INTRODUCTION
The National Academy of Medicine released a report in 2010 highlighting recommendations with regards to what the United States Department of Health and Human Services can do to improve population health[1]. One of the suggested approaches in the report highlighted that the biological and environmental causes of poor health are complex and inter-related. Computer simulation models and other novel analytical tools such as artificial intelligence (AI) can potentially elucidate these relationships and help us better understand the underlying pathophysiology. The main pre-requisite for such models is that they should be built on the foundation of plausible biological and physiological understanding and algorithms.
In a world increasingly surrounded by data, digital twins have been used in everything from wind turbines to cities to spacecraft to model processes and preempt problems[2]. The European Union has even been attempting to create a digital twin model of planet earth to better forecast weather and predict climate change[3]. It would not be unreasonable to think that these technological advances could be applied to the field of healthcare as well. With the recent rise of electronic medical records, more sophisticated monitoring, and molecular biology in healthcare, digital twin technology provides a unique opportunity to personalize medicine to the level of the individual patient[4]. Digital twins are able to integrate vast amounts of data to create digital replicas of the physical environment and acts as models that are able to inform clinical decision making in an actionable way[5].
There is a need to evaluate the status of research on the use of simulation applications by various medical and surgical specialties to identify and recommend areas of research wherein there is a significant knowledge gap. This urgency is further compounded by the issue that medical errors are one of the leading causes of death in the United States[6]. Whether the use of simulation models by expert clinicians (or trainees) will improve the overall patient outcomes in clinical practice remains a challenging research question. Yet, it would be unquestionably helpful to test medical decisions in an “in silico” environment before attempting our treatment strategies on real patients. Such a testing environment would be especially useful to evaluate management decisions of uncertain benefit the patients.
WHAT IS A DIGITAL TWIN?
Digital twins are a concept from engineering whereby digital models of a system are built to allow testing of products more efficiently and economically[2]. The development of the use of a “Twin AI” for predictive modeling in health care first caught attention in 2003 with the Archimedes project, which sought to model the complicated management of diabetes and was validated to 18 different trials involving diabetes with a very high correlation despite the fact that the trial data was not used to develop the model[7]. These new digital twin AI models are able to integrate the various demographic and individual-specific factors that complicate diabetes management on a level that the human brain cannot[8]. In addition to proving an accurate predictive model at the population level, Archimedes has also been shown to make accurate predictions for individuals[9]. The high accuracy of prediction and fidelity of the model led to its use in in-silico clinical trials, thereby saving crucial time, millions of dollars and most importantly shielding patients from being exposed to harm from interventions that may or may not have been beneficial[8,10].
In clinical practice, the concept of digital twins has also been applied to the fields of cardiology and endocrinology[11-13]. In cardiology, a few digital twin models have recently been developed to allow clinicians to provide precise care tailored to the patient by considering inter-individual variability and integrating the wide spectrum of biologic, environmental, and lifestyle data that influence cardiovascular outcomes. However, there is still much work to be done before these models become common in clinical practice[12]. Additionally, AI has been used to create large-scale synthetic data for training of other machine learning algorithms[14]. In Endocrinology, an AI model of the pancreas has been developed for use in the critical care setting to manage patients’ glucose levels[13].
In the field of undergraduate medical education, programs that utilize an AI model of physiology, such as justphysiology and sycamore, have recently been incorporated in curricula[15]. These simulations afford the benefits of providing a safe practice environment for trainees, exposing students to a range of pathology that is not restricted to the available patient population, and getting students to engage actively with the underlying physiological principles involved in chronic disease management. While these models are based on solid mathematical models of human physiology, they are focused on chronic disease management rather than the acute pathology seen in critical care units and are unable to adapt to prospective data from real-time patients.
Digital twin AI models can be developed as “associative models” (mostly data driven) or “actionable models” (based on causal inference). Associative models are built using retrospective electronic health record data, which is more readily available. Utilizing a database of 703782 patients, Tomašev et al[16] created an associative AI model that was able to predict 55.8% of inpatient acute kidney injury events at 48 h. While these models are great at providing prognostic information, they do not offer information on the effects of different interventions on patient care. Additionally, these models are purely data-driven and do not consider the underlying physiology or causal pathways of disease in their development. The clinical utility of these models is limited by the lack of precision and underperformance in the clinical setting. In comparison, actionable AI models (or, as we have previously coined them, “Causal AI” models) are developed with explicit consideration of causal pathways, providing greater clinical utility in predicting the outcome of a given intervention as well as providing clinicians a better understanding of how the AI model is reaching its conclusions[17,18].
AI APPLICATIONS IN NEUROCRITICAL CARE
While digital twin models have been developed and tested for use in the fields of diabetes, cardiology, and sepsis management, this model has not yet been tested in the neurocritical care (NCC) unit. Yet, the NCC unit is an optimal place to develop “Twin AI” model. Within the NCC unit, there is a large need to integrate vast amounts of data including intracranial pressure, electroencephalography, hemodynamics, ventilation parameters, body temperature, and fluid balance, along with the neurological exam to allow neurointensivists to make time-sensitive and impactful decisions for patient care[19,20]. Use of AI to augment clinical decision making also has the potential to reduce costs and improve access to quality care for patients in areas where the expertise of a NCC physician is not readily available[21].
In NCC, current AI technology focuses on interpreting electroencephalography, monitoring intracranial pressure (ICP), and prognosticating outcomes[22]. AI models have been developed to interpret electroencephalograms by helping to annotate the tracings, detecting seizures, and identifying brain activation in unresponsive patients[23-26]. More specific models have been developed to analyze waveforms of ICP to detect artifact in ICP measurements, predict future ICP levels, determine which patients are at risk of increased ICP, and prognosticate mortality[27-30]. AI models are able to provide prognostic information for patients with subarachnoid hemorrhage, traumatic brain injury, or who are at risk for health-care associated ventriculitis and meningitis[31-33]. In the European Union, technologies such as Avert-IT have been developed for use in the critical unit to predict hypotensive events in patients with traumatic brain injury[34]. Still, to our knowledge, a model that integrates all the measures available in the NCC unit to create a broad digital twin model of the patient does not yet exist.
Having a digital twin model that can accurately replicate patient physiology in the NCC environment would have distinct advantages. Such a model would allow training physicians to sharpen their clinical decision making and provide opportunities to trial different treatments without ever risking patient safety. Preliminary results of a digital twin model used to predict response to treatments in patients in the intensive care unit with sepsis within the first 24 h have shown that creating such a model is possible[18].
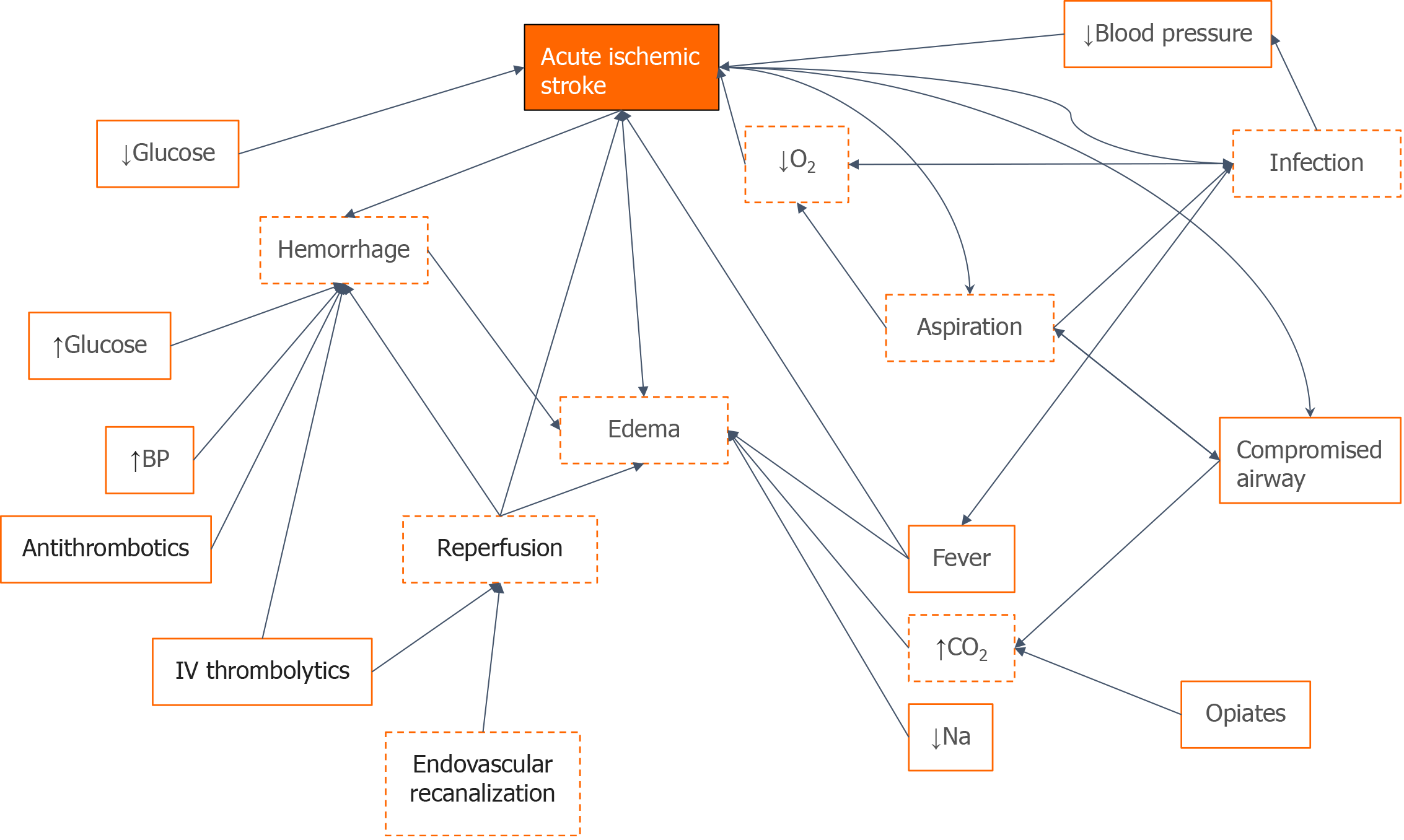
A similar approach should be feasible for neurocritical diseases and illustrations of how these models could be conceptually built for application in NCC are shown in Figures 1 and 2. In applying this model to a patient with ischemic stroke, for example, factors such as blood pressure, glucose levels, securing an airway, and giving anticoagulation, thrombolytics, or opiate medication are all actionable factors that can be input into the AI model. These actions will affect certain semi-actionable factors and the overarching concept in the digital twin AI model such as hemorrhage, edema, aspiration, and, ultimately, ischemic stroke, all connected by Bayesian networks. Similar models such as this will be built for other disease states within the NCC unit as well. With this digital twin of the patient, trainees will be able to test different interventions and get real-time feedback on the effects of their intervention without ever having to worry about potential harm to the actual patient.
Figure 1 A directed acyclic graph for stroke patients that link concepts through Bayesian networks built from an underlying understanding of disease processes.
Orange boxes represent concepts, orange solid lines represent actionable factors, dashed red lines represent semi-actionable factors, arrows represent Bayesian connections between different variables. O2: Oxygen; CO2; Carbon dioxide; BP: Blood pressure; Na: Sodium.
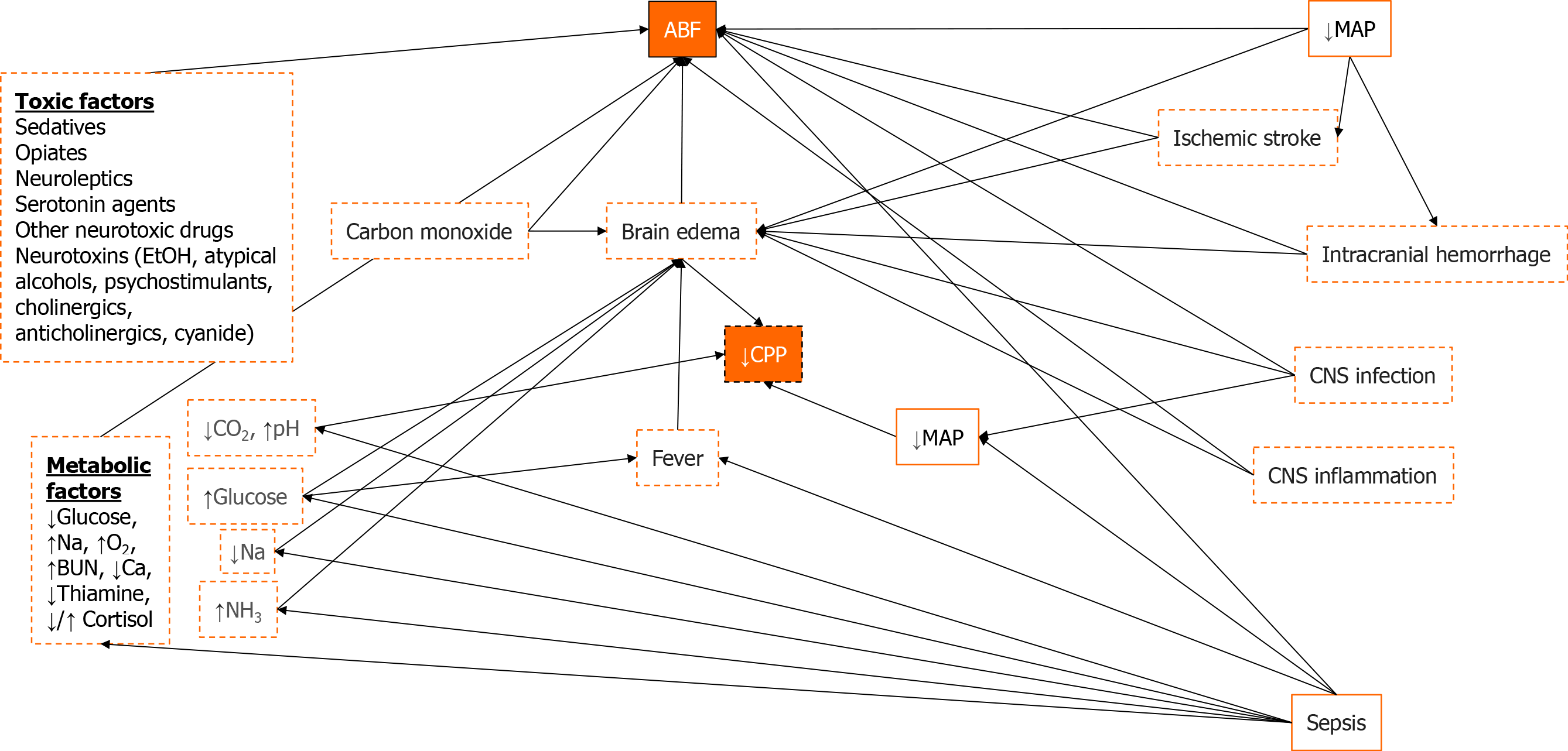
Figure 2 A directed acyclic graph for acute brain failure that links concepts through Bayesian networks built from an underlying understanding of disease processes.
Orange boxes represent concepts, orange solid lines represent actionable factors, dashed red lines represent semi-actionable factors, arrows represent Bayesian connections between different variables. MAP: Mean arterial pressure; CPP: Cerebral perfusion pressure; NH3: Ammonium; Na: Sodium; BUN: Blood urea nitrogen; Ca: Calcium; O2: Oxygen; ABF: Acute brain failure; CNS: Central nervous system.
UTILITY IN MEDICAL EDUCATION
The central purpose of medical education, learning and assessment is to optimize patient care, avoid harm to the patients, and improve the cognitive skills of practitioners and learners alike. Continual learning and retooling are a vital aspect of practicing medicine. A major concern in healthcare and medical education is that initial training must be provided with minimal risk to patients. Moreover, maintenance of skills among busy physicians practicing in the community is an ever-growing concern.
The utilization of a virtual environment to enhance the procedural performance through simulation is not a new concept. High-fidelity simulators are now a prerequisite for gaining proficiency in endoscopic, laparoscopic, and robotic surgery[35]. With the advent of minimally invasive surgical procedures, it became evident that there is a dire need for skill acquisition outside the operating theater before attempting a similar procedure on real patients[36]. Despite the compelling evidence in various areas of clinical medicine, the world of critical care medicine has lagged in providing a well-equipped platform for cognitive training and skill acquisition in the virtual environment.
Creating an “in-silico” model or a “digital twin” allows learning, cognitive skill acquisition and refinement in an environment that does not expose patients to the risk of uncertain interventions and offers the ability to test the cognitive domains of decision making in real time with rapid assessment and perceptible metrics. We envision creating such an educational tool with potential refinement to a level that it can be used as a digital twin to assess the effect of an intervention in the virtual environment without exposing actual patients to risk. Early in the medical education program, even low fidelity patient presentations can be a good fit for assessment purposes if appropriately matched for the level of learner and educational level. The digital twin AI model can not only be used for medical education but can also be utilized for summative assessment where the cognitive competency of the critical care trainees can be assessed in an objective manner to determine if he/she can be graduated to the next level.
BUILDING THE AI MODEL–CHALLENGES AND ETHICAL CONSIDERATIONS
AI model should be constructed in such a way that they augment, rather than attempt to replace, the clinician’s judgment[37]. Transparent AI models based on our understanding of pathophysiology are more likely to be trusted, and consequently implemented into practice, by clinicians than “black-box” AI models that reach their conclusions through multiple layers of neural networks. Actionable AI models should therefore be based on sound biology and should aim to replicate real-life disease processes.
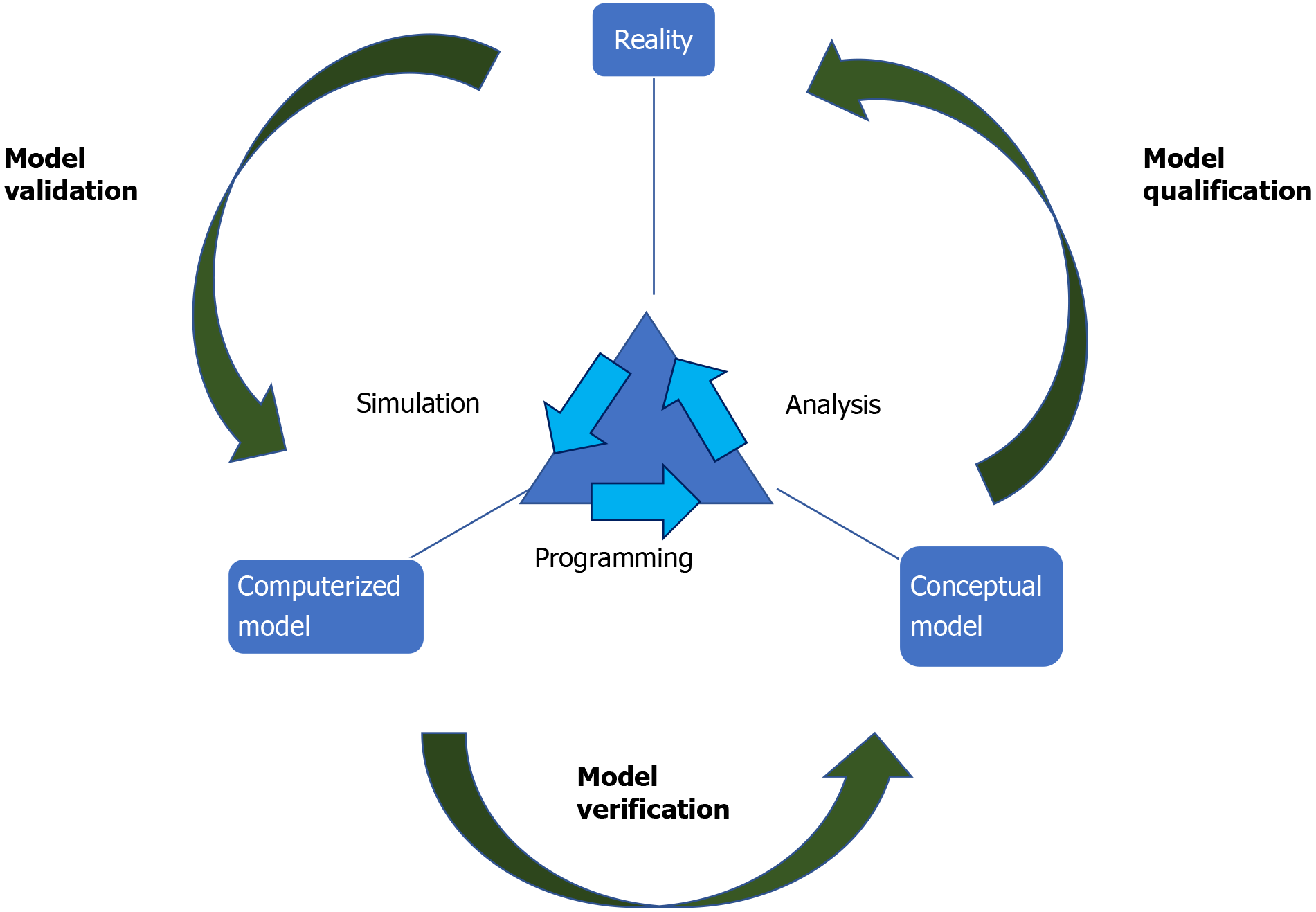
Building these models starts with directed acyclic graphs (DAGs). DAGs are diagrams that connect concepts (defined as variables) through Bayesian networks that represent the probabilistic relationship between those concepts (Figures 1 and 2). These DAGs, built from an understanding of underlying pathophysiology and in collaboration with content experts act as a base for the development of the AI model. Expert knowledge is necessary to develop the rules that will connect the variables (i.e., what would be expected to happen to the connected variables after a certain change in one of them). To avoid bias, we intend to gain expert consensus on our rules using DELPHI method, an iterative process of surveying experts that seeks to integrate knowledge about a specific field, before constructing the AI models. These DAGs are then converted into statements that can then be transformed into code and incorporated into the AI model. Once the model is developed, it will be prospectively validated by comparing its predictions to the actual clinical findings in real patients, the irreplaceable gold standard for any AI application to health care. This process will go through multiple cycle or iterations of computer modeling (programming), comparing the performance of the digital twin in an “in-silico” environment (simulation) and gathering of qualitative and quantitative data to improve the performance of the model (analysis) (Figure 3). This process was piloted in our feasibility study for the digital twin of critically ill sepsis patients[18].
Figure 3 Accurate verification and validation of the model using the iterative steps of programming, simulation, and analysis[39].
While a digital twin model in healthcare could lead to a more accurate, individualized model of health and diseased states, this new technology also brings with it ethical questions, such as who will have access to this new technology, how this technology may lead to a deemphasizing of patient autonomy in favor of algorithms, and how compiling large amounts of health data may lead to identification of trends that may justify future divisiveness and segregation[38]. In creating any new AI technology, we must be cognizant of the ethical and safety implications of the new technology and ensure that any new AI model acts to augment rather than supersede clinician judgement. Like any nascent technology, AI models can be initially erroneous or insufficiently accurate; validation is therefore essential for their refinement and must always be conducted before their implementation.
CONCLUSION
While digital twin models have been established in the fields of cardiology, endocrinology, and undergraduate medical education, a validated model has not yet been adopted to training and clinical practice in the field of NCC. We propose to develop actionable digital twin models based on an understanding of the underlying pathophysiology of disease to train future physicians and potentially inform clinical decision making in the complex environment of NCC.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Society of Critical Care Medicine; American College of Chest Physicians; and American College of Physicians.
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cavdar SC, Ma PL S-Editor: Zhang H L-Editor: A P-Editor: Wang LL