, United States) and visualized in the FluorChem E Digital Darkroom (Protein Simple, San Jose, CA, United States), as described previously[23-26]. Densitometry was performed with the AlphaView software (Protein Simple) in the linear range of signal detection (on non-saturated bands).
All animal procedures and experiments were performed in accordance with the guidelines of the IACUC committee of Nova Southeastern University. Adrenal-specific in vivo siRNA delivery in -300 g adult (3-month-old) male Sprague-Dawley rats was done, essentially as described[23,30,33], via direct injection of 1 μg total siRNA [dissolved in sterile phosphate-buffered saline], in each of the two adrenal glands of each animal with a 31-gauge needle. Daily i.p. injections of 1 mg/kg nicotine (or saline), starting on the day of the adrenal-specific siRNA delivery, followed for 7 d in a row. Groups of five animals per treatment were generally used for analysis.
Two-dimensional guided M-mode and Doppler echocardiography using a 14-MHz transducer (Vevo 1100 Echograph, FUJIFILM Visualsonics, Inc., Toronto, ON, Canada) were performed in rats, as described previously[23,25,30]. Three independent echocardiographic measurements were taken in both modes. Echocardiography was performed immediately prior to the adrenal siRNA in vivo deliveries and then again at the end of the nicotine (or saline) treatments. The operator was blind regarding the type of treatment (Arrb1 or scrambled siRNA and drug or saline) each animal that was echo’d had received.
Data are generally expressed as mean ± SEM. Unpaired 2-tailed Student’s t test and one- or two-way ANOVA with Bonferroni or Dunnett’s test was performed for statistical comparisons using the SPSS 23 software (SPSS, Inc., Chicago, IL, United States). For all tests, a P < 0.05 was generally considered to be significant. All sample sizes were calculated for a one-way ANOVA with equal sample sizes in each group and based on previous publications and preliminary data. For the animal experiments, estimation of sample size was done using nQuery Advisor 7.0 software (Informer Technologies, Inc.).
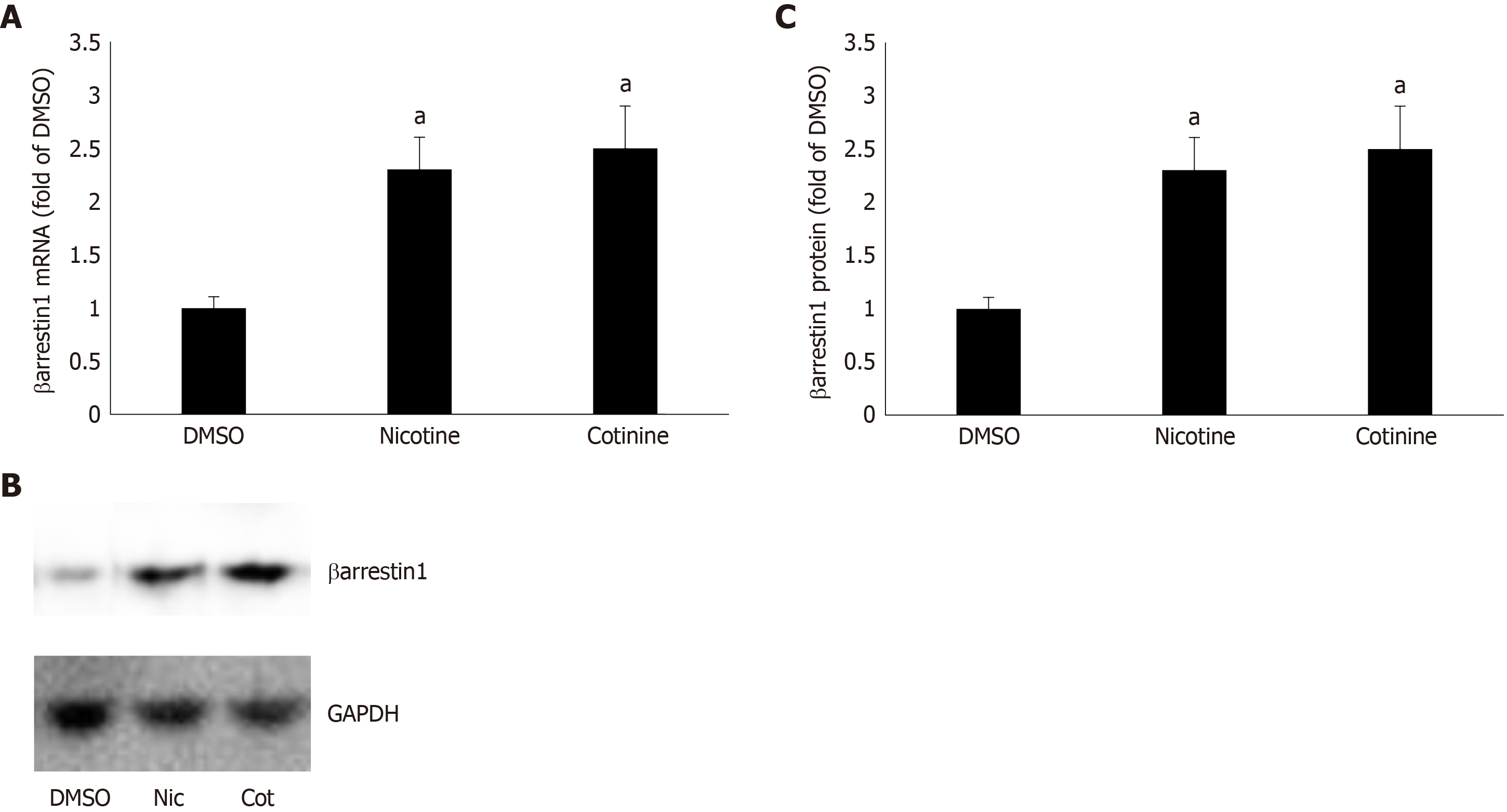
To determine whether βarrestin1 is involved in tobacco-dependent adrenal aldosterone production, we took advantage of the human AZG cell line H295R, which endogenously expresses the AT1R (but not the AT2R) and βarrestin1[15,22]. This cell line produces and secretes aldosterone in response to AngII stimulation[15]. Treatment of H295R cells with standard concentrations (10 μM) of either nicotine or cotinine (10 µmol/L is very close to the cotinine concentration attained in chronic smokers[9]) for 24 h led to significant upregulation of both mRNA (Figure 1A) and protein (Figure 1B and C) levels of βarrestin1.
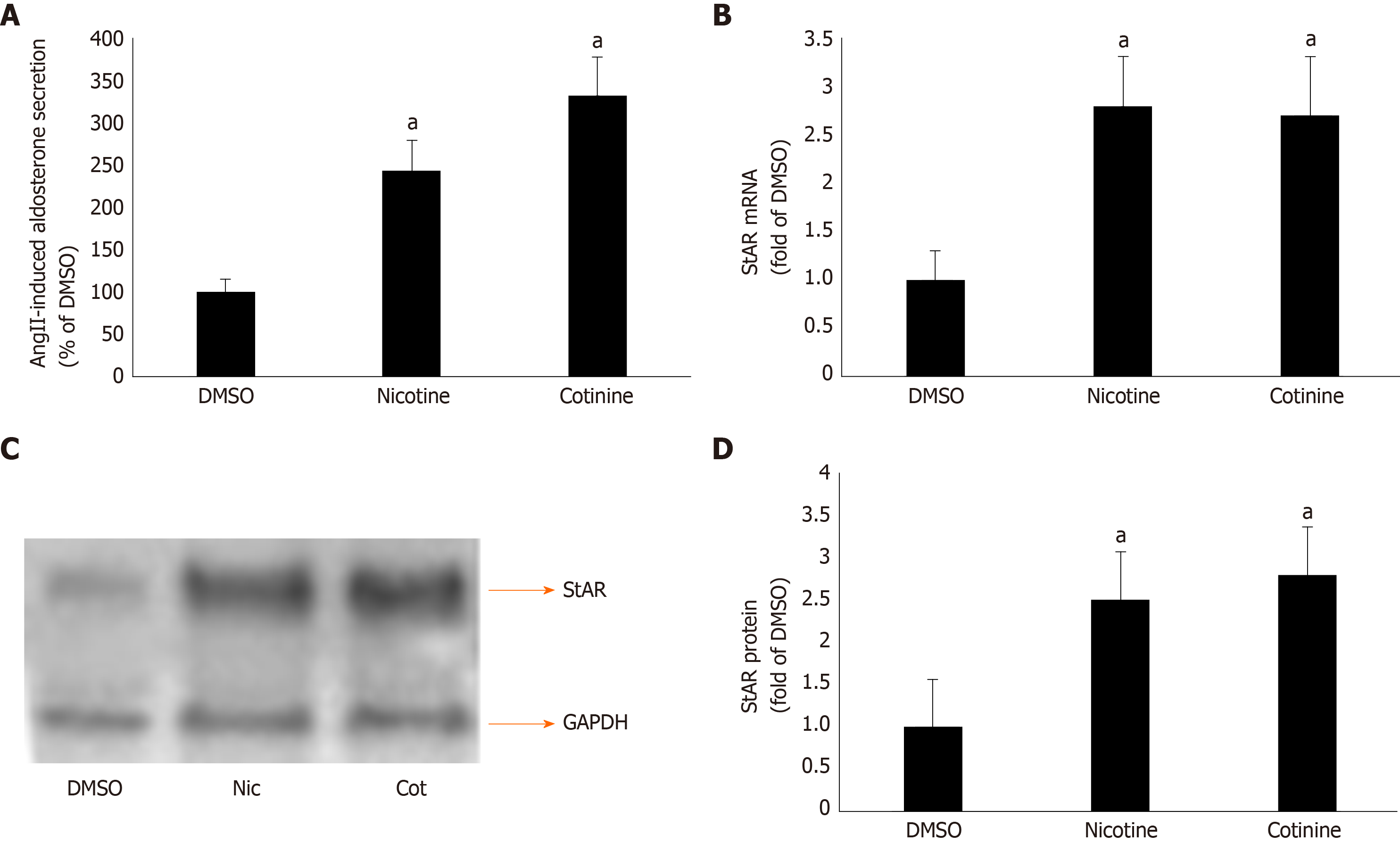
Given that βarrestin1 is critically involved in AngII-dependent aldosterone production in AZG cells[34,35], we next examined the impact of its tobacco-induced upregulation on aldosterone turnover in H295R cells. As expected, both nicotine and cotinine markedly enhanced AngII-induced aldosterone secretion (Figure 2A) from H295R cells, as well as StAR mRNA (Figure 2B) and protein (Figure 2C and D) levels in these cells.
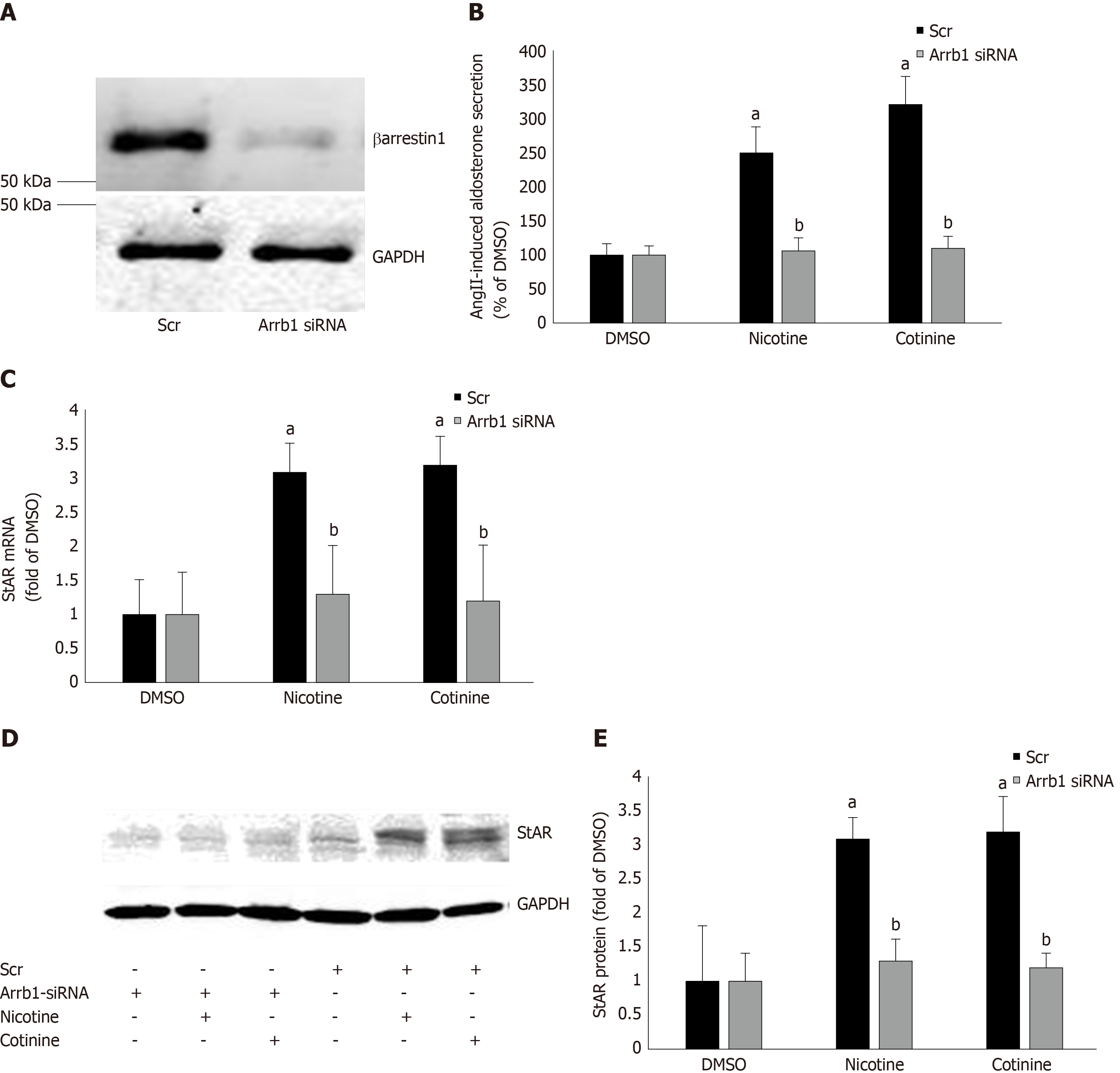
Since StAR upregulation signals aldosterone biosynthesis, these results suggest that tobacco compounds significantly enhance aldosterone synthesis and secretion in AZG cells. To prove that this augmentation of aldosterone production by nicotine/cotinine is mediated by the tobacco-upregulated βarrestin1, we knocked it down via siRNA in H295R cells (Figure 3A) and treated them again with nicotine and cotinine to examine the effect on aldosterone synthesis and secretion. Indeed, βarrestin1 knockdown almost completely abrogated the nicotine- and cotinine-induced enhancement of AngII-dependent aldosterone secretion (Figure 3B), StAR mRNA levels (Figure 3C), and StAR protein levels (Figure 3D and E) in H295R cells.
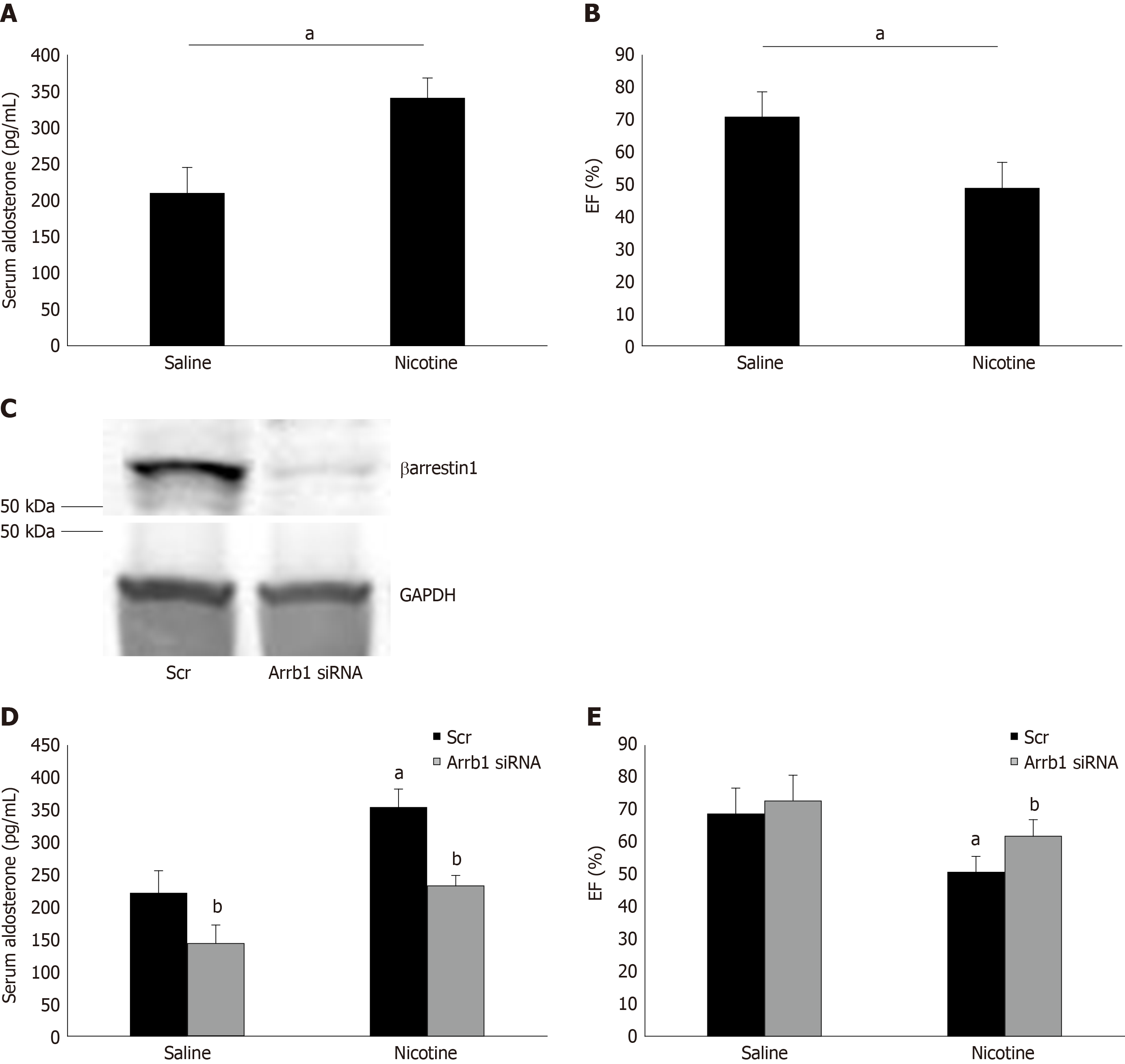
To demonstrate the physiological significance of our in vitro findings in H295R cells, we also treated adult male (otherwise healthy) rats with daily i.p. injections of nicotine for 7 d and, at the end of the 7-d-long treatment period, we measured their circulating aldosterone levels and cardiac function [ejection fraction (EF %)]. Of note, the daily dose of nicotine administered (1 mg/kg per day) is known to lead to blood circulating levels of nicotine comparable to those of chronic human smokers[9]. As shown in Figure 4A, nicotine exposure led to a significant hyperaldosteronism in these animals after one week of treatment. This led to cardiac dysfunction beginning to set in, since the nicotine-exposed animals displayed slightly but, nevertheless, significantly less EF compared to control saline-injected animals (Figure 4B). Notably, all animals used in the study had comparable EF at the beginning of the 7-d-long treatments (i.e., prior to group randomization) (69% ± 4.2%).
Importantly, in rats having βarrestin1 knocked-down via siRNA specifically in their adrenals (Figure 4C), nicotine exposure at the same concentration and for the same time-period (7 d) led to substantially less hyperaldosteronism (Figure 4D) and better cardiac function (higher EF) (Figure 4E) than in control animals receiving scrambled siRNA in their adrenals at the end of the 7-day-long treatments. Of note, adrenal βarrestin1 knockdown significantly lowered circulating aldosterone levels even in saline-treated animals (Figure 4D), which is consistent with βarrestin1’s essential role in adrenal aldosterone production in vivo[24]. However, this did not translate into better cardiac function of saline-treated animals (Figure 4E), probably because these animals were overall healthy and their cardiac function was optimal to begin with (there was a non-statistically significant trend toward higher EF though also in the saline-treated, Arrb1-siRNA rats, see Figure 4E). In any case, taken together, the in vivo results of Figure 4 strongly suggest that adrenal βarrestin1 mediates tobacco-related hyperaldosteronism and cardiac dysfunction in vivo, as well.
In the present study, we have identified adrenal βarrestin1 as a novel molecular target for mitigating the aldosterone-dependent cardiotoxic effects of tobacco. AngII induces aldosterone production in AZG cells by binding to its adrenal AT1R, which then activates βarrestin1[22]. Nicotine and cotinine are known to activate RAAS[13], promoting hyperaldosteronism. We report herein that these tobacco compounds chronically upregulate adrenal βarrestin1, promoting excessive aldosterone synthesis and secretion from human AZG cells in vitro and from adrenal glands in vivo. Thus, adrenal βarrestin1 appears to be a crucial component of tobacco-induced RAAS activation, which contributes to heart disease development/progression.
AngII-dependent aldosterone secretion and aldosterone biosynthesis, as measured by the expression levels of StAR, the rate-limiting enzyme in aldosterone biosynthesis, were found significantly higher in H295R cells treated with either nicotine or cotinine compared to control, vehicle-treated cells. Importantly, we uncovered that this was due to significant upregulation of βarrestin1, at both the mRNA and protein levels, in these cells, since βarrestin1 siRNA-mediated knockdown reversed the tobacco compound-induced increases in AngII-dependent aldosterone secretion and in StAR expression (i.e. aldosterone biosynthesis).
Of note, chronic nicotine exposure increased adrenal βarrestin1-mediated aldosterone synthesis and secretion, causing hyperaldosteronism, also in vivo. This led to development of cardiac dysfunction (ejection fraction drop/functional decline) in animals in vivo. As a proof of concept, adrenal-specific βarrestin1 siRNA-mediated knockdown in vivo normalized the elevated circulating aldosterone levels and significantly attenuated the cardiac functional decline induced by the chronic nicotine exposure in vivo. These findings strongly suggest that adrenal βarrestin1 inhibition (or genetic knockdown/ablation) might be of value in prevention or amelioration of tobacco-related heart disease progression and risk elevation.
The mineralocorticoid receptor (MR) is known to underlie HF pathology and it was recently documented to promote cardiac dysfunction and cardiomyopathy in transgenic mice, even in the absence of a cardiac insult[36]. Thus, aldosterone, the endogenous natural MR agonist, needs to be suppressed for heart disease therapy. Given various reports that this hormone oftentimes acts in an MR-independent manner[37], which circumvents the actions of MR antagonist (MRA) drugs, cutting aldosterone production at its source, i.e. the adrenal cortex, via βarrestin1 inhibition poses as an even more efficient approach to combat aldosterone’s (and tobacco’s) cardiotoxic actions than simply using MRA’s.
Adrenal βarrestin1 blockade has been shown to effectively suppress adrenal aldosterone production[28]. This is obviously feasible via gene therapy to knock down or knock out the protein specifically from the adrenal glands. Pharmacologic blockade of the adrenal AT1R with candesartan or valsartan, which are very potent βarrestin1 inhibitors, is another possible approach[25,26]. Of course, whether any ARB, like candesartan or valsartan, can effectively suppress the nicotine (tobacco)-induced elevation in aldosterone production from AZG cells is an open question right now and one that needs to be addressed in future studies. Alternatively, the βarrestin1-mediated signaling to aldosterone synthesis could be targeted with barbadin, a compound that was recently identified as an inhibitor of βarrestin-dependent internalization and signaling[38].
Finally, nicotine (and tobacco in general) has been known for decades to cause sympathetic activation and to increase circulating catecholamines, e.g., by stimulating catecholamine secretion from the adrenal medulla via direct agonism of nicotinic cholinergic receptors expressed on chromaffin cell membranes[30,39,40]. We report here that it can also promote production and secretion of another major adrenal hormone with important effects on the myocardium and the vasculature, i.e., aldosterone from the adrenal cortex. Nicotine achieves this thanks to direct upregulation of βarrestin1 in AZG cells, an AT1R-adapter protein that is an essential transducer linking the AngII hormone signal with aldosterone production in these cells[28]. Therefore, βarrestin1 might be a crucial molecular master-switch controlling tobacco-dependent stimulation of hormone production in the adrenal gland, which has enormous repercussions for cardiovascular homeostasis, in general, and for the function of the myocardium.
The present study has two major limitations: (1) The small animal group sizes-more data are needed in more animals and with other nicotine doses and routes of administration to fully confirm the present results; and (2) The study has to be repeated in larger animals (e.g., pigs or rabbits) that more closely resemble human physiology and disease conditions. In addition, all of the animals used in the present study were male; experiments need to be repeated in female rats, as well. Finally, AngII is only one of several stimuli for adrenal nicotine-induced aldosterone production. Perhaps different results will be obtained, if the aldosterone response to a different hormone is studied.
In conclusion, nicotine, and its major metabolite in humans cotinine, induce hyperaldosteronism via adrenal βarrestin1 upregulation, which mediates enhanced AngII-dependent aldosterone production from the adrenal cortex in vitro and in vivo. Thus, adrenal βarrestin1 upregulation is an essential biological mechanism underlying the tobacco-induced increase in RAAS activity observed in chronic smokers and in chronically tobacco-exposed animals. This results in raised aldosterone levels and thus, in increased cardiac dysfunction and cardiovascular risk. Therefore, adrenal βarrestin1 inhibition, either pharmacologically or genetically (via siRNA-mediated knockdown or even CRISPR/Cas9-mediated gene excision), poses as an attractive therapeutic or even preventive strategy for mitigating the devastatingly toxic effects of tobacco on the heart, by reducing the neurohormonal (aldosterone) burden of the myocardium.
Nicotine, the main addictive compound in tobacco, is associated with major cardiovascular adverse events, such as heart failure and hypertension. One of the molecular mechanisms underlying nicotine-induced cardiotoxicity is elevation of renin-angiotensin-aldosterone system (RAAS) activity. Nicotine, and its major metabolite in humans cotinine, have been reported to induce RAAS activation, resulting in hyperaldosteronism. Aldosterone has myriad adverse cardiac effects and is produced by the adrenal cortex in response to angiotensin II (AngII) acting through its type 1 receptors (AT1Rs). AT1Rs induce aldosterone production via both Gq/11 proteins and βarrestin1 (Arrestin-2).
It was hypothesized that nicotine activates adrenal ßarrestin1, thereby contributing to RAAS activation and heart disease development.
We tested our hypothesis by investigating the effects of nicotine on aldosterone production in vitro and on aldosterone levels and cardiac function of experimental animals in vivo.
We used the human adrenocortical zona glomerulosa (AZG) cell line H295R, in which we performed real-time polymerase chain reaction (PCR) and western blotting to measure βarrestin1 mRNA and protein levels, respectively, as well as ELISA to measure aldosterone secretion. We also manipulated βarrestin1 expression via siRNA-mediated knockdown in H295R cells. For the in vivo studies, we used adult male Sprague-Dawley rats, which we exposed to chronic nicotine administration after adrenal-specific, βarrestin1 siRNA-mediated knockdown or control scrambled siRNA delivery in vivo.
Nicotine and cotinine upregulate βarrestin1 mRNA and protein levels in AZG cells, which augments aldosterone synthesis and secretion. In contrast, siRNA-mediated βarrestin1 knockdown mitigates the effects of nicotine on AngII-induced aldosterone production. In vivo, nicotine-exposed experimental rats with adrenal-specific βarrestin1 knockdown display lower circulating aldosterone levels and better cardiac function than nicotine-exposed control animals with normal adrenal βarrestin1 expression.
Adrenal βarrestin1 upregulation is one of the mechanisms by which tobacco, i.e. nicotine, promotes cardio-toxic hyperaldosteronism that accelerates cardiac functional decline, both in vitro and in vivo.
Adrenal βarrestin1 pharmacological blockade or genetic deletion (or knockdown) represents a novel therapeutic strategy to ameliorate tobacco-related heart disease morbidity and mortality.
The authors would like to acknowledge Dr. Lina Shehadeh, Univ. of Miami Miller School of Medicine (Miami, FL, United States) and members of her laboratory for excellent technical assistance.
Manuscript source: Invited Manuscript
Corresponding Author's Membership in Professional Societies: American Heart Association (000144729187); Heart Failure Society of America; American Association for the Advancement of Science; American Society of Pharmacology and Experimental Therapeutics; British Pharmacological Society; Endocrine Society of the United States of America; American Association of Colleges of Pharmacy; and American Chemical Society and European Society of Cardiology.
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kharlamov AN, Teragawa H S-Editor: Wang J L-Editor: A E-Editor: Qi LL
| 1. | Weber KT. Aldosterone in congestive heart failure. N Engl J Med. 2001;345:1689-1697. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 537] [Cited by in F6Publishing: 543] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 2. | Connell JM, Davies E. The new biology of aldosterone. J Endocrinol. 2005;186:1-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 237] [Cited by in F6Publishing: 241] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | Marney AM, Brown NJ. Aldosterone and end-organ damage. Clin Sci (Lond). 2007;113:267-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 163] [Cited by in F6Publishing: 161] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 4. | Zhao W, Ahokas RA, Weber KT, Sun Y. ANG II-induced cardiac molecular and cellular events: role of aldosterone. Am J Physiol Heart Circ Physiol. 2006;291:H336-H343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Swedberg K, Eneroth P, Kjekshus J, Wilhelmsen L. Hormones regulating cardiovascular function in patients with severe congestive heart failure and their relation to mortality. CONSENSUS Trial Study Group. Circulation. 1990;82:1730-1736. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 821] [Cited by in F6Publishing: 861] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 6. | Pitt B, Remme W, Zannad F, Neaton J, Martinez F, Roniker B, Bittman R, Hurley S, Kleiman J, Gatlin M; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309-1321. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3634] [Cited by in F6Publishing: 3289] [Article Influence: 156.6] [Reference Citation Analysis (0)] |
| 7. | Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, Palensky J, Wittes J. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709-717. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6409] [Cited by in F6Publishing: 5887] [Article Influence: 235.5] [Reference Citation Analysis (0)] |
| 8. | de Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev. 2000;52:415-472. [PubMed] [Cited in This Article: ] |
| 9. | Benowitz NL, Porchet H, Jacob III P. Pharmacokinetics, metabolism, and pharmacodynamics of nicotine. In: Wonnacott 5, Russell MAH, Stolerman IP. Nicotine Psychopharmacology; Molecular, Cellular and Behavioral Aspects. Oxford, UK: Oxford University Press, 1990: 112-157. [Cited in This Article: ] |
| 10. | Rubin RP, Warner W. Nicotine-induced stimulation of steroidogenesis in adrenocortical cells of the cat. Br J Pharmacol. 1975;53:357-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Oakes JM, Fuchs RM, Gardner JD, Lazartigues E, Yue X. Nicotine and the renin-angiotensin system. Am J Physiol Regul Integr Comp Physiol. 2018;315:R895-R906. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 12. | Skowronski RJ, Feldman D. Inhibition of aldosterone synthesis in rat adrenal cells by nicotine and related constituents of tobacco smoke. Endocrinology. 1994;134:2171-2177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Laustiola KE, Lassila R, Nurmi AK. Enhanced activation of the renin-angiotensin-aldosterone system in chronic cigarette smokers: a study of monozygotic twin pairs discordant for smoking. Clin Pharmacol Ther. 1988;44:426-430. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Yuan YM, Luo L, Guo Z, Yang M, Ye RS, Luo C. Activation of renin-angiotensin-aldosterone system (RAAS) in the lung of smoking-induced pulmonary arterial hypertension (PAH) rats. J Renin Angiotensin Aldosterone Syst. 2015;16:249-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Rainey WE, Saner K, Schimmer BP. Adrenocortical cell lines. Mol Cell Endocrinol. 2004;228:23-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 16. | Ganguly A, Davis JS. Role of calcium and other mediators in aldosterone secretion from the adrenal glomerulosa cells. Pharmacol Rev. 1994;46:417-447. [PubMed] [Cited in This Article: ] |
| 17. | Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308:512-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1331] [Cited by in F6Publishing: 1329] [Article Influence: 69.9] [Reference Citation Analysis (0)] |
| 18. | Luttrell LM, Gesty-Palmer D. Beyond desensitization: physiological relevance of arrestin-dependent signaling. Pharmacol Rev. 2010;62:305-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 307] [Cited by in F6Publishing: 321] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 19. | Lymperopoulos A, Bathgate A. Pharmacogenomics of the heptahelical receptor regulators G-protein-coupled receptor kinases and arrestins: the known and the unknown. Pharmacogenomics. 2012;13:323-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153-183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 1318] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 21. | Osman H, Murigande C, Nadakal A, Capponi AM. Repression of DAX-1 and induction of SF-1 expression. Two mechanisms contributing to the activation of aldosterone biosynthesis in adrenal glomerulosa cells. J Biol Chem. 2002;277:41259-41267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Lymperopoulos A, Rengo G, Zincarelli C, Kim J, Soltys S, Koch WJ. An adrenal beta-arrestin 1-mediated signaling pathway underlies angiotensin II-induced aldosterone production in vitro and in vivo. Proc Natl Acad Sci USA. 2009;106:5825-5830. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Lymperopoulos A, Rengo G, Zincarelli C, Kim J, Koch WJ. Adrenal beta-arrestin 1 inhibition in vivo attenuates post-myocardial infarction progression to heart failure and adverse remodeling via reduction of circulating aldosterone levels. J Am Coll Cardiol. 2011;57:356-365. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 68] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Bathgate-Siryk A, Dabul S, Pandya K, Walklett K, Rengo G, Cannavo A, De Lucia C, Liccardo D, Gao E, Leosco D, Koch WJ, Lymperopoulos A. Negative impact of β-arrestin-1 on post-myocardial infarction heart failure via cardiac and adrenal-dependent neurohormonal mechanisms. Hypertension. 2014;63:404-412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 25. | Lymperopoulos A, Sturchler E, Bathgate-Siryk A, Dabul S, Garcia D, Walklett K, Rengo G, McDonald P, Koch WJ. Different potencies of angiotensin receptor blockers at suppressing adrenal β-Arrestin1-dependent post-myocardial infarction hyperaldosteronism. J Am Coll Cardiol. 2014;64:2805-2806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 26. | Dabul S, Bathgate-Siryk A, Valero TR, Jafferjee M, Sturchler E, McDonald P, Koch WJ, Lymperopoulos A. Suppression of adrenal βarrestin1-dependent aldosterone production by ARBs: head-to-head comparison. Sci Rep. 2015;5:8116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 27. | Maning J, Negussie S, Clark MA, Lymperopoulos A. Biased agonism/antagonism at the AngII-AT1 receptor: Implications for adrenal aldosterone production and cardiovascular therapy. Pharmacol Res. 2017;125:14-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 28. | Lymperopoulos A, Aukszi B. Angiotensin receptor blocker drugs and inhibition of adrenal beta-arrestin-1-dependent aldosterone production: Implications for heart failure therapy. World J Cardiol. 2017;9:200-206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Valero TR, Sturchler E, Jafferjee M, Rengo G, Magafa V, Cordopatis P, McDonald P, Koch WJ, Lymperopoulos A. Structure-activity relationship study of angiotensin II analogs in terms of β-arrestin-dependent signaling to aldosterone production. Pharmacol Res Perspect. 2016;4:e00226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Lymperopoulos A, Rengo G, Funakoshi H, Eckhart AD, Koch WJ. Adrenal GRK2 upregulation mediates sympathetic overdrive in heart failure. Nat Med. 2007;13:315-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 31. | Lymperopoulos A, Rengo G, Gao E, Ebert SN, Dorn GW 2nd, Koch WJ. Reduction of sympathetic activity via adrenal-targeted GRK2 gene deletion attenuates heart failure progression and improves cardiac function after myocardial infarction. J Biol Chem. 2010;285:16378-16386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 88] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 32. | McCrink KA, Maning J, Vu A, Jafferjee M, Marrero C, Brill A, Bathgate-Siryk A, Dabul S, Koch WJ, Lymperopoulos A. β-Arrestin2 Improves Post-Myocardial Infarction Heart Failure via Sarco(endo)plasmic Reticulum Ca2+-ATPase-Dependent Positive Inotropy in Cardiomyocytes. Hypertension. 2017;70:972-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 33. | Lymperopoulos A, Rengo G, Zincarelli C, Soltys S, Koch WJ. Modulation of adrenal catecholamine secretion by in vivo gene transfer and manipulation of G protein-coupled receptor kinase-2 activity. Mol Ther. 2008;16:302-307. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Markan U, Pasupuleti S, Pollard CM, Perez A, Aukszi B, Lymperopoulos A. The place of ARBs in heart failure therapy: is aldosterone suppression the key? Ther Adv Cardiovasc Dis. 2019;13:1753944719868134. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Solesio ME, Mitaishvili E, Lymperopoulos A. Adrenal βarrestin1 targeting for tobacco-associated cardiac dysfunction treatment: Aldosterone production as the mechanistic link. Pharmacol Res Perspect. 2019;7:e00497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Oakley RH, Cruz-Topete D, He B, Foley JF, Myers PH, Xu X, Gomez-Sanchez CE, Chambon P, Willis MS, Cidlowski JA. Cardiomyocyte glucocorticoid and mineralocorticoid receptors directly and antagonistically regulate heart disease in mice. Sci Signal. 2019;12:eaau9685. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 60] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 37. | Parker BM, Wertz SL, Pollard CM, Desimine VL, Maning J, McCrink KA, Lymperopoulos A. Novel Insights into the Crosstalk between Mineralocorticoid Receptor and G Protein-Coupled Receptors in Heart Adverse Remodeling and Disease. Int J Mol Sci. 2018;19:3764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Beautrait A, Paradis JS, Zimmerman B, Giubilaro J, Nikolajev L, Armando S, Kobayashi H, Yamani L, Namkung Y, Heydenreich FM, Khoury E, Audet M, Roux PP, Veprintsev DB, Laporte SA, Bouvier M. A new inhibitor of the β-arrestin/AP2 endocytic complex reveals interplay between GPCR internalization and signalling. Nat Commun. 2017;8:15054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 39. | Lymperopoulos A, Rengo G, Koch WJ. Adrenal adrenoceptors in heart failure: fine-tuning cardiac stimulation. Trends Mol Med. 2007;13:503-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 40. | Lymperopoulos A, Brill A, McCrink KA. GPCRs of adrenal chromaffin cells & catecholamines: The plot thickens. Int J Biochem Cell Biol. 2016;77:213-219. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |